Difference between revisions of "ANYL Sem Abstracts"
(→Monday, October 17, 2016: Added 1st yr student abstracts for 31Oct16.) |
(→4 November 2024: Natalie's title) |
||
| (816 intermediate revisions by 6 users not shown) | |||
| Line 3: | Line 3: | ||
A shortcut to this page is: [http://tinyurl.com/anylsem http://tinyurl.com/anylsem] | A shortcut to this page is: [http://tinyurl.com/anylsem http://tinyurl.com/anylsem] | ||
| − | == | + | ==4 November 2024== |
| − | + | <B>Freedom from Fluorocarbons: 2D Perovskites as the Next Generation of Refrigerants</b> | |
| − | + | ||
| − | + | Leo Bloxham, <br> | |
| − | + | CU ANYL 1st year | |
| − | + | ||
| − | + | Phase change materials (PCMs) absorb, store, and release thermal energy as latent heat when transitioning between different physical states. Currently, hydrofluorocarbons (HFCs) are the most common PCM used for refrigeration purposes. Since HFCs are known greenhouse gasses, any alternative PCM which does not release gas into the atmosphere is of very high interest. Two-dimensional (2D) perovskites with n-alkylammonium cations often undergo a solid-solid phase transition upon changes in pressure or temperature, during which the organic cations undergo an order-to-disorder partial melting transition. Here, we demonstrate tunability of this phase transition by various alloying methods, including blending organic cations of varying lengths and blending the central metal cations. We see that blending organic cations significantly decreases the phase transition temperature of Cu and Mn-based 2D perovskites. The magnitude of this decrease depends on the relative lengths of the two n-alkylammonium cations, as well as the molar ratio of each organic cation included. Furthermore, we found that blending Cu2+ and Mn2+ has a slight effect on the phase transition temperature due to similar lattice spacing between the inorganic layers in DA2CuCl4 and DA2MnCl4. Our study suggests new design principles for tuning structural phase transitions in 2D perovskites through alloying organic and metal cations, proving 2D perovskites to be strong candidates for the next generation of refrigerants. | |
| − | + | ||
| − | + | and | |
| − | + | ||
| − | <B>Measurements of Peroxy Radical Loss Rates on Laboratory Surfaces</B> | + | <b>Rotational Spectroscopy of Water-Ketone Clusters</b> |
| − | + | ||
| − | Benjamin Deming, First Year Graduate Student | + | Natalie Couch, <br> |
| − | <br>Department of Chemistry and Biochemistry | + | CU ANYL 1st year |
| − | <br>University of Colorado Boulder | + | |
| − | + | Gas phase microwave spectroscopy is a powerful tool for investigating intermolecular interactions such as hydrogen bonding. These weak interactions are difficult to investigate in solution, where, for example, hydrogen bonds are constantly being broken and reformed. In the ultrahigh vacuum (~10-6 Torr) inside my undergraduate lab’s microwave spectrometer, clusters of molecules connected via hydrogen bonds exist long enough for us to probe their rotational transitions. The microwave region of the electromagnetic spectrum corresponds to energy differences between clusters’ rotational states, which give precise information on the structure of the cluster. In this presentation, I will discuss interactions between water and 2-methylcyclopentanone, a ketone that acts as a hydrogen bond acceptor. I will describe my experimental setup to acquire spectra of these clusters and show some preliminary results. | |
| − | Peroxy radicals are important intermediates in the oxidative processing of volatile organic compounds in the atmosphere. Numerous instruments to measure atmospheric concentrations of these short-lived species are in active development. Wall losses of these compounds should be considered when designing such an instrument due to losses within the inlet. In this work, an ECHAMP peroxy radical detector was used to determine the wall-loss rates of HO 2 , CH 3 O 2 , C 2 H 5 O 2 , and isoprene peroxy radicals on PFA Teflon, quartz, halocarbon wax, and other materials. In addition to concentration and relative humidity dependencies, the use of FEP vs PFA Teflon was investigated. | + | |
| − | + | ==28 October 2024== | |
| − | = | + | <b>The Airborne and Remote sensing Methane and Air Pollutant Surveys (AiRMAPS) and Initial Results from Colorado and Utah</b> |
| − | + | ||
| − | <B>Liquid water on Mars? Stable or metastable salt solutions formed via deliquescence</B> | + | Steven S. Brown, <br> |
| − | + | Tropospheric Chemistry and Atmospheric Remote Sensing Program Lead, <br> | |
| − | Raina Gough | + | NOAA Chemical Sciences Laboratory, Boulder, CO <br> |
| − | <br>Department of Chemistry and Biochemistry and CIRES | + | Adjoint Professor of Chemistry, <br> |
| − | <br>University of Colorado Boulder | + | University of Colorado, Boulder, CO |
| − | + | ||
| − | Several different hygroscopic salts are known to exist in the Martian soil. Although Mars is a cold, dry planet where pure liquid water is not stable, deliquescence of perchlorate and other species can likely provide small amounts of liquid water temporarily. We use Raman microscopy and an environmental cell to probe the conditions under which such liquid brines can form and persist under low Martian temperatures. We will discuss the brine-forming ability of several different Mars-relevant salts and salt mixtures, as well as the potential habitability or toxicity of these aqueous solutions to bacterial spores. We discuss the relevance of these studies to the recently publicized “recurring slope lineae” (RSL) on Mars that may be evidence of liquid water flows. We also discuss the results of experiments we have done on salty sediment samples from the Dry Valleys of Antarctica, the only terrestrial analog site for the Martian RSL. | + | The Airborne and Remote Sensing Methane and Air Pollutant Surveys (AiRMAPS) is a 3-5 year initiative led by the NOAA Office of Oceanic and Atmospheric Research (OAR) and National Environmental and Satellite Data Information Service (NESDIS) to comprehensively characterize U.S. emissions and impacts of methane and co-emitted air pollutants. The 2024 AiRMAPS campaign consisted of two separate deployments in Colorado (June 28 - July 14) and Utah (July 15 – August 18). The Colorado campaign included a NOAA Twin Otter equipped with in-situ instruments and a Doppler lidar for 3-dimensional winds for boundary layer mass balance, a NASA-supported B-200 with the AVIRIS-3 imaging spectrometer for detailed methane mapping, three mobile laboratories driving at surface level, and images from the GHGSat spaceborne spectrometer. The Colorado campaign surveyed oil and gas, agricultural, landfill and urban emissions in the Denver-Julesburg basin and Denver metro area. The Utah campaign consisted of mass balance flights with a NOAA Twin Otter, three mobile laboratories and surface networks, mapping overflights of the NSF G-V aircraft with the MethaneAir instrument, and satellite overpasses of MethaneSat. This campaign included detailed surveys of the Salt Lake City urban area, and coordinated aircraft measurements in the Uinta Basin oil and gas producing region. This presentation will outline the goals of and strategy for AiRMAPS and present initial results from the 2024 campaigns in Colorado and Utah. |
| − | + | ||
| − | + | and | |
| − | <B>Electronic Properties and Composition of GaAs1-xPx Grown by Close-Spaced Vapor Transport</B> | + | |
| − | + | <B>Towards the ideal CIMS</b> | |
| − | Allison Davis, First Year Graduate Student | + | |
| − | <br>Department of Chemistry and Biochemistry and CIRES | + | Henning Finkenzeller, <br> |
| − | <br>University of Colorado Boulder | + | Postdoctoral researcher at University of Helsinki, INAR/Physics, Finland, and Karsa Ltd, Helsinki, Finland |
| − | + | ||
| − | Abstract | + | Chemical Ionization Mass Spectrometry has arguably enabled a “molecular revolution” in atmospheric sciences and has become an analytical workhorse in the field. Still, achieving sensitivity that covers the spectrum of chemically diverse trace gases has required combining multiple CIMS instruments with different ionization schemes which differ regarding both the reagent ions and the IMR pressure. For example, Proton Transfer Mass Spectrometry (PTR-MS) achieves sensitivity to VOCs employing a rather unselective ionization at low pressure, while clustering with NO3- at high pressure ionizes highly oxygenated compounds. |
| − | + | ||
| − | Semiconductor thin films are widely utilized in the production of photovoltaic devices. GaAs is a III-V binary semiconductor with exceptional optoelectronic properties such as a direct band gap of 1.42eV and a lattice parameter of 5.6Å, making it an attractive material for the construction of high efficiency solar cells. GaAs can be combined with GaP, another III-V semiconductor with a 2.25 eV band gap, which allows the band gap and the lattice parameter to be tuned. Combining these binary materials to make the ternary alloy GaAs1-xPx is useful for multi-junction cells. This study aims to produce and characterize GaAs1-xPx thin films grown via CSVT. | + | In my presentation, I will present how multi-pressure chemical ionization synergizes the advantages of different ionization chemistries within a single instrument to attain sensitivity to a broad range of compounds. Additionally, I show exploratory measurements with novel reagent ions. |
| − | + | ||
| − | = | + | ==7 October 2024== |
| − | + | <b>Chemistry of Volatile Organic Compounds in the Atmosphere </b><br> | |
| − | <B>Characterization of Organic Nitrogen in the Atmosphere</B> | + | [https://sites.google.com/view/de-gouw-lab Joost de Gouw], <br> |
| − | + | ANYL Faculty, CU Boulder | |
| − | Ellie Browne | + | |
| − | <br>Department of Chemistry and Biochemistry and CIRES | + | Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products. |
| − | <br>University of Colorado Boulder | + | |
| − | + | In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments. | |
| − | Abstract | + | |
| + | Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments to better understand their sources and fate. One particular study involves the impact of the Marshall Fire in Boulder County on indoor air in homes that were near the burnt area. Second, we are working on the emissions and chemistry of VOCs in urban air. One study that will be highlighted involved field measurements in Los Angeles in the Summer of 2022. Third, we are working on the indoor air chemistry induced by air cleaners such as germicidal UV lamps that are effective at inactivating airborne viruses. Finally, we are also using data from satellite remote sensing instruments to measure the pollutants from oil and natural gas production, in urban air and from wildfires. | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Understanding aerosols for indoor and outdoor air quality and climate</b><br> | ||
| + | [http://cires1.colorado.edu/jimenez/ Jose-Luis Jimenez], <br> | ||
| + | ANYL Faculty, CU Boulder | ||
| + | |||
| + | Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly summarize ongoing projects in our group, with more emphasis on projects of interest to first year PhD students. | ||
| + | |||
| + | We frequently perform ambient aerosol composition measurements. We recently deployed an AMS in the NASA DC8 aircraft during the ASIA-AQ campaign in Spring 2024, investigating air pollution in South Korea, the Philippines, Taiwan, and Thailand; we also continue the analysis of data from previous aircraft campaigns. Since May 2023 we have been operating continuous measurements of aerosol composition in Denver with multiple instruments (ACSM, SMPS, Aethalometer, XAct) as part of the NSF ASCENT long-term monitoring program, which we are going to continue until at least 2030. We also collected data to characterize the precursors responsible for SOA formation in the Los Angeles area in Summer of 2022. I will summarize recent results from ongoing analyses of these projects. | ||
| + | |||
| + | Indoor air chemistry and airborne disease transmission has also been a focus over the last decade. Over the last 3 years we have been investigating the formation and/or removal of air pollutants by different “air cleaner” technologies, with a special focus on germicidal ultraviolet light (GUV). I will present results from recent experimental and modeling projects, including an analysis of GUV tradeoffs across all possible wavelengths, and the characterization of a catalyst to remove ozone from indoor air. | ||
| + | |||
| + | Dual frequency combs (DFC) are new light sources with unique properties for analytical spectroscopy. The mid-IR contains important chemical information about organic functional groups, but analysis of aerosols suspended in air in this region with FTIR was considered infeasible, due to interferences from narrow absorption lines of abundant gases such as CO2. As part of the collaborative IARPA SAURON project led by CU-Boulder Engineering, we are working to test the aerosol measurement capabilities of new DFC systems. | ||
| + | |||
| + | ==30 September 2024== | ||
| + | <b>Multiphase laboratory studies of wildfire smoke plume aging under wet and dry conditions</b> | ||
| + | |||
| + | Prof. [https://www.sandiego.edu/cas/faculty/biography.php?profile_id=39 David O. De Haan], <br> | ||
| + | Department of Chemistry & Biochemistry, University of San Diego | ||
| + | |||
| + | "Field studies have shown that when wildfire smoke plumes age under dry conditions in the atmosphere, approximately half of the total brown carbon (BrC) absorption tends to fade with time due to photobleaching. Cloud-processed smoke plumes, however, do not fade noticeably, but the chemical or physical transformations behind this effect are unknown. To address this gap, we burned four types of biomass samples under simulated smoldering conditions (400 C) and fractionated the smoke for a series of chamber experiments. In one set of experiments, whole smoke, the VOC fraction, and denuded smoke where oxidized by OH, ozone, or NO3 under wet and dry conditions in a steel cloud chamber. In a second set of experiments, smoke POA, denuded POA, and water-soluble gas components of smoke were collected and re-aerosolized at constant rates into an oxidation flow reactor (OFR) for integrated OH exposure of 2 – 9 d of atmospheric oxidation. Observations of aerosol bounce indicated that aerosol particles had viscosities of > 1 Pa s even before photooxidation. No bleaching at 405 nm and little chemical reactivity was observed when smoke aerosol was exposed to the highest levels of OH radicals under dry conditions in the OFR. These observations suggest that diffusion is limited in smoke OA and that OH reactivity is limited to smoke aerosol surfaces. Ozone exposure, on the other hand, oxidized smoke OA on a 12-min timescale in the steel chamber., especially under dry conditions. " | ||
| + | |||
| + | ==23 September 2024== | ||
| + | <b>Environmental aquatic chemistry and source water protection</b> | ||
| + | |||
| + | Prof. [https://fernandorosario.wordpress.com/ Fernando Rosario-Ortiz], <br> | ||
| + | Environmental Engineering, CU Boulder | ||
| + | |||
| + | "Source water protection is an important consideration for the continued production of potable water in the US and abroad. Source waters can be impacted by contamination from anthropogenic sources as well as natural perturbations such as from wildland and urban fires. | ||
| + | |||
| + | The presence of organic contaminants in source waters is a concern for potable water systems. These organic contaminants can be removed via advanced oxidation processes, as well as via sunlight driven photochemical processes. In this later case, reactions are promoted via the formation of reactive intermediates. These reactive intermediates include short lived radical species that are formed from photochemical processes within dissolved organic matter. In my group, we study the formation of these reactive intermediates. | ||
| + | |||
| + | We also study how fires impact source water quality and ultimately potable water production. In this respect, we look at how compounds are formed, mobilized and degraded as well as how treatment systems can remove them. Lastly, we also study how compounds mobilized after fires can impact the formation of regulated disinfection byproducts." | ||
| + | |||
| + | |||
| + | and | ||
| + | |||
| + | |||
| + | <b>Particle Formation and Growth in New Chemical Regimes</b> | ||
| + | |||
| + | Prof. [https://sites.google.com/view/brownelab/people Ellie Browne], <BR> | ||
| + | CU ANYL Faculty | ||
| + | |||
| + | "Understanding the mechanisms through which aerosol particles form and grow is critical for constraining a planet’s energy budget, however, our knowledge of the key processes governing particle formation and growth in diverse environments remains weak. In the coming decades, our knowledge of particle formation and growth will continue to be challenged as changing climate and anthropogenic emissions alter the chemical regimes of modern-day atmospheric chemistry on Earth and measurements from, for instance, the James Webb Space Telescope and NASA’s Dragonfly mission offer increasingly chemically resolved insights into planetary atmospheres much different from our own. | ||
| + | |||
| + | In this talk I will highlight our projects on particle formation and growth in new and/or understudied chemical regimes. I will briefly discuss our recent advances in three different areas: how reactive nitrogen chemistry is important in agricultural regions, how the oxidation of volatile methyl siloxanes contributes to SOA in urban regions, and how organosulfur compounds affect gas- and particle-phase composition in anoxic planetary atmospheres." | ||
| + | |||
| + | ==16 September 2024== | ||
| + | <B>Chemistry at Aqueous Interfaces: Iodine, Ammonia, and Organics in the Anthropocene</b> | ||
| + | |||
| + | Prof. [http://volkamergroup.colorado.edu/ Rainer Volkamer] <BR> | ||
| + | CU ANYL Faculty | ||
| + | |||
| + | "Anthropogenic enhancements of natural processes modify atmospheric chemistry in the urban, remote marine, forested, and upper atmosphere. For example, the deposition of ozone at the air-sea interface leads to marine iodine emissions that have tripled in recent decades due to increases in pollution ozone over oceans, affecting particle formation, tropospheric and stratospheric ozone. In the upper troposphere, high concentrations of ammonia, NH3, drive the formation of the Asian Tropopause Aerosol Layer (ATAL), suggesting efficient transport through convective clouds despite the extremely high water solubility (Henry’s Law) of this gas. Can we measure the ice retention efficiency of NH3 experimentally in the laboratory? What are the implications for cloud processing for other water soluble oxygenated VOCs, like glyoxal and fatty acids? The Volkamer group is interested in studying the fundamentals of atmospheric processes that affect radiation in the atmosphere. We develop advanced instrumentation (optical spectroscopy and mass spectrometry) to measure small molecules and condensable vapors relevant to particle formation, ozone, public health and climate discussions. Our molecular perspective and focus on fundamentals aims to advance atmospheric models, and has helped satellites measure new species (e.g., glyoxal, HONO, NH3). Opportunities for graduate research in field measurements, laboratory experiments, and chemical calculations are discussed (i.e., CLOUD/CERN; TI3GER2; AIRMAPS; TEMPO)." | ||
| + | |||
| + | ==Tuesday, 27 August 2024== | ||
| + | <b>Iodine activation and recycling driven by Fe(III)-carboxylate photochemistry</b> | ||
| + | |||
| + | [https://www.psi.ch/en/lac/multiphase-chemistry-research-group Lucia Iezzi], <br> | ||
| + | Paul Scherrer Institut <br> | ||
| + | 2:00 PM <br> | ||
| + | Ekeley S274 <br> | ||
| + | |||
| + | "Iodine represents a globally important sink for ozone, influences the oxidative capacity of the troposphere and can efficiently nucleate particles. Nevertheless there is still lack of information on reactive iodine precursor gas fluxes. Recent field measurements have found indication of active chemical recycling of iodine from the particulate phase back to gas phase. This recycling may be driven by still not well understood reductive processes involving particulate iodate. In our work we used Coated Wall Flow Tube experiments to explore the role of Fe(III)-carboxylate photochemistry for iodine activation and recycling, and found that the coupling between ROS, iron and iodine species can efficiently reduce iodate and give unexpected feedbacks to the Fe(II)/(III) redox cycling." | ||
| + | |||
| + | ==Thursday, 15 August 2024== | ||
| + | <b>Utilizing the Properties of Functional Groups to Understand Indoor, High NOx, and Low NOx Atmospheric Chemistry</b> | ||
| + | |||
| + | Anna Ziola, <br> | ||
| + | [https://sites.google.com/site/ziemanngroup/ Ziemann group]<br> | ||
| + | ANYL Dissertation Defense<br> | ||
| + | 2:00pm<br> | ||
| + | CASE E240<br> | ||
| + | https://cuboulder.zoom.us/j/96404067692 (Meeting ID: 964 0406 7692) | ||
| + | |||
| + | "Over 1000 Tg of volatile organic compounds (VOCs) are emitted into the atmosphere every year from both biogenic (Guenther et al., 2012) and anthropogenic (Huang et al., 2023) sources, an equivalent weight of about 200,000 school buses per day. Depending on the structure of these compounds and conditions in which they exist, they can remain in the atmosphere for seconds to years. This thesis investigates the fate of selected volatile organic compounds in three types of conditions: low NOx, high NOx, and indoor environments. | ||
| + | |||
| + | The first study was inspired by the significant decrease of nitrogen oxides (NOx) in the U.S. in the last few decades. This reduction has led to the oxidation of VOCs – crucial for the formation of ozone and fine particles – occurring in urban areas under conditions historically associated with remote rural locations. To gain a better understanding of VOC oxidation mechanisms, I investigated the fate of two model linear alkenes that were chosen due to the high abundance of alkenes in atmospheric emissions and the simplicity of the selected compounds. By reacting 1-pentene and 3-butenoic acid with OH radicals in an environmental chamber under low NOx conditions, monitoring the gas- and particle-phase products (and 3-butenoic acid) using real-time mass spectrometry and offline techniques, and kinetics modeling, I was able to elucidate the oxidation mechanisms of these compounds. This allowed for a better understanding of the effect of a carboxyl group on reaction branching ratios and product yields. | ||
| + | |||
| + | In the second study, I investigated the OH radical-initiated oxidation of a model ether compound under high NOx conditions. Ether groups are commonly found in more functionalized volatile chemical products (VCPs) – anthropogenic sources of VOCs that come from cleaning products, personal care products, adhesives, and elsewhere (Seltzer et al., 2021). Although it is known that ether groups affect reaction kinetics, nitrate yields, and SOA yields, the mechanism and degree to which a single ether group impacts the products formed from a large ether has not been determined or quantified. Therefore, for this study I reacted dioctyl ether (a symmetric C16 compound with a central ether group) with OH radicals under high NOx conditions; identified and quantified the gas- and particle-phase products using mass spectrometry, gas chromatography, liquid chromatography, and spectrophotometric techniques; and developed a quantitative reaction mechanism to explain their formation which could be used in models. | ||
| + | |||
| + | In the final chapter I investigated the fate of carboxylic acids and ozone in simulated indoor conditions. Humans spend the majority of their life indoors, yet we have limited knowledge about the chemistry that occurs in these spaces. One major process that impacts the composition of indoor air is surface chemistry. Indoor spaces can have dozens of different surfaces, each with unique properties and compositions. Here, I investigated the mechanisms by which carboxylic acids and ozone interact with various wood species and wood coatings, including lacquer and shellac. An iodide chemical ionization mass spectrometer (I-CIMS) and ozone monitor were used to measure uptake coefficients and deposition velocities in uncoated and coated tubes, an attenuated total reflectance-Fourier transform infrared spectrometer was used to measure diffusion coefficients of carboxylic acids in wood and coatings, and a multi-layer model was used to extract compound diffusion coefficients and material absorbance capacities from experiments. The results were then used to develop a conceptual model for describing the interactions of carboxylic acids wood and coatings." | ||
| + | |||
| + | ==Spring 2024== | ||
| + | <font size="4">10 May 2024</font size="4"><br> | ||
| + | <b>Mountaintop DOAS observations of trace gases in the remote atmosphere: Relevance for ozone and mercury oxidation</b> | ||
| + | |||
| + | Christopher F. Lee, <br> | ||
| + | [https://volkamergroup.colorado.edu/ Volkamer group], <br> | ||
| + | ANYL Dissertation Defense | ||
| + | |||
| + | "Although ozone is a ground-level pollutant, ozone in the stratosphere absorbs solar radiation and therefore reduces the amount of harmful ultraviolet light reaching the Earth’s surface. Mercury is a potent neurotoxin and global environmental hazard which enters the ecosystem following oxidation and subsequent deposition from the atmosphere. While ozone chemistry is relatively well-understood, atmospheric mercury chemistry is highly uncertain. | ||
| + | |||
| + | The chemistry of atmospheric halogens and their impact on ozone and mercury oxidation are investigated using Differential Optical Absorption Spectroscopy (DOAS) measurements of trace gases in conjunction with other measurements at mountaintop observatories on remote islands in the northern and southern hemisphere tropics (5 years of observations), and over the central continental United States (1.5 years of observations). Total ozone (O<sub>3</sub>) and stratospheric nitrogen dioxide (NO<sub>2</sub>) vertical columns are presented for the entire measurement period at all three sites. Other trace gases, including iodine monoxide (IO), bromine monoxide (BrO), sulfur dioxide (SO<sub>2</sub>), formaldehyde (HCHO), and water vapor (H<sub>2</sub>O) are presented for case study periods. | ||
| + | |||
| + | The first ground-based observations of IO radicals in the continental troposphere are presented, alongside co-located measurements of gas-phase elemental and oxidized mercury. The observed levels of tropospheric IO were up to three times higher than predicted by the GEOS-Chem global chemical transport model. Furthermore, the observed levels of oxidized mercury were up to ten times higher than predicted by the same model, which considers only bromine and hydroxyl radicals as major mercury oxidants. The role of iodine as a mercury oxidant was evaluated using a chemical box model of gas-phase mercury chemistry which included iodine, bromine, and hydroxyl radicals as mercury oxidants. | ||
| + | |||
| + | Observations of the 2023 Hunga Tonga volcanic plume in the stratosphere over the southern hemisphere site are presented. The eruption injected an enormous amount of water vapor into the stratosphere, increasing the total amount of stratospheric water vapor by 10% and resulting in a 5% reduction of ozone in the tropical stratosphere. A suite of ground-based, balloon-borne, and satellite observations revealed that the stratospheric ozone depletion was likely due to chlorine reservoir species being efficiently converted into reactive chlorine radical species as a result of the water vapor injection. Zenith-sky DOAS measurements of stratospheric NO<sub>2</sub> and BrO constrained the role of NO<sub>x</sub> and BrO<sub>x</sub> in the observed ozone depletion." | ||
| + | |||
| + | <font size="4">22 April 2024</font size="4"><br> | ||
| + | <b>A partial story of an aircraft-based laminar gas sampling inlet</b> | ||
| + | |||
| + | Da Yang, <br> | ||
| + | [https://volkamergroup.colorado.edu/ Volkamer group] | ||
| + | |||
| + | "Aircraft-based measurements allow for large spatial-scale characterization of atmospheric aerosol and gas, but these measurements, under high-speed flow conditions, complicate efforts to maintain sample integrity through the inlet transport process. Of particular concern is the role of turbulence in driving loss of gas-phase species and aerosol particles. While a significant amount of research has gone into understanding aerosol sampling efficiency for aircraft inlets, a similar research investment has not been made for gas sampling. To study the gas sampling loss of aircraft-based measurements, we used computational simulations and wind tunnel experiments to analyze the gas sampling performance of a laminar gas inlet developed and used in the Ti<sup>3</sup>GER project. We conducted measurements of H<sub>2</sub>SO<sub>4</sub> in a high-speed wind tunnel. The gas transmission efficiency of H<sub>2</sub>SO<sub>4</sub> through different sampling lines was measured using CIMS, and the experimental results are being compared to simulations of flow and mass diffusion modelling in the sampling line. Both experimental data and simulation results show the gas transmission efficiency increases with an increased sampling flow rate, including turbulent flow. In this presentation, I would like to give a short introduction to my work on studying aircraft-based sampling systems and focus on sharing the information I learned from gas-phase H<sub>2</sub>SO<sub>4</sub> measurement results." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Characterisation of Br-CIMS response under different humidity level and its application in CLOUD 16</b> | ||
| + | |||
| + | Yandong Tong, <br> | ||
| + | [https://volkamergroup.colorado.edu/ Volkamer group] | ||
| + | |||
| + | "Chemical ionisation mass spectrometry (CIMS) exhibits high sensitivity and | ||
| + | versatility in gaseous species, therefore, it has been widely used in atmospheric | ||
| + | chemistry studies, especially in new particle formation and secondary organic | ||
| + | aerosol formation studies. Among the different ionisation methods, CIMS using | ||
| + | bromide (Br-) as reagent ion has been recently developed, optimised, and | ||
| + | characterised, which is suitable to detect various organic and inorganic species, | ||
| + | e.g., iodine and its oxidation products. However, Br-CIMS shows humidity | ||
| + | dependence in many studies. In this study, we developed a new water regulation | ||
| + | system for our CIMS and implemented it in laboratory experiments and in the | ||
| + | CLOUD 16 campaign at CERN in Geneva, Switzerland. By taking advantage of | ||
| + | the water regulation system and CLOUD chamber facility, we explored the Br- | ||
| + | CIMS sensitivity to different species in a wide range of humidity levels. For | ||
| + | strongly bonded species, e.g., iodine (I<sub>2</sub>), Br-CIMS sensitivity to these species | ||
| + | increases as the humidity level increases, whereas Br-CIMS sensitivity to | ||
| + | weakly bonded species, e.g., glyoxal (CHOCHO), exhibits the opposite trend. A | ||
| + | decent understanding of Br-CIMS response to different species under various | ||
| + | humidity conditions allows us to provide valuable measurements in CLOUD 16, | ||
| + | especially helping us understand the role of CHOCHO in nucleation events." | ||
| + | |||
| + | <font size="4">15 April 2024</font size="4"><br> | ||
| + | <b>Laboratory and mechanistic studies of complex VOC oxidation systems</b> | ||
| + | |||
| + | [https://staff.ucar.edu/users/qingye Dr. Qing Ye], <br> | ||
| + | NCAR Atmospheric Chemistry Observations & Modeling Lab | ||
| + | |||
| + | "Improving our knowledge of the atmospheric oxidation chemistry of volatile organic compounds (VOCs) is crucial to our understanding of how air pollutants form and evolve. One major challenge in the understanding of VOC oxidation arises from the extreme complexity in the oxidation processes which convert the precursor to new oxidized products and exponentially increase the number and diversity of species in the reaction mixture. To study the complex oxidation processes, laboratory chamber experiments and mechanistic simulations are two commonly used tools that have different advantages and limitations. In this seminar, I will compare a chamber dataset on alpha-pinene oxidation with a mechanistic dataset generated by a hyper-explicit mechanism generator, GECKO-A. The measurement dataset was collected by a suite of advanced analytical instruments, with the goal of achieving a near-complete description of the reactive carbon. The measurement-mechanism comparisons on a species-to-species level and an ensemble property level will be discussed. I will then show how targeted adjustments to the mechanisms in GECKO-A affect its overall agreement with chamber observations." | ||
| + | |||
| + | <font size="4">5 April 2024</font size="4"><br> | ||
| + | <b>New observations and models of ozone photochemistry in wildfire and urban plumes</b> | ||
| + | |||
| + | Michael Robinson, <br> | ||
| + | [https://www.colorado.edu/lab/browngroup/members Brown Group], <br> | ||
| + | ANYL Dissertation Defense | ||
| + | |||
| + | "Atmospheric reactions of nitrogen oxides (NOx = NO + NO2) and volatile organic compounds significantly impact the composition of the troposphere in the form of ozone (O3) and secondary organic aerosol. Despite decades of research and precursor regulation, O3 pollution in the United States continues to be a problem in urban areas. Increasing wildfire occurrence and intensity in the western United States raises the question of wildfires' impact on this air quality issue. Critical questions regarding this topic include: 1) which photochemical regime does O3 production occur in western wildfires, 2) how do O3 photochemical regimes compare across four major cities in North America, 3) does changing O3 photochemical regimes in North America necessitate new approaches to quantifying chemical regimes. In this defense, I address these questions with 1) aircraft observations and modeling of western wildfires to describe and quantify the photochemical regime and 2) an analysis of isoprene peroxy radical fate in four urban areas from aircraft and ground site observations." | ||
| + | |||
| + | <font size="4">2 April 2024</font size="4"><br> | ||
| + | <b>Oxidation of Monoterpenes in the Atmosphere and Indoor Environments</b> | ||
| + | |||
| + | Olivia Jenks, <br> | ||
| + | [https://sites.google.com/view/de-gouw-lab/people de Gouw group], <br> | ||
| + | ANYL Dissertation Defense | ||
| + | |||
| + | "Monoterpenes are emitted into the atmosphere from vegetation and indoor products like personal care items and building materials. Once in the atmosphere, monoterpenes undergo oxidation by ozone (O3), hydroxyl radicals (OH), and nitrate radicals (NO3), forming secondary organic aerosol (SOA). These aerosols play crucial roles in the climate system, in limiting visibility, and impact human health. Aerosols reflect sunlight, contributing to a cooling of climate, and influence cloud properties. However, their small particle size enables them to penetrate deep into the lungs of humans, posing risks to cardiovascular and respiratory health. My first project involved characterizing the gas-phase products of monoterpene oxidation, focusing on Δ-3-carene, α-pinene, β-pinene, ocimene, and limonene. Innovative techniques were developed to identify and quantify these products, including the use of tubing delay experiments with the Vocus mass spectrometer. Field studies in Los Angeles corroborated findings from the lab, highlighting the importance of both daytime and nighttime chemistry. The composition of the monoterpene oxidation products observed in the field were compared to that of the lab studies, contributing to a comprehensive understanding of atmospheric VOC oxidation processes. My second project investigated the impact of germicidal ultraviolet (GUV) irradiation on indoor VOC oxidation. GUV has been widely used for air disinfection, particularly in public settings like hospitals and schools. However, its effect on indoor air quality remains poorly understood. By irradiating air that contains common indoor volatile organic compounds (VOCs), like limonene, in a controlled chamber, the impact of GUV light on the monoterpene ozonolysis process was studied. This work contributes to a better understanding of the implications of GUV disinfection on indoor air quality and human health and will inform strategies for improving indoor air quality and reducing health risks associated with VOC exposure in indoor environments." | ||
| + | |||
| + | <font size="4">1 April 2024</font size="4"><br> | ||
| + | <b>Investigating SOA Formation from Volatile Methyl Siloxanes</b> | ||
| + | |||
| + | Hanalei Lewine, <br> | ||
| + | ANYL 3rd year, Browne group<br> | ||
| + | |||
| + | "Volatile methyl siloxanes (VMS) are anthropogenic organosilicon molecules that are used in a variety of applications including in personal care products. Decamethylcyclopentasiloxane (D5) has been detected at high mixing ratios indoors and outdoors, with 90% of the D5 in cosmetics being released to the atmosphere. VMS have recently been identified as precursors to aerosol in urban areas, however lab studies of D5 oxidation have been unable to fully characterize D5’s SOA yield. The dominant oxidation product, the siloxanol, has been observed in lab and ambient aerosol, however absorptive partitioning alone cannot explain the amounts measured. Previous SOA experiments did not control for seed and wall effects, and did not measure gas phase chemistry leading to SOA. Here, we performed chamber experiments to investigate how the presence of seed aerosols impacts D5 SOA formation, if multigenerational chemistry is required for D5 SOA, and how RO<sub>2</sub> fate impacts D5 SOA. We measured the gas phase products of D5 oxidation using chemical ionization mass spectrometry (CIMS) and measured particle size distribution using SMPS. We find that at lower OH exposures, with only one generation of oxidation chemistry, we still make SOA. Despite challenges in data analysis, it is clear that seed aerosol is important for D5 SOA formation." | ||
| + | |||
| + | <font size="4">18 March 2024</font size="4"><br> | ||
| + | <b>Comparison of Common Vapor Pressure Estimation Methods through Modeling of the Reactions of Linear and Branched Alkenes with OH/NOx</b><br> | ||
| + | |||
| + | Emmaline Longnecker, <br> | ||
| + | ANYL 3rd year, Ziemann group<br> | ||
| + | |||
| + | "Modeling atmospheric reactions that lead to the formation of secondary organic aerosol (SOA) is an important tool for understanding the current and future impacts of human activity on the environment. Vapor pressure is a key parameter in modeling these reactions, as it largely determines the gas-particle partitioning of atmospheric oxidation products. However, the vapor pressures of many atmospherically relevant molecules are still poorly constrained. To aid modeling efforts, several structure-activity relationships (SARs) based on group contribution methods have been developed for estimating compound vapor pressures. The current study evaluates how four of these SARs: SIMPOL, EVAPORATION, SPARC, and Nannoolal, impact the modeled predictions of SOA yields for reactions of C<sub>8</sub>-C<sub>14</sub> 1-alkenes and C<sub>9</sub>-C<sub>15</sub> 2-methyl-1-alkenes with OH radicals in the presence of NO<sub>x</sub>. The models include well-constrained, quantitative reaction mechanisms developed by our research group from several previous environmental chamber studies of product yields, gas-particle and gas-wall partitioning, and secondary reactions with OH radicals. Based on our previous studies, there was no need to account for particle-phase oligomer formation. Comparison of modeled and measured SOA yields provided insight into the major products responsible for SOA formation over the large range of carbon numbers, and the sources of discrepancies between model predictions and measurements. The wide range of agreement exemplifies the impact of vapor pressure in modeling atmospheric reactions and indicates the need for further development of estimation methods." | ||
| + | |||
| + | <font size="4">11 March 2024</font size="4"><br> | ||
| + | <b>Career panel with ANYL alumni: </b><br> | ||
| + | |||
| + | We have asked the speakers to present for 12-15 min on their perspective on careers outside academia for PhD chemists, and in particular for graduates of our ANYL PhD program. For example, they may address the following questions: what path took you to where you are? What strengths from the ANYL program helped you in your career? What could have the program done to make that better? What do you wish someone had told you when you were a PhD student? What advice would you give to current PhD students and postdocs? A Q&A session will follow, prioritizing questions from current students and postdocs.<br> | ||
| + | |||
| + | <b>Ingrid Ulbrich</b>, <br> | ||
| + | Achieving Academic Success | ||
| + | |||
| + | "Ingrid Ulbrich (Ph.D., Jimenez Group, 2011) discovered in grad school that teaching was a much greater passion than research. And that she needed the Ph.D. for teaching jobs in higher ed. So she pushed through to complete her research (applying PMF to tropospheric organic aerosol; H-index = 26 as of 2020) and began helping students get better at problem solving. After teaching CU (Gen Chem to grad-level data analysis), she became the General Chemistry Lecture Coordinator at Colorado State University. While teaching first-year courses she saw the true struggles of students – not underdeveloped math skills or challenges dissecting word problems – but lacking a life vision, believing the voice that says they’re not smart enough, and inability to manage major life events while prioritizing academics. After encountering a curriculum that helps students learn 'how' to learn and become self-growers, Ingrid discovered how to empower students to overcome those struggles. Ingrid founded a nonprofit in 2021, [https://achievingacademicsuccess.org/ Achieving Academic Success], to develop students as master learners who pursue their life visions on a path of continued growth and create the lives they want to live." | ||
| + | |||
| + | & | ||
| + | |||
| + | <b>Erin McDuffie</b>, <br> | ||
| + | US EPA | ||
| + | |||
| + | "Dr. Erin McDuffie is a physical scientist at the U.S. Environmental Protection Agency. Erin received her PhD from the Analytical, Environmental, and Atmospheric Chemistry program at the University of Colorado Boulder in 2018. During her PhD, Erin worked with Dr. Steve Brown in NOAA’s Chemical Sciences Laboratory, where she focused on in situ sampling and simple chemical box modeling to better understand the sources and chemical transformations of ozone and fine particulate matter. After graduate school, Erin transitioned to using global atmospheric chemistry transport models to study air pollution impacts on human health as a post-doctoral research fellow at Dalhousie University with Dr. Randall Martin (now at Washington University in St. Louis). Erin completed a 2020-2022 AAAS Science and Technology Policy Fellowship at the U.S. EPA in Washington, D.C., and is currently an atmospheric scientist in EPA’s Climate Change Division, working on a wide range of science and policy questions related to better understanding, communicating, and mitigating future risks of climate change. " | ||
| + | |||
| + | <font size="4">4 March 2024</font size="4"><br> | ||
| + | <b>From the air to the sea: Addressing environmental challenges through fundamental chemistry</b><br> | ||
| + | [https://www.sandia.gov/people/staff/ryan-dustin-davis/ Dr. Ryan Davis], <br> | ||
| + | Sandia National Laboratories, <br> | ||
| + | University of New Mexico | ||
| + | |||
| + | "Environmental systems, such as the hydrosphere and atmosphere, are a complex balance of chemical | ||
| + | and physical processes that impact nearly every facet of life. In the atmosphere, aerosol particles – tiny | ||
| + | particles of liquid and/or solid floating in the air -- have a strong influence on air quality, climate, and | ||
| + | human health. For example, the aerosol effect on climate is one of the largest sources of uncertainty in | ||
| + | climate models. Human respiratory aerosol can also transmit certain diseases. Understanding aerosol | ||
| + | particles is a unique challenge because they can exhibit chemical and physical properties that are not | ||
| + | observed in bulk solution. In the first part of this talk, I will discuss efforts to understand and | ||
| + | characterize the unique properties of aerosol particles using levitation-based techniques to understand | ||
| + | aerosol phase state. With this approach we have demonstrated unique supramolecular effects on the | ||
| + | phase state of aqueous aerosol particles composed of organic and inorganic compounds. The | ||
| + | environmental and public health implications for droplet-based supramolecular chemistry will be | ||
| + | discussed. | ||
| + | In the second part of the talk, we will turn discussion to the hydrosphere, where anthropogenic activities | ||
| + | have contaminated global water sources with environmentally-persistent chemical species. One such | ||
| + | class of “forever chemicals” are per- and poly-fluoroalkyl substances (PFAS), which are highly resistant to | ||
| + | natural degradation processes. PFAS are used widely in a range of consumer and industrial products and | ||
| + | their widespread use has led to a global distribution. Certain PFAS have been linked to a myriad of | ||
| + | environmental and human health problems, so there is a need to remove these compounds from water | ||
| + | supplies. Here, I will discuss ongoing efforts to destroy, sequester, and detect PFAS. | ||
| + | Along the way, I will highlight other areas of my ongoing research at Sandia National Labs, including gas- | ||
| + | surface interactions on metal surfaces, machine-learning based climate models, and the development of | ||
| + | analytical chemistry capabilities." | ||
| + | |||
| + | Sandia National Laboratories is a multimission laboratory managed and operated by National | ||
| + | Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International | ||
| + | Inc., for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE- | ||
| + | NA0003525. This talk describes objective technical results and analysis. Any subjective views or opinions | ||
| + | that might be expressed in the talk do not necessarily represent the views of the U.S. Department of | ||
| + | Energy or the United States Government. | ||
| + | |||
| + | <font size="4">28 February 2024</font size="4"><br> | ||
| + | <b>Atmospheric Aerosols: Quantification and Method Development</b> | ||
| + | |||
| + | Melinda Schueneman, <br> | ||
| + | [https://cires1.colorado.edu/jimenez-group/ Jimenez Group], <br> | ||
| + | CU ANYL Dissertation Defense | ||
| + | |||
| + | "The composition of atmospheric aerosols is influenced by the source/atmospheric conditions (e.g. rural, polluted, and/or biomass burning) as well as gas (g)↔particle (p) partitioning in an air mass. Several analytical techniques can be used to measure aerosol chemical composition, including Extractive Electrospray Ionization Mass Spectrometers (EESI, molecular ions of components of aerosols) and Aerosol Mass Spectrometers (AMS, typically bulk aerosol composition). Here, two methods for understanding aerosols generated in laboratory experiments are introduced. The first method focuses on a procedure that allows for calibration of molecular species. The second involves the quantification of secondary organic aerosol (SOA) formation and g↔p partitioning for known Volatile Organic Compound (VOC) precursors. | ||
| + | |||
| + | In part I, a new calibration technique is designed and tested, which combines High Performance Liquid Chromatography (HPLC), aerosolization, SMPS, and Positive Matrix Factorization (PMF) to calibrate the EESI and AMS. Species in complex aerosol mixtures (like SOA) can be separated and calibrated for, in the absence of reference standards. | ||
| + | |||
| + | In part II, the SOA formation potential from the oxidation by OH of different biomass burning VOC precursors was quantified. An iterative solver within a kinetic model was developed, including the effect of vapor wall loss (VWL) in atmospheric chambers. This allowed constraining g↔w↔p partitioning for the reaction products of each VOC. I compared the volatility of SOA to that of primary OA (POA) in a simulated wildfire and found that POA appears to be less volatile, thus more likely to be retained in the particle phase as wildfire smoke ages. | ||
| + | |||
| + | The results of this work will help to better understand and model aging biomass burning emissions and provide new methods for online aerosol chemical composition measurements." | ||
| + | |||
| + | <font size="4">26 February 2024</font size="4"><br> | ||
| + | <b>Efficiency of Urban Ozone Photochemistry during the 2023 AEROMMA and CUPiDS Airborne Field Campaigns</b> | ||
| + | |||
| + | Wyndom Chase, <br> | ||
| + | CU ANYL 3rd year, Brown group | ||
| + | |||
| + | "The summer 2023 Airborne Emissions and Reactions Observed from Megacities to Marine Areas (AEROMMA) and Coastal Urban Plume Dynamics (CUPiDS) field campaigns provided extensive in-situ observations of ozone (O<sub>3</sub>) and its precursors in the largest United States urban areas (New York City, Chicago, and Los Angeles). We present the distribution of O<sub>3</sub> in these megacities, along with the distribution of its precursor nitrogen oxides (NO<sub>x</sub> = NO + NO<sub>2</sub>) and total reactive nitrogen (NO<sub>y</sub>), as measured from the NASA DC-8 and NOAA Twin Otter aircraft platforms during AEROMMA and CUPiDS, respectively. Several metrics calculated from in-situ trace gas measurements can aid the understanding of the photochemical regimes in which O<sub>3</sub> production takes place. The O<sub>3</sub> production efficiency (OPE) is a measure of the amount of O<sub>3</sub> produced per unit NO<sub>x</sub> that is emitted and oxidized and is often taken as the ratio of enhanced O<sub>3</sub> or O<sub>x</sub> (= O<sub>3</sub> + NO<sub>2</sub>) to NO<sub>z</sub> (= NO<sub>z</sub> - NO<sub>x</sub>) within an urban plume transect. The spatial and temporal variability of OPE probes the evolution of O<sub>3</sub> sensitivity within a given urban area. Comparison of this metric between urban areas, in this case New York City, Chicago, and Los Angeles, elucidates differences or similarities across major U.S. urban areas. This analysis establishes a foundation for future work that will incorporate additional O<sub>3</sub> sensitivity metrics, as well as chemical box modeling and remote sensing observations, to gain greater insight into the current landscape of U.S. O<sub>3</sub> photochemical pollution." | ||
| + | |||
| + | <font size="4">19 February 2024</font size="4"><br> | ||
| + | <b>Observing Wildfire Emissions and Improving Air Quality Forecasting Using Remote Sensing Data</b><BR> | ||
| + | |||
| + | Lindsey Anderson, <br> | ||
| + | CU ANYL 3rd year, de Gouw group | ||
| + | |||
| + | "Human-caused climate change and the buildup of fuel from fire suppression practices has led to an increase in the frequency and intensity of wildfires in the western United States. Wildfire emissions of particulate matter and reactive gases impact human health, affect local and downwind ozone (O<sub>3</sub>) formation, and influence the climate. The magnitude and chemical composition of wildfire emissions in operational air quality forecasts depend on the vegetation type and the burned area. However, laboratory and field measurements have shown that the composition of wildfire emissions also depends on whether the wildfire is experiencing mainly flaming combustion or mainly smoldering combustion, which changes throughout its lifetime. Including a parametrization of how the composition of wildfire emissions changes with evolving combustion conditions could improve air quality forecasts, especially for pollutants such as O<sub>3</sub>. Here, we will show how remote sensing observations of trace gases from the Tropospheric Monitoring Instrument (TROPOMI) can be used to observe changes in the composition of wildfire emissions over time, as wildfires transitions from mainly flaming to mainly smoldering combustion. We will also discuss how the Hourly Wildfire Potential (HWP) index, which is derived from NOAA’s High Resolution Rapid Refresh (HRRR) model predictions of wind speed, temperature, humidity, and soil moisture, relates to trace gas composition from TROPOMI and can be used to parametrize how NO<sub>x</sub> emissions vary with forecasted changes in combustion conditions. Using this technique, we have found improvements in forecasted NO<sub>x</sub> and O<sub>3</sub> during the FIREX-AQ field study. These are the first experimental simulations in which the chemical composition of wildfire emissions varies online in a high-resolution (3km x 3km) convection-allowing air quality forecast. Looking forward, we also plan to use HWP in order to parametrize the vertical distribution of emissions, because this is also dependent on the dominant combustion type." | ||
| + | |||
| + | <font size="4">12 February 2024</font size="4"><br> | ||
| + | <b>Making Air Quality Count: Low-cost Sensors. Public Health and Urban Planning</b><br> | ||
| + | |||
| + | [https://architectureandplanning.ucdenver.edu/our-people/person-profile/deSouza-Priyanka-EXTQTN1F7 Prof. Priyanka deSouza], <br> | ||
| + | Urban and Regional Planning Department, <br> | ||
| + | CU-Denver | ||
| + | |||
| + | "Air pollution is responsible for ~ 7 million premature deaths every year. 90% of these deaths occurred in the Global South. How can countries in the Global South improve air quality? This talk takes a multipronged approach to answer this question. First: air pollution data is critical for the development of effective air quality management plans. Few cities in the Global South have extensive regulatory air pollution monitor networks, and when they do, the data are often not publicly available. This talk proposes novel methodologies to leverage data from low-cost sensors and satellite data to fill in the air pollution data gaps. However, air pollution data is only one piece of the puzzle. To convince policy-makers of the urgency of tackling air pollution this talk also evaluates the health impact of air pollution in the Indian and African contexts. Finally, how does data ultimately lead to action? This talk ends by describing what actions air quality measurements have led to in Kenya, and what existing barriers to effective policy actions are." | ||
| + | |||
| + | ==Fall 2023== | ||
| + | |||
| + | <font size="4">4 December 2023</font size="4"><br> | ||
| + | <b>Ozone in wildfire smoke and its influence on regional and global ozone</b><br> | ||
| + | Steven S. Brown<br> | ||
| + | Tropospheric Chemistry and Atmospheric Remote Sensing Program Lead, <br> | ||
| + | NOAA Chemical Sciences Laboratory, Boulder, CO<br> | ||
| + | Adjoint Professor of Chemistry, <br> | ||
| + | University of Colorado, Boulder, CO | ||
| + | |||
| + | The frequency, burned area and emissions from wildfires have been increasing in North | ||
| + | America for the last four decades. Biomass burning is a known sources of ozone precursors, | ||
| + | nitrogen oxides (NO<sub>x</sub>) and volatile organic compounds (VOCs). Increasing wildfire emissions | ||
| + | have influenced trends in North American urban ozone. The pyrogenic influence on ozone | ||
| + | occurs either through ozone production within smoke plumes that is then transported to urban | ||
| + | regions, or through the mixing of pyrogenic VOCs with urban NO<sub>x</sub> to enhance local and regional | ||
| + | ozone production. This presentation will use data from recent airborne and ground-based field | ||
| + | campaigns to quantify these processes. The 2016-2017 Atmospheric Tomography mission | ||
| + | (ATom) assessed the influence of biomass burning at hemispheric and global scales. The 2019 | ||
| + | Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ) sampled wildfire | ||
| + | smoke across the U.S. with multiple research aircraft. The 2022 California Fire Dynamics | ||
| + | Experiment (CalFiDE) conducted focused in-situ and remote sensing measurements in | ||
| + | California and Oregon. Ground-based measurements in Boulder, Colorado intercepted periods | ||
| + | of smoke influence in the Northern Front Range urban area in 2020 and 2021. Finally, the 2023 | ||
| + | Atmospheric Emissions and Reactivity Observed from Megacities to Marine Areas (AEROMMA) | ||
| + | campaign on the NASA DC-8 and the Coastal Urban Plume Dynamics Study (CUPiDS) on the | ||
| + | NOAA Twin Otter observed long range smoke transported to U.S. urban areas and the | ||
| + | associated impacts on ozone. | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>The Physical and Electrochemical Studies of Perylene Diimide Compounds</b><BR> | ||
| + | Jim Hall,<br> | ||
| + | CU 1st Year | ||
| + | |||
| + | Solar photovoltaics are an important part of the worlds climate change mitigation strategies; however, the solar cells being use commercially only perform at 10-15% efficiency. Improving these cells to reduce the energy lost to thermalization can be an important step in increasing the efficiency of these cells. Perylene diimides (PDIs), a class of organic industrial dyes, have shown great promise in their ability to harness this energy loss through a process called singlet fission. This talk looks into the transient absorption measurements of some of these PDI compounds and shows their ability to downconvert this energy to be better used by a solar cell. In addition, we will explore the cyclic voltammetry (CV) of these compounds; despite several rounds of troubleshooting and problem solving, the set up being used did not show any significant oxidation or reduction events within the voltages being scanned. This is likely due to reactions with oxygen during the CV process, despite best efforts to remove it. | ||
| + | |||
| + | <font size="4">27 November 2023</font size="4"><br> | ||
| + | <b>Indoor Air Quality in Western Canada During Wildfire Episodes</b><br> | ||
| + | Rebecca Mesburis, <br> | ||
| + | CU ANYL 1st year | ||
| + | |||
| + | The increased frequency, duration, and intensity of wildfires has raised public awareness of | ||
| + | the impact of wildfire smoke on air quality and human health. The 2023 wildfire season in | ||
| + | Canada broke the record for the most area burned in North America’s history at about 18.5 | ||
| + | million hectares. The main threat to public health from wildfire smoke is particulate matter, | ||
| + | with particulate matter that have a diameter of 2.5 micrometers or less (PM<sub>2.5</sub>) contributing to | ||
| + | most of the total particulate mass and travelling significant distances from the source of the | ||
| + | fire. Recently, low-cost air quality sensors have been used in air quality studies due to their | ||
| + | ability to capture high-resolution spatiotemporal data. We used the publicly available low- | ||
| + | cost PurpleAir sensor network to gather indoor and outdoor PM<sub>2.5</sub> data in Kamloops, Canada | ||
| + | from January to December 2021 with the goal to assess the level of exposure to wildfire PM<sub>2.5</sub> | ||
| + | relative to other sources of PM<sub>2.5</sub>. Given that we obtain most of our particulate inhalation | ||
| + | exposure when indoors, changes to the indoor environment during wildfire episodes were | ||
| + | emphasized. On wildfire-influenced days, wildfire PM<sub>2.5</sub> dominated the indoor exposure | ||
| + | sources and indoor PM<sub>2.5</sub> was almost always less than outdoor PM<sub>2.5</sub>. On non-wildfire- | ||
| + | influenced days, no typical relationship was established between indoor and outdoor PM<sub>2.5</sub>. | ||
| + | The analysis was limited by the number of PurpleAir sensors and knowledge of the indoor | ||
| + | environments studied. The findings indicate that remaining indoors during wildfire events is | ||
| + | currently an effective but finite strategy to limit PM<sub>2.5</sub> exposure in Kamloops. | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Exploring Alcohol Adsorption to Gold Induced by Applied Potential</b><br> | ||
| + | Drew Blauth, <br> | ||
| + | CU ANYL 1st year | ||
| + | |||
| + | Self-assembled monolayers (SAMs) play vital roles in battery development, biosensor function, and drug delivery. Extensive research has been directed towards adsorbate and substrate pairs that spontaneously form SAMs, such as thiols and gold, leaving pairs that do not spontaneously form SAMs relatively understudied. This project investigated alcohol adsorption to gold, which has been ignored by researchers due to oxygen - gold bond being relatively weak. By applying potential to gold substrates immersed in alcohol solutions and observing alcohol adsorption to gold using Surface Enhanced Raman Spectroscopy (SERS), it was determined that alcohol adsorption to gold could be induced and interrupted repeatedly by cycling between positive and negative applied voltages. While it is unclear exactly how the alcohols interact with the gold, these results suggest that applied potential could be used to create alcohol and gold SAMs, greatly broadening the applications SAMs could be used for. | ||
| + | |||
| + | <font size="4">13 November 2023</font size="4"><br> | ||
| + | <b>Impact of Cleaning on Concentrations of Volatile and Semivolatile Organic Compounds in A Normally-Occupied Residence</b><br> | ||
| + | Nathan Sweet, <br> | ||
| + | CU ANYL 1st year | ||
| + | |||
| + | The use of cleaning products alters the chemical composition of indoor air. Utilizing data from detailed observational monitoring conducted over multiple months, we explore the influence of cleaning activities in a normally occupied single-family house. To study emissions and chemistry, we quantified more than 200 VOCs using a proton-transfer reaction time-of-flight mass spectrometer and 52 SVOCs using semivolatile thermal-desorption gas chromatography. During regular professional home cleaning, we observed concentration enhancements in ~60% of observed VOCs and ~80% of reported SVOCs. Most of these concentration enhancements were not clearly linked to either primary emission from cleaning products or secondary formation through reactive chemistry. Instead, shifts in the sorptive properties of indoor surfaces may account for these observations. Individual concentrations of four chlorinated compounds, including dichloramine, increased by up to 0.8 ppb during bleach cleaning. Most cleaning-associated compounds returned to pre-event concentrations within a few hours, with some VOCs and lower volatility SVOCs persisting more than 5 hours, longer than would be expected for removal by ventilation alone. Concentrations of ultrafine particles also increased during professional cleaning, likely from nucleation events associated with bleach cleaning. Use of carpet cleaner was associated with emission of hexanediol, which persisted at elevated concentrations for days after the initial event. We infer that surface sorption dynamics influence the composition of indoor air after cleaning a home. Cleaning events can affect indoor air quality long after the cleaning is completed. | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Using <i>Caulobacter crescentus</i> as a Model to Probe the Reactivity of Silver Nanoparticles</B><br> | ||
| + | Maddie Farber, <br> | ||
| + | CU ANYL 1st year | ||
| + | |||
| + | Silver nanoparticles (AgNPs) are an increasingly common environmental pollutant with antimicrobial and antibacterial properties. The elucidation of their interaction with cellular membranes and subsequent mechanisms of toxicity are critical areas of research that need to be better understood in order to manage potential adverse environmental effects. This work investigates the interaction of AgNPs with large unilamellar vesicles (LUVs) as a model membrane system. Similar studies conducted by others in this field have largely been conducted with uncoated AgNPs, ignoring the effect of environmental conditions on the formation of an eco-corona on AgNPs. Thus, in this study, the spent medium (SM) of a relevant environmental bacterium, <i>Caulobacter crescentus</i>, was used to form a complex eco-corona. We hypothesized that the eco-corona would mediate the <i>in vivo</i> reactivity of AgNPs, specifically through distinct interactions at the cell membrane. The differential reactivity of AgNPs and SM-AgNPs is shown through an <i>in vivo</i> toxicity study using <i>C. crescentus</i>. Model membranes were analyzed using dynamic light scattering (DLS), in which AgNP and LUV size and charge are characterized, and fluorescence anisotropy, where changes to LUV membrane fluidity and dynamics are interrogated. Results of the <i>in vivo</i> study are presented in tandem with model membrane studies in order to correlate toxicity effects seen <i>in vivo</i> with potential mechanisms of reactivity at the cell membrane. | ||
| + | |||
| + | <font size="4">6 November 2023</font size="4"><br> | ||
| + | <b>Explorations into the Composition of Brake Wear Particles from Semi-Metallic and Ceramic Brake Pads</b><br> | ||
| + | Maxwell Lee, <br> | ||
| + | CU ANYL 1st year | ||
| + | |||
| + | Vehicle based air pollution has always been a major contributor to air pollution from combustion-based exhaust particulate matter and emissions. As vehicle trends shift away from combustion-based drive trains, other sources of vehicle-based pollutants will become the main source of vehicle air pollution. Particularly, particulate matter is of great concern to study because it is known that brake pad wear can be a source of these hazardous particles. While a general idea of elemental composition is known for brake pad particles, the chemical composition of organic particles emitted from braking events is largely unknown. Additionally, there is no general consensus on whether brake pads marketed as “ceramic” and “semi-metallic” differ in elemental composition. These unknowns create multiple issues when attempting to find the concentration of brake pad pollution in the air near areas of high traffic. We found that the elemental composition of “ceramic” and “semi-metallic” brake pads does differ and are linked by the presence of common elements like Iron, Magnesium, and Barium, making these elements potential ways to track brake pollution. Additionally, we discovered that organic particles from brake pads form from unique tribological processes, and that using H/C and O/C ratios may be a possible method for tracking organic brake pad pollution. These results can be used to inform possible field studies near high traffic areas to determine how concentrated brake pollution is in those areas. Ideally, these tracers can be used to find trends in brake pad pollution in the context of environmental justice and show whether there are trends between brake pad pollution concentrations and the health of low-income communities. | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Comparative Analysis of AVIRIS and WorldView-3 SWIR for Geologic Mapping in Anza-Borrego Desert State Park</b><br> | ||
| + | Jeffrey Price, <br> | ||
| + | CU ANYL 1st year | ||
| + | |||
| + | A geologic map is both a visual depiction of the lithologies and structures occurring at the Earth’s surface and a representation of a conceptual model for the geologic history in a region. The work needed to capture such multifaceted information in an accurate geologic map is time consuming. Remote sensing can complement traditional primary field observations, geochemistry, chronometry, and subsurface geophysical data in providing useful information to assist with the geologic mapping process. Two novel sources of remote sensing data are particularly relevant for geologic mapping applications: decameter-resolution imaging spectroscopy (spectroscopic imaging) and meter-resolution multispectral shortwave infrared (SWIR) imaging. Decameter spectroscopic imagery can capture important mineral absorptions but is frequently unable to spatially resolve important geologic features. Meter-resolution multispectral SWIR images are better able to resolve fine spatial features but offer reduced spectral information. Such disparate but complementary datasets can be challenging to integrate into the geologic mapping process. Here, we conduct a comparative analysis of spatial and spectral scaling for two such datasets: one Airborne Visible/Infrared Imaging Spectrometer—Classic (AVIRIS-classic) flightline, and one WorldView-3 (WV3) scene, for a geologically complex landscape in Anza-Borrego Desert State Park, California. In this talk, I will discuss the physically based portion of our approach: the spectral mixture residual. The mixture residual uses the wavelength-explicit misfit of a linear spectral mixture model to capture low variance spectral signals. For this study area, the spectral mixture residual revealed greater spectral dimensionality in AVIRIS than WorldView (99% of variance in 39 versus 5 residual dimensions). These results illustrate the potential of recent and planned imaging spectroscopy missions to complement high-resolution multispectral imagery—along with field and lab observations—in planning, collecting, and interpreting the results from geologic field work. | ||
| + | |||
| + | <font size="4">30 October 2023</font size="4"><br> | ||
| + | <b>Drivers of Biogenic Reactive Nitrogen Oxide Emissions in Southern Indiana Deciduous Forests</b><br> | ||
| + | Clara Lietzke, <br> | ||
| + | CU ANYL 1st year | ||
| + | |||
| + | In the troposphere, reactive nitrogen oxides (NO<sub>y</sub> = NO, NO<sub>2</sub>, HONO) are harmful pollutants. Conversion of NO to NO<sub>2</sub> propagates the radical reactions that oxidize volatile organic compounds (VOCs) and generate hydroxyl radical (OH). NO<sub>2</sub> is directly photolyzed to produce ozone (O<sub>3</sub>), a harmful air pollutant and main component of smog. Anthropogenic emissions of nitrogen oxide and nitrogen dioxide (NO<sub>x</sub> = NO + NO<sub>2</sub>) have declined with the enactment of air pollution control policy, increasing the importance of biogenic NO<sub>y</sub> emissions. Previous work characterizing sources and sinks of NO<sub>y</sub> has been focused on soil microbial emissions, although emissions of NO<sub>x</sub> from plant matter have also been directly observed. To determine the short-term impact of leaf senescence on local NO<sub>y</sub> levels, we conducted the Fall Forest Exchange of Reactive Nitrogen (FFERN) field campaign, where we observed substantial NO emissions from American Sycamore leaf litter, particularly under specific stressors like dew and freezing events. Here, we investigate the mechanism of NO<sub>y</sub> emissions from American Sycamore and other tree species leaves in addition to their response to different stressors, such as drying, rehydration, freezing, and cutting. | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Base-mediated depolymerization of amine-cured epoxy resins</b><br> | ||
| + | Katherine Stevenson, <br> | ||
| + | CU ANYL 1st year | ||
| + | |||
| + | Carbon fiber-reinforced epoxy composites are used in multiple industries, including for aerospace, automotive, and wind energy applications, due to their excellent strength-to-weight ratios and tunable material properties. Fortunately, recycling strategies for carbon fiber-based composites are emerging, with the primary focus on recovery of fibers due to the cost and energy intensity in their production. In addition to fiber recovery, there is an opportunity to recycle the epoxy components, such that ideal recycling strategies would yield both post-consumer fibers and epoxy monomers for reuse. Here, we examine potassium tert-butoxide-mediated cleavage of C–O and C–N bonds in amine-cured epoxy resins. We accomplish this via developing model compounds that reflect both C–O and C–N linkages in amine-cured epoxy composites, before expanding to both model linear thermoplastics and thermosets. We obtain excellent yields of both phenol (up to 97% molar yield) and amine products (up to 99 mol%) from aromatic and/or aliphatic amine-based model compounds. This system enables up to quantitative yield of bisphenol-A and up to 58% molar yield of aniline from model thermoplastic epoxy amines and 71% molar yield of BPA from a reaction with a thermoset substrate. These data correspond to 15% mass recovery of BPA from a commercial epoxy thermoset. | ||
| + | |||
| + | <font size="4">23 October 2023</font size="4"><br> | ||
| + | <b>Impact of Sulfur on Planetary Haze: Implications for Habitability</b><br> | ||
| + | [https://sites.google.com/view/brownelab/people Eleanor Browne] <br> | ||
| + | CU ANYL Faculty | ||
| + | |||
| + | Atmospheric composition plays a critical role in creating and maintaining a habitable environment. For instance, the formation of aerosol particles (“hazes”) may affect the surface ultraviolet radiation flux and temperature and may serve as a source of complex organic molecules including nutrients. Although the composition of aerosols in planetary atmospheres is diverse, organic aerosols are of particular interest for our understanding of habitable worlds. Organic aerosol may serve as a potential biosignature for certain anoxic atmospheric compositions, and it may have played an important role in the climate and biogeochemistry of early Earth. Recently, we have explored how the addition of trace H<sub>2</sub>S (0-10 ppmv) affects organic haze analogs produced from ultraviolet photochemistry of reducing (N<sub>2</sub>/0.1% CH<sub>4</sub>) and weakly reducing (N<sub>2</sub>/0.1% CH<sub>4</sub>/0-2% CO<sub>2</sub>) gas mixtures. In these laboratory experiments, we analyze the aerosol products in real time using a suite of analytical instrumentation developed for studying modern Earth’s atmospheric chemistry. Specifically, we measure aerosol chemical composition using a quadrupole aerosol mass spectrometer, aerosol size and number with a scanning mobility particle sizer, and aerosol optical properties and water uptake using photoacoustic and cavity ringdown spectroscopies. We find that the inclusion of trace amounts of H<sub>2</sub>S in the precursor mixture significantly enhances the formation of organic aerosol mass, increases particle effective density, alters aerosol optical properties, and leads to the formation of organosulfur aerosol. Notably, we find no evidence of S<sub>8</sub> aerosol – a finding that challenges predictions that H<sub>2</sub>SO<sub>4</sub> and S<sub>8</sub> were the primary sulfur reservoirs in Earth's Archean atmosphere. We further explore how the changes in aerosol optical properties could influence the radiative forcing of a planet using a modified gray radiative scheme and we discuss the implications of our water uptake measurements on cloud formation and radiative forcing. Overall, our results suggest that coupled carbon-sulfur atmospheric chemistry significantly impacts organic haze with implications for planetary climate, habitability, biosignature detection, and prebiotic chemistry. | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>The pollution impacts of disinfection-only air cleaners, and applying frequency combs for aerosol analysis</b><br> | ||
| + | [http://cires1.colorado.edu/jimenez/ Jose-Luis Jimenez] <br> | ||
| + | ANYL Faculty | ||
| + | |||
| + | Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly summarize ongoing projects such as the upcoming NASA ASIA-AQ campaign and the ASCENT long-term monitoring site in Denver. However, I will mostly focus on upcoming projects of direct interest to first year PhD students. | ||
| + | Airborne disease transmission was successfully labeled as a superstition by American epidemiologist Charles Chapin in 1910, with limited progress until the COVID-19 pandemic. The pandemic has ushered in a paradigm shift in which most respiratory viruses are understood to have important or dominant airborne transmission. For this reason, many types of air cleaners focused only on disinfection have been developed and sold during the pandemic. Over the last year we have investigated the formation of pollutants from many of these air cleaners, including germicidal ultraviolet light (GUV). Multiple types of air cleaners form O<sub>3</sub> and PM (SOA) indoors. I will summarize recent experimental and modeling results, showing that multiple types of air cleaners may kill more people from pollution than they save from disinfection. A likely PhD position in our group will expand recent and ongoing GUV experimental work to multiple types of air cleaners, and will quantify the SOA formation potential of indoor air. | ||
| + | |||
| + | Dual frequency combs are new light sources with unique properties for analytical spectroscopy. The mid-IR contains important chemical information about organic functional groups, but analysis of aerosols suspended in air in this region with techniques such as FTIR was considered infeasible, due to interferences from narrow absorption lines of abundant gases such as CO<sub>2</sub>. As part of the collaborative IARPA SAURON project led by CU-Boulder Engineering, a PhD student in our group will collaborate with the SAURON team to use new and emerging DFC mid-IR sources to explore the analysis of aerosols in an environmental chamber (and eventually simple field experiments), exploring the ability to subtract interfering gases and measure the spectrum of aerosols of different composition, concentration, and mixing state. | ||
| + | |||
| + | <font size="4">10 October 2023</font size="4"><br> | ||
| + | <b>Particle Formation and Growth in New Chemical Regimes</b><br> | ||
| + | [https://sites.google.com/view/brownelab/people Eleanor Browne] <br> | ||
| + | CU ANYL Faculty | ||
| + | |||
| + | Understanding the mechanisms through which aerosol particles form and grow is critical for constraining a planet’s energy budget, however, our knowledge of the key processes governing particle formation and growth in diverse environments remains weak. In the coming decades, our knowledge of particle formation and growth will continue to be challenged as changing climate and anthropogenic emissions alter the chemical regimes of modern-day atmospheric chemistry on Earth and measurements from, for instance, the James Webb Space Telescope and NASA’s Dragonfly mission offer increasingly chemically resolved insights into planetary atmospheres much different from our own. | ||
| + | |||
| + | In this talk I will highlight three of our projects on particle formation and growth in new and/or understudied chemical regimes. In the first part I will discuss our field measurements in an agricultural region, focusing on reactive nitrogen and carbon species that contribute to particle formation and growth. I will briefly touch on how vertical gradients in atmospheric composition and physical properties (e.g., temperature) affect particle formation and growth. Next, I will discuss our laboratory investigations of the atmospheric chemistry of volatile methyl siloxanes. These solely anthropogenic compounds have recently come under scrutiny for their potential toxicity and environmental persistence. Moreover, field measurements suggest that they are present in urban nanoparticles. Our work investigating the kinetics, mechanism, and aerosol yield of volatile methyl siloxane oxidation addresses key gaps in our understanding of their environmental chemistry. Lastly, I will describe our laboratory experiments exploring aerosol formation in anoxic planetary atmospheres. I will primarily focus on how organosulfur formation affects aerosol mass, composition, and optical properties in Archean Eon analog experiments and will discuss the implications for habitability and the understanding of the evolution of Earth’s atmosphere.<br> | ||
| + | |||
| + | <font size="4">9 October 2023</font size="4"><br> | ||
| + | <b>Laboratory studies of the formation of atmospheric aerosol under different chemical regimes</b><br> | ||
| + | [http://krollgroup.mit.edu/ Jesse Kroll],<br> | ||
| + | MIT Dept. of Civil and Environmental Engineering<br> | ||
| + | MIT Dept. of Chemical Engineering | ||
| + | |||
| + | A large fraction of fine particulate matter (aerosol) in the atmosphere is secondary in nature, formed from the atmospheric oxidation of gas-phase compounds. This oxidation chemistry can govern the amount and properties of aerosol particles, and hence their impacts on climate and health. Much of our understanding of aerosol formation derives from laboratory studies, which to be most useful for atmospheric modeling should cover the range of atmospheric oxidation conditions as well as possible. One particular challenge is matching the chemistry of organic peroxy (RO<sub>2</sub>) radicals; these key intermediates can react via a number of channels – bimolecular reactions with different radical species (NO, HO<sub>2</sub>, RO<sub>2</sub>…) as well as unimolecular (isomerization) reactions – each of which may have its own reaction product distribution and aerosol yields. This talk will describe our group’s efforts to measure aerosol formation in an environmental (“smog”) chamber, across the full range of RO<sub>2</sub> conditions found in the atmosphere. For the oxidation of a given aerosol precursor, we vary RO<sub>2</sub> chemistry in the chamber by changing concentrations of various reactants, and estimate RO<sub>2</sub> fate using chemical ionization mass spectrometry (CIMS) of gas-phase products as well as mechanistic modeling of the oxidation chemistry. Chemical systems examined include the oxidation of dimethyl sulfide (CH<sub>3</sub>SCH<sub>3</sub>, emitted to the atmosphere by phytoplankton) to form sulfate aerosol, and the oxidation of isoprene and alpha-pinene (C<sub>5</sub>H<sub>8</sub> and C<sub>10</sub>H<sub>16</sub>, emitted by plants) to form organic aerosol.<br> | ||
| + | |||
| + | <font size="4">2 October 2023</font size="4"><br> | ||
| + | <b>Long-path atmospheric measurements using dual frequency comb spectroscopy</b> | ||
| + | |||
| + | [https://www.nist.gov/people/kevin-cossel Kevin Cossel]<br> | ||
| + | NIST, Boulder, CO | ||
| + | |||
| + | Open-path measurements of atmospheric gas species over km-scale path lengths are well suited to quantify emissions from sources like oil and gas production, agricultural activities, forest fires, and industry. Our group at NIST has developed open-path dual frequency comb spectroscopy (DCS) as a tool to provide accurate measurements of multiple trace gas species simultaneously across path lengths ranging from 100 m to >10 km. | ||
| + | |||
| + | We have used these systems for a number of field measurements. In the first campaign, we deployed a prototype system in the mid-infrared spectral region to a new oil and gas well installation in order to measure emissions during the different stages of unconventional well development. In another measurement, we deployed to the Platteville Atmospheric Observatory in north-eastern Colorado or 4 months. This site is located in the Denver-Julesburg oil and gas basin and in an area with a large number of confined animal feeding operations, leading to a complex mixture of trace gas emissions. By using measurements of ethane and NH<sub>3</sub>, we can attribute the observed CH<sub>4</sub> to the oil and gas and agricultural sectors. We also see HCHO plumes that are correlated with C<sub>2</sub>H<sub>6</sub>, indicating oil and gas related sources of HCHO (likely from combustion). Finally, we took a system to a beef cattle stocker site to measure emissions of methane and NH<sub>3</sub> over several months. | ||
| + | |||
| + | Current opportunities in our group include field measurements working to understand urban greenhouse gas | ||
| + | emissions and air quality links, testing mitigation of greenhouse gas emissions in agriculture, and measuring | ||
| + | emissions from forests as well as laboratory studies looking at emissions of greenhouse gases and reactive nitrogen | ||
| + | from cyanobacteria and combustion. | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Expanding the Use of <sup>19</sup>F NMR Spectroscopy in Per- and Polyfluoroalkyl Substance Analysis</b><br> | ||
| + | Andre Schaum, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | Aqueous film-forming foams (AFFFs) have been used to extinguish liquid-fuel fires since the 1960s and | ||
| + | are significant historical sources of per- and polyfluoroalkyl substances (PFAS) in the environment. | ||
| + | Though in the process of being phased out of use in the United States, large stockpiles of AFFFs still exist | ||
| + | nationwide, often stored in poorly labeled containers and tanks. For proper assessment and disposal, rapid | ||
| + | and inexpensive analytical methods are needed to quantify total fluorine, determine PFAS composition, | ||
| + | and identify AFFF type. Current analytical methods that provide quantitative measures of individual | ||
| + | PFAS in AFFFs, such as liquid chromatography – tandem mass spectrometry (LC-MS/MS), are often | ||
| + | complicated, time-consuming, and expensive. Methods for total fluorine analysis, though quantitative, | ||
| + | provide relatively little information on the chemical nature of PFAS in AFFFs. Fluorine Nuclear Magnetic | ||
| + | Resonance Spectroscopy (<sup>19</sup>F NMR) has previously been used to characterize PFAS in environmental | ||
| + | matrices, technical mixtures, and analytical standards, though its application to AFFFs has been limited. | ||
| + | Here, a <sup>19</sup>F NMR method was developed for rapid qualitative and quantitative analysis of fluorine content | ||
| + | in AFFFs and used to identify the manufacturing method of two AFFFs of unknown origin.<br> | ||
| + | |||
| + | <font size="4">18 September 2023</font size="4"><br> | ||
| + | <b>Chemistry of Volatile Organic Compounds in the Atmosphere </b><br> | ||
| + | [https://sites.google.com/view/de-gouw-lab Joost de Gouw], <br> | ||
| + | ANYL Faculty, CU Boulder | ||
| + | |||
| + | Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products. | ||
| + | |||
| + | In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments. | ||
| + | |||
| + | Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments to better understand their sources and fate. One particular study involves the impact of the Marshall Fire in Boulder County on indoor air in homes that were near the burnt area. Second, we are working on the emissions and chemistry of VOCs in urban air. One study that will be highlighted involved field measurements in Los Angeles in the Summer of 2022. Third, we are working on the indoor air chemistry induced by air cleaners such as germicidal UV lamps that are effective at inactivating airborne viruses. Finally, we are also using data from satellite remote sensing instruments to measure the pollutants from oil and natural gas production, in urban air and from wildfires. | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>From Coastal Megacities to Remote Oceans: Small Molecules in the Anthropocene</b><br> | ||
| + | [http://volkamergroup.colorado.edu/ Rainer Volkamer], <br> | ||
| + | ANYL Faculty, CU Boulder | ||
| + | |||
| + | Anthropogenic enhancements of natural processes modify atmospheric chemistry in the urban and remote marine atmosphere. For example, marine iodine emissions have tripled in recent decades due to enhanced deposition of pollution ozone over oceans, affecting particle formation and the ozone layer. Furthermore, increasing emissions from wildfires pose challenges to public health and urban air quality. The Volkamer group develops advanced instrumentation (optical and mass spectrometric) to measure small molecules and condensable vapors relevant to particle formation, public health and climate discussions. We seek to better quantify and understand the interconnections between marine ecosystem, aerosols and their control on atmospheric chemistry. We also study the emissions and oxidative chemistry of coastal Megacities using research aircraft and satellites. Examples from recent field and laboratory experiments are discussed that aim to develop a molecular level understanding to test and advance atmospheric models. Opportunities for graduate research exist in 1) field measurements using research aircraft (i.e., TI3GER-2022, CUPiDS-2023, TI3GER2-2025 projects), 2) laboratory experiments of particle formation and multiphase chemistry (incl. at CLOUD/CERN), and to 3) develop instrumentation by exploiting synergies between optical spectroscopy and mass spectrometry. | ||
| + | |||
| + | |||
| + | <font size="4">11 September 2023</font size="4"><br> | ||
| + | <b>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</b> | ||
| + | <br> | ||
| + | [https://sites.google.com/site/ziemanngroup/ Paul Ziemann], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative studies of indoor air chemistry at CU. In this talk I will provide a brief overview of recent research in my lab for the purpose of informing first-year chemistry graduate students." | ||
| + | |||
| + | ==Summer 2023== | ||
| + | |||
| + | <font size="4">21 July 2023</font size="4"> <br> | ||
| + | <b>It’s Okay to be Salty: Salt Deliquescence and Efflorescence Relevant to Mars and Earth</b> | ||
| + | |||
| + | Marium Fernanders, <br> | ||
| + | [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Tolbert group], <br> | ||
| + | ANYL PhD thesis | ||
| + | |||
| + | "The presence of salts in Martian soil gives rise to new mechanisms of liquid water formation through deliquescence and efflorescence of sediment salts. Observations by the Phoenix lander, Curiosity rover, and Mars Reconnaissance Orbiter (MRO) have detected hydrated salts in the Martian regolith, including chloride, perchlorate, and chlorate compounds. This thesis uses laboratory studies to investigate water uptake and loss by model systems to gain insight into the water cycle on Mars. | ||
| + | |||
| + | With recent discoveries of chlorine in the Martian soil, past research has focused on the deliquescence and efflorescence of perchlorate and chloride salts. However, less is known about chlorate salts. Chlorate is one of several intermediate oxidation states between perchlorate and chloride, however of the other oxychloride species chlorate is the more stable species and therefore more likely to exist on the Martian surface. Here, sodium chlorate and magnesium chlorate were studied to observe their moisture absorption and release behavior. It was found that sodium chlorate displayed temperature-dependent deliquescence and magnesium chlorate exhibited water uptake regardless of temperature. This could mean that magnesium chlorate can take up water at single digit relative humidities, making it an extremely Mars-relevant salt for study. | ||
| + | |||
| + | Understanding the behavior of pure salts is crucial, but it's also important to consider real-world conditions that influence water uptake and release in sediment salts. Additionally, this thesis investigated the behavior of magnesium nitrate hexahydrate under Martian and Atacama Desert conditions. It was found that pure magnesium nitrate is unlikely to undergo aqueous cycling on Mars because the temperature and relative humidity required for nitrate deliquescence happens at relative humidities that is to wet at the same temperature present on Mars. However, in the Atacama Desert, nitrates in the soil can uptake water throughout the year, with peak uptake occurring in the fall season, potentially forming stable or metastable brines. A potentially year-round source of water could be used for microbial life to survive under the hyper-arid conditions present on the desert surface. | ||
| + | |||
| + | Finally, complex sediment samples from Don Juan Pond's slope streaks were investigated as a model for Recurring Slope Lineae present on Mars. It was found that chlorine-bearing salts, such as sodium and calcium chloride, drive water uptake and deliquescence, while insoluble mineral components do not hinder water absorption. Efflorescence and deliquescence occurred at similar relative humidities, preventing supersaturation. Based on laboratory data and environmental conditions in the Lake Vanda region, episodic occurrence of slope streaks throughout the year, with higher prevalence in the southern summer and spring seasons, was predicted." | ||
| + | |||
| + | <font size="4">15 May 2023</font size="4"><br> | ||
| + | <b>Formation, Abundance, and Evolution of Molecular Products in α-Pinene and β-Pinene Secondary Organic Aerosol</b> | ||
| + | |||
| + | Dr Christopher Kenseth, <br> | ||
| + | Department of Atmospheric Sciences, University of Washington | ||
| + | |||
| + | "Secondary organic aerosol (SOA) contributes substantially (15–80% by mass) to the global | ||
| + | burden of atmospheric fine particulate matter (PM<sub>2.5</sub>), which exerts large but uncertain effects on | ||
| + | climate as well as adverse impacts on air quality and human health. The oxidation of α-pinene | ||
| + | and β-pinene (C<sub>10</sub>H<sub>16</sub>), emitted in appreciable quantities from forested regions (~85 Tg y<sup>-1</sup>), | ||
| + | represents a dominant source of SOA. Deciphering the molecular composition, and in turn | ||
| + | formation and aging mechanisms, of α-pinene and β-pinene SOA is essential to reducing | ||
| + | uncertainty in assessment of their environmental and health impacts. However, molecular | ||
| + | characterization of α-pinene and β-pinene SOA, which generally consist of hundreds or more | ||
| + | compounds of diverse classes, is significantly hindered by their chemical complexity. This | ||
| + | seminar will present an overview of research aimed at constraining the formation, abundance, | ||
| + | and evolution of molecular products in SOA derived from the ozonolysis and photooxidation of | ||
| + | α-pinene and β-pinene using a combination of environmental chamber and flow tube | ||
| + | experiments, liquid chromatography/electrospray ionization mass spectrometry (LC/ESI-MS), | ||
| + | and organic synthesis." | ||
| + | |||
| + | ==Spring 2023== | ||
| + | |||
| + | <font size="4">1 May 2023</font size="4"><BR> | ||
| + | <b>Air-Sea Exchange of Reactive Gases: Chemistry at Interfaces and Partitioning in Aqueous Droplets</b> | ||
| + | |||
| + | Randall Chiu, <br> | ||
| + | [https://volkamergroup.colorado.edu/ Volkamer group], <br> | ||
| + | ANYL PhD thesis | ||
| + | |||
| + | "Many puzzles remain regarding the interaction of halogens with oxygenated volatile organic compounds (OVOC) in the marine boundary layer (MBL). Observations of the OVOC glyoxal (CHOCHO) over the remote Pacific Ocean are difficult to reconcile with its brief (2-4 hour) atmospheric lifetime and high water solubility (Henry’s law constant ~4×10<sup>5</sup> M/atm). In those same airmasses, concentrations of reactive halogen species, specifically bromine monoxide (BrO) are below detection limits even though models predict up to 1.3 pptv. Presented here is a surface reaction that holds promise for explaining one or both puzzles. In the lab, fatty acids on a simulated sea surface microlayer (SML) undergo photochemical transformation to unsaturated aldehydes (2-alkenals), subsequent ozonolysis of which produces glyoxal. In a follow-up study, 2-alkenals were shown to react with Br to produce HBr in high yield. Depending on the concentration of fatty acids in SML, fatty acid photochemistry holds promise as a candidate for the missing BrO sink. | ||
| + | |||
| + | The second work presented are aircraft ozone eddy covariance (EC) flux over the Pacific Ocean during the 2023 Technical Innovation Into Iodine and GV Environmental Research (TI<sup>3</sup>GER) technical campaign. Ozone EC fluxes were measured with three independent ozone sensors of two different designs (NCAR Fast O<sub>3</sub>, a nitric oxide chemiluminescence instrument, and KIT Fast Airborne O<sub>3</sub>, a coumarin dry chemiluminescence instrument), which has not been done before. All three instruments were shown to be suitable for EC flux experiments. Particularly interesting case studies are presented in which ozone EC flux exhibits different spatial variability than water vapor EC flux. In addition, the availability of three independent EC flux measurements was used to constrain the uncertainty and limit of detection (LOD) methods in the literature." | ||
| + | |||
| + | <font size="4">24 April 2023</font size="4"><BR> | ||
| + | <b>The Role of Organic Heterogeneous Nuclei in Contact Crystallization of Atmospherically Relevant Salts</b> | ||
| + | |||
| + | Kyle McMillan, <br> | ||
| + | ANYL 3rd year, [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Tolbert group] | ||
| + | |||
| + | The overall radiative effect of atmospheric aerosols on climate is currently a major source of | ||
| + | uncertainty in climate models. Essential in resolving this uncertainty is a complete understanding | ||
| + | of the particle phase state in the atmosphere. Often overlooked in current studies on the particle | ||
| + | phase is the role of collisions in inducing contact nucleation. Thus, more research that focuses on | ||
| + | determining which atmospherically relevant compounds are most likely to be involved in contact | ||
| + | nucleation under varying conditions is warranted. Of interest here was evaluating the | ||
| + | effectiveness of organic-containing contact nuclei (CN) in inducing contact efflorescence of | ||
| + | aqueous salt-containing droplets. To do this, contact efflorescence was probed on freely floating | ||
| + | aqueous ammonium sulfate (AS) droplets using a long-working distance optical trap. In this | ||
| + | setup, optically levitated droplets were contacted with both glassy (D-(+)-raffinose and citric | ||
| + | acid) and surface-active (stearic acid and cis-pinonic acid) organic CN over a range of relative | ||
| + | humidity (RH) values above the homogeneous efflorescence RH of AS. The purpose of these | ||
| + | experiments was to determine if certain organic-containing CN could induce contact | ||
| + | efflorescence of aqueous droplets at an RH significantly above the homogeneous efflorescence | ||
| + | RH of AS. Far-field imaging was employed as a way of probing crystallization either in the | ||
| + | contact or homogeneous modes, and was supplemented with near-field imaging, which was used | ||
| + | to track contact events. Droplet compositions before and after crystal nucleation were | ||
| + | characterized using Raman spectrometry to track the liquid water content of the droplet. | ||
| + | Promising in these experiments is the effectiveness of surface-active organics—in particular, cis- | ||
| + | pinonic acid—in inducing contact efflorescence of AS, which has major implications for the | ||
| + | particle phase state of the troposphere. Continued experiments in this area will provide key | ||
| + | insight into the interactions that cause contact to be effective at initiating nucleation and will | ||
| + | ultimately lay the groundwork for analogous contact ice nucleation studies. | ||
| + | |||
| + | <font size="4">17 April 2023 - special seminar</font size="4"><BR> | ||
| + | <b>Environmental Analytical Chemistry in an Art Museum</b> | ||
| + | |||
| + | Julia Bakker-Arkema, Ph.D., <br> | ||
| + | Associate Research Scientist, Metropolitan Museum of Art <br> | ||
| + | (Ziemann Group alum, 2020) | ||
| + | |||
| + | As a preventive conservation scientist at a museum, my work is centered around improving environmental conditions during the conservation, transportation, storage, and display of cultural heritage objects. I will share some current work using gas-chromatography-mass-spectrometry (GC-MS) to identify and quantify volatile organic compounds emitted from conservation materials that have the potential to damage the surfaces of art and artifacts, and hopefully I’ll give you a sense of what it means to be an analytical chemist in an art museum. During my Ph.D. research in the Ziemann lab, I investigated the oxidation of volatile organic compounds and the formation of secondary organic aerosol through environmental chamber studies. My experience and interest in environmental analytical chemistry led me down various paths both in and out of the laboratory, including science policy on ozone management in the front range, an indoor air field campaign in Texas, science communication workshops for Women in Science & Engineering, and website design and video production with the Sloan Foundation. I’ll discuss each of these pursuits, and how I applied the skills I learned to launch a career in teaching and research at the Metropolitan Museum of Art in New York City. This talk is aimed at graduate students, and all questions are welcome! | ||
| + | |||
| + | <font size="4">17 April 2023</font size="4"><BR> | ||
| + | <b>Design and Characterization of the CU-Boulder Environmental Chamber Facility & Long-term and High-time-resolution Measurement for the Characterization of Aerosol Properties in the Denver Metropolitan Area</b> | ||
| + | |||
| + | Seonsik Yun, <br> | ||
| + | ANYL 3rd year, [http://cires1.colorado.edu/jimenez-group/ Jimenez group] | ||
| + | |||
| + | "Environmental reaction chambers are essential tools for studying atmospheric chemistry and physics based on laboratory experiments. The design of chambers varies concerning the chamber sizes, temperature/humidity controls, light sources, air purification, and operating conditions to simulate atmospheric chemistry reactions and are often used to investigate secondary organic aerosol formation. Here we present a new state-of-the-art CU-Boulder environmental chamber facility and its design and characterizations. | ||
| + | |||
| + | The Atmospheric Science and Chemistry mEasurement NeTwork (ASCENT) is a new comprehensive, high-time-resolution, long-term measurement network in the U.S. to characterize aerosol chemical composition and physical properties. As one of 12 sites of the ASCENT, La Casa site is located near downtown Denver, Colorado, and will be equipped with a suite of advanced aerosol instrumentation: Aerosol Chemical Speciation Monitor (ACSM, non-refractory aerosols), Xact (trace metals), Aethalometer (black/brown carbon), and Scanning Mobility Particle Sizer (SMPS, aerosol number size distribution and concentration). Additional instrumentation, such as a Vocus Proton Transfer Reaction Time-of-Flight Mass Spectrometer (Vocus PTR-TOF, gas phase volatile organic compounds) and a radon detector (RAD7), will also be deployed to address a comprehensive data analysis on Denver urban aerosols. In this talk, I will present the preliminary results from the ASCENT instrumentation and future research opportunities." | ||
| + | |||
| + | <font size="4">11 April 2023</font size="4"><BR> | ||
| + | |||
| + | <b>Measurements of Volatile Organic Compounds in Urban Air by Proton-Transfer Reaction Mass Spectrometry</b> | ||
| + | |||
| + | Andrew Jensen, <br> | ||
| + | [https://sites.google.com/view/de-gouw-lab/people de Gouw Group] <br> | ||
| + | CU ANYL Dissertation Defense | ||
| + | |||
| + | "Volatile organic compounds (VOCs) in urban environments are emitted to the atmosphere from many biogenic and anthropogenic sources. VOC oxidation contributes to the formation of secondary products, including ozone and secondary organic aerosol, which negatively impact air quality and human health. As emissions change, VOC measurements are necessary to reassess their role in urban air quality. Here, I present results from measurements of ambient VOCs in three urban environments made by proton-transfer reaction mass spectrometry (PTR-MS) with part-per-trillion by volume detection limits. | ||
| + | |||
| + | I first detail the quantification of VOCs measured in Boulder, CO in spring 2021 with a focus on instrument characterization. I developed a PTR Data Tool to parameterize instrument response which, in turn, informs the quality of the measurements and the quantification of VOCs which cannot be directly calibrated. These findings help streamline and improve future PTR-MS measurements. | ||
| + | |||
| + | VOCs were also measured in Changzhou, an industrial Chinese city, before, during, after the 2020 COVID-19 lockdowns which shut down non-essential activities. I quantified strong reductions in industrial and transportation emissions. These estimates help inform emission inventories and chemistry-transport models during this global perturbation of air pollution, as well as possible future scenarios with reduced emissions. | ||
| + | |||
| + | Finally, VOCs were measured in the Los Angeles basin, California during summer 2022. Ambient concentrations of primary VOCs from vehicles have declined over the preceding decade due to emission regulations while concentrations of secondary VOCs were largely unchanged, in part, due to faster oxidation and unregulated emission sources. This study expands the detected subset of urban VOCs and allows for a more detailed understanding of atmospheric sources and fates." | ||
| + | |||
| + | <font size="4">10 April 2023</font size="4"><BR> | ||
| + | |||
| + | <b>Pollution distribution in the Denver metroplex: Chemical, sociological, and historical insights</b> | ||
| + | |||
| + | Alexander Bradley, <br> | ||
| + | ANYL 3rd year, [https://sites.google.com/view/de-gouw-lab de Gouw group] | ||
| + | |||
| + | "Prior studies have shown that people of color in the United States are exposed to higher levels of pollution than non-Hispanic White people. We show that the city of Denver, Colorado displays similar race and ethnicity-based air pollution discrepancies by using a combination of high-resolution satellite data, air pollution modeling, historical demographic information, and areal apportionment techniques. TROPOMI NO2 columns and modeled PM2.5 concentrations from 2019 are higher in communities subject to redlining, a discriminatory mortgage appraisal practice from the 1930s by the federal Home Owners’ Loan Corporation (HOLC). We calculated and compared Spearman coefficients for pollutants and race at the census tract level for every city that underwent redlining to contextualize the disparities in Denver. We find inequitable siting of polluting infrastructure leading to higher populations of people of color living near point sources, including 40% higher Hispanic and Latino populations; Traffic analysis and emission inventory data show that people of color are more likely to live near busy highways. Unequal opportunities for people of color has allowed for pollution disparities to persist despite attempts by the city to rectify them . Finally, we identify core causes of the pollution disparities to provide direction for remediation." | ||
| + | |||
| + | <font size="4">4 April 2023</font size="4"><br> | ||
| + | |||
| + | <b>Optical Properties of Brown Carbon Found in Earth and Planetary Haze Aerosol</b> | ||
| + | |||
| + | Kevin Jansen,<br> | ||
| + | [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Tolbert group] <br> | ||
| + | CU ANYL Dissertation Defense | ||
| + | |||
| + | "Atmospheric aerosol has long been documented to be capable of both scattering and absorbing incoming solar radiation, leading to either a net cooling or warming effect on planetary atmospheres. Despite the optical properties of some forms of aerosol such as inorganic salts being well characterized, the optical properties of organic aerosol are not as well established. While most organic aerosol is only capable of scattering light in the atmosphere, a fraction of organic aerosol does absorb light. Unlike inorganic “black carbon” which absorbs light throughout the visible wavelength range, so-called organic “brown carbon” is capable of absorbing light at in the near ultraviolet tailing off in the visible. Brown carbon aerosol can account for a significant fraction of the total light absorption at lower wavelengths in various atmospheres. For example brown carbon account for up to twenty percent of Earth’s light absorption at wavelengths <400nm, implying brown carbon can have a major effect on a planet’s total radiative forcing. | ||
| + | |||
| + | Part of the uncertainty in the magnitude and direction of brown carbon forcing stems from the fact that atmospheric aerosol contains numerous organic compounds, each with their own optical properties. Depending on the aerosol source, ambient conditions, and any potential atmospheric processing, the optical and chemical properties of brown carbon can vary dramatically over time. | ||
| + | |||
| + | This thesis examines the effects of both formation conditions and atmospheric processing on two different brown carbon aerosol systems. First, the optical properties of brown carbon formed via aqueous reactions of carbonyls and ammonium found in Earth’s atmosphere were characterized under varying pH conditions. It was found that brown carbon formation is heavily dependent on the pH, with brown carbon formation being favored under basic conditions. At the same time, brown carbon was also formed under acidic, atmospheric conditions in aerosol particles that are incapable of forming in acidic bulk phase solutions, indicating a unique reaction only accessible in aerosol particles. In addition, the ozonolysis of this brown carbon in the atmosphere was simulated in a flow tube to monitor the potential loss of the aerosol’s light absorption. It was found that despite readily reacting with ozone, a recalcitrant fraction of brown carbon retains its ability to absorb light, indicating it can have a persistent warming effect as it remains in Earth’s atmosphere. | ||
| + | |||
| + | Brown carbon is not limited to the Earth's atmosphere, but has been detected in the atmospheres of other planets as well. For instance, organic brown carbon has been found on Titan, Saturn's largest moon and may have been important on the Archean Earth as well. For planetary atmospheres without obvious sources of large organic molecules, brown carbon is likely formed through photochemical reactions of small molecules including methane. Here, light absorbing aerosol was generated by exposing mixtures of CH4/CO2/H2S in N2 to deep UV light to mimic aerosol formation potentially found in planetary hazes. It was found that the light absorption of the haze aerosol decreased as the concentration of CO2 increased. This trend was found to be due to the formation of non-absorbing, sulfate salts. In addition, the aerosol was found to be more hygroscopic as the CO2 concentration increased, implying the aerosol could grow in size and scatter light more efficiently, implying the CO2 could have a significant effect on the optical properties of planetary haze aerosol and subsequently its radiative forcing. | ||
| + | |||
| + | Because brown carbon has been found in both Earth's atmosphere and in the atmospheres of other planets, these studies of the optical properties of brown carbon could assist in modeling the radiative forcing and climate trends of both Earth and various planetary systems." | ||
| + | |||
| + | <font size="4">3 April 2023</font size="4"><BR> | ||
| + | |||
| + | <b>Chemical and Meteorological Controls on New Particle Formation in the Southern Great Plains</b> | ||
| + | |||
| + | Bri Dobson, ANYL 3rd year, <br> | ||
| + | [https://sites.google.com/view/brownelab/people Browne group] | ||
| + | |||
| + | "New particle formation (NPF) is estimated to account for 40 – 70 % of cloud condensation nuclei and therefore plays a central role in our understanding of aerosol-cloud interactions. It is well established that the likelihood of NPF is influenced by both chemical precursors, such as sulfuric acid, ammonia, amines, and organic compounds, as well as physical atmospheric conditions. However, the complexity of the process leads to large uncertainties in the representation of NPF in models. A complete understanding of the conditions that lead to NPF requires observations of both gas-phase chemistry as well as physical and meteorological conditions. Additionally, these measurements must be made in diverse ecosystems as both chemical and physical conditions vary by region. | ||
| + | |||
| + | We investigated NPF in agricultural regions, which have been understudied compared to urban and forested regions. To do so, we deployed an atmospheric-pressure interface time-of-flight mass spectrometer (APi-TOF) and a chemical ionization time-of-flight mass spectrometer with ethanol reagent ion (EtOH-CIMS) to the US Department of Energy Atmospheric Radiation Measurement (ARM) Southern Great Plains (SGP) site in rural Oklahoma in October 2021 and Spring 2022. These mass spectrometry measurements of gas-phase compounds along with the long-term meteorological measurements from the ARM SGP site allow us to further investigate the chemical and physical conditions that contribute to NPF. In this talk, I will present a case study of two of the observed NPF events during our spring observing period. Changes in transported gases and atmospheric conditions cause differences in new particle formation and growth over the course of a few days at the same location." | ||
| + | |||
| + | <font size="4">20 March 2023</font size="4"><BR> | ||
| + | |||
| + | <b>Ozone photochemistry and free radical budgets in the Los Angeles basin: A comparison of ground-based observations in 2021 and 2010</b> | ||
| + | |||
| + | Michael Robinson, <br> | ||
| + | ANYL 3rd year, [https://www.colorado.edu/lab/browngroup/ Brown group] | ||
| + | |||
| + | "Radical precursors and termination products are important factors to understanding sensitivities of ozone (O3) production to nitrogen oxides (NO + NO2 = NOx) and volatile organic compounds (VOCs). During the summer of 2021, an extensive set of photochemical measurements were conducted at a ground site on the Caltech campus in Pasadena, CA to evaluate emissions and photochemistry in the Los Angeles (LA) basin. Here we present the calibration and measurement of important radical precursors and compare them to observations from the CalNex 2010 field intensive. Major radical sources include formaldehyde (HCHO) and other aldehydes, nitrous acid (HONO), nitryl chloride (ClNO2), ozonolysis of alkenes, and O(1D) + H2O. In addition to radical precursors, radical termination products were measured, including peroxy acetyl nitrates (PAN), nitric acid (HNO3), organic nitrates and organic peroxides. The comparison of radical precursors and termination products illustrates shifts in photochemical regime resulting from NOx and VOC emissions changes over the past ten years in the LA basin, as well as other influences such as temperature, seasonality and day of week." | ||
| + | |||
| + | <font size="4">14 March 2023</font size="4"><BR> | ||
| + | |||
| + | <b>The Effects of Trace H<sub>2</sub>S in Laboratory Experiments of Planetary Organic Haze Chemistry</b> | ||
| + | |||
| + | Nathan William Reed, <br> | ||
| + | [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Tolbert group] / [https://sites.google.com/view/brownelab/people Browne group]<br> | ||
| + | CU ANYL Dissertation Defense | ||
| + | |||
| + | Planetary organic hazes from methane (CH<sub>4</sub>) photochemistry and atmospheric sulfur | ||
| + | gases are each common in planetary atmospheres, including the Archean Earth and, likely, | ||
| + | exoplanetary atmospheres. A planetary organic haze can affect a planet’s radiative forcing and | ||
| + | interpretations of observable spectra, as well as act as a source for prebiotic chemistry and | ||
| + | nutrients for life. However, cross reactions between inorganic sulfur and organic molecules to | ||
| + | form organosulfur have yet to be explored in haze chemistry. The objective of my thesis is to | ||
| + | explore the coupling between hydrogen sulfide (H<sub>2</sub>S) and organic haze chemistry. Here I present | ||
| + | the results of laboratory experiments using aerosol mass spectrometry, differential mobility | ||
| + | analysis, and optical measurements to explore how trace H<sub>2</sub>S influences the compositional, | ||
| + | physical, and optical properties of aerosol from organic haze analogs produced by CH<sub>4</sub> photochemistry. I investigated the chemistry of aerosol formed in reducing (CH<sub>4</sub>/H<sub>2</sub>S gas | ||
| + | mixtures in N<sub>2</sub>, varying trace H<sub>2</sub>S) and weakly reducing (CO<sub>2</sub>/CH<sub>4</sub>/H<sub>2</sub>S gas mixtures in N<sub>2</sub>, varying CO<sub>2</sub>) atmospheric conditions. | ||
| + | |||
| + | When I included trace H<sub>2</sub>S in precursor mixtures, the total organic aerosol mass increased | ||
| + | in both conditions, despite H<sub>2</sub>S not being a carbon source. Moreover, I found evidence for the | ||
| + | formation of organic reduced sulfur (ORS) and organic oxidized sulfur (OOS) compounds in the | ||
| + | reducing and weakly reducing conditions, respectively. At lower CO<sub>2</sub> mixing ratios, I attributed | ||
| + | the total sulfate signal to be entirely OOS. Thus, in contrast to previous thought, I show that ORS | ||
| + | and OOS are potentially significant atmospheric sulfur reservoirs. Further, I found the | ||
| + | compositional changes in the reducing conditions lead to changes in the aerosol optical properties, as the total extinction (absorption plus scattering) of light increased with increasing | ||
| + | H<sub>2</sub>S mixing ratios. | ||
| + | I found that trace H<sub>2</sub>S dramatically influences the organic aerosol composition, mass, and | ||
| + | optical properties by the formation of organosulfur (ORS and OOS) compounds and enhancing | ||
| + | organic aerosol formation. These results have implications for Archean atmospheric chemistry, | ||
| + | prebiotic chemistry, planetary climate, and spectral interpretations for exoplanetary atmospheres. | ||
| + | |||
| + | Thus, future work should consider the potential impacts of trace H<sub>2</sub>S on organic haze chemistry. | ||
| + | |||
| + | <font size="4">13 March 2023</font size="4"><br> | ||
| + | |||
| + | <b>Metal Oxide Particles as Atmospheric Nuclei: Exploring the Role of Metal Speciation in Heterogeneous Efflorescence and Ice Nucleation</b> | ||
| + | |||
| + | Zachary Schiffman, ANYL 3rd year, <br> | ||
| + | [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Tolbert group] | ||
| + | |||
| + | "ABSTRACT: Mineral dust can indirectly impact climate by nucleation of atmospheric solids, for example by heterogeneously nucleating ice in mixed-phase clouds or by impacting the phase of aerosols and clouds through contact nucleation. The effectiveness toward nucleation of individual components of mineral dust requires further study. Here, the nucleation behavior of metal oxide nanoparticle components of atmospheric mineral dust is investigated. A long working distance optical trap is used to study contact and immersion nucleation of ammonium sulfate by transition metal oxides, and an environmental chamber is used to probe depositional ice nucleation on metal oxides particles. Previous theory dictates that ice nucleation and heterogeneous nucleation of atmospheric salts can be impacted by several factors including morphology, lattice match, and surface area. Here, we observe a correlation between the cationic oxidation states of the metal oxide heterogeneous nuclei and their effectiveness in causing nucleation in both contact efflorescence mode and depositional freezing mode. In contrast with the activity of contact efflorescence, the same metal oxide particles did not cause a significant increase in efflorescence relative humidity when immersed in the droplet. These experiments suggest that metal speciation, possibly as a result of cationic charge sites, may play a role in the effectiveness of nucleation that is initiated at particle surfaces." | ||
| + | |||
| + | <font size="4">6 March 2023</font size="4"><br> | ||
| + | |||
| + | <b>Advancing Process-based Understanding of How Humans Are Changing the Global Sulfur Cycle</b> | ||
| + | |||
| + | [https://www.colorado.edu/ebio/eve-lyn-hinckley Prof. Eve-Lyn Hinckley], <br> | ||
| + | Univ. of Colorado Ecology and Evolutionary Biology | ||
| + | |||
| + | "Today, the nature of how humans alter the global sulfur (S) cycle is changing. As atmospheric S | ||
| + | deposition has declined in response to air quality regulation in the United States and Europe, | ||
| + | there has been an increase in S fertilizer applications reported in many large-scale regional crop | ||
| + | systems. In addition, intensification of agriculture has driven increased S inputs for other uses: | ||
| + | as a pesticide, regulator of soil pH, and soil conditioner. Given that excess S can cause soil | ||
| + | acidification and mobilization of heavy metals in ecosystems, it is critical to develop methods to | ||
| + | trace the “fingerprint” of agricultural S through complex landscapes, quantify S forms and | ||
| + | transformations in soils and surface waters, and determine the consequences of its use. In this | ||
| + | talk, I will describe both new trend analyses and process-based studies that provide compelling | ||
| + | evidence for how the forms, amounts, flows, and consequences of S have changed from what | ||
| + | they were in the 1960s and 1970s when the dominant human manipulation of the S cycle was | ||
| + | through mining and fossil fuel emissions. I will highlight studies from my research group that | ||
| + | show exciting new methodological developments using radio- and stable isotopes of S adapted | ||
| + | from the marine literature to trace S applications through large agricultural regions. I will also | ||
| + | discuss the collaborative actions that researchers, land managers, and regulators may take to | ||
| + | address the consequences of excess S in the environment. Ultimately, I will make the case for | ||
| + | how an element that has been far less investigated than carbon, nitrogen, and phosphorus, | ||
| + | should be a priority for study in the coming years." | ||
| + | |||
| + | <font size="4">27 February 2023</font size="4"><br> | ||
| + | |||
| + | <b>Investigations into the health effects of wildfire smoke</b> | ||
| + | |||
| + | [https://www.colorado.edu/geography/colleen-reid-0 Prof. Colleen Reid], <br> | ||
| + | Univ. of Colorado Geography and Institute for Behavioral Science | ||
| + | |||
| + | "Wildfires have been increasing in frequency and duration in the western U.S. and the wildfire | ||
| + | season has been increasing in length such that many regions now claim that there is no wildfire | ||
| + | season anymore but that wildfires have become a year-round threat. While there are many causes | ||
| + | of the increase in wildfires in the western U.S., it is becoming clear that wildfires not only affect | ||
| + | forests and grasslands, but also have impacts on the health of populations downwind. Indeed | ||
| + | studies have shown that fine particulate air pollution () is decreasing in most areas of the United | ||
| + | States, except for areas most affected by wildfires, where an increasing trend in can be attributed | ||
| + | to wildfire smoke. Studies of the health impacts of wildfire smoke are challenging due to lack of | ||
| + | sufficient air pollution monitoring across space, the complexity of the components of the smoke | ||
| + | that could affect human health, the numerous health outcomes that have been found to be linked | ||
| + | to wildfire smoke, and more. In her talk, Dr. Reid will dive into her research that investigates | ||
| + | how to better assess population exposure to wildfire smoke, how it impacts human health, and | ||
| + | which communities are more affected by wildfire smoke. She will also provide a glimpse into | ||
| + | her ongoing work to understand the health tradeoffs of interventions to protect health from | ||
| + | wildfire smoke." | ||
| + | |||
| + | <font size="4">20 February 2023</font size="4"><br> | ||
| + | |||
| + | <b>Assessment of indoor air quality using substance-specific guide values and TVOC</b> | ||
| + | |||
| + | [https://www.tu-braunschweig.de/en/oekochemie/mitarbeiter/t-salthammer Prof. Tunga Salthammer]<br> | ||
| + | Department of Material Analysis and Indoor Chemistry, Fraunhofer WKI | ||
| + | |||
| + | "The primary goal of indoor hygiene studies is to create a pleasant and healthy environment | ||
| + | for people. This requires a sufficient supply of fresh air and the setting of climatic parameters | ||
| + | that lead to thermal comfort. However, the concentration of air pollutants must also be kept | ||
| + | as low as possible. A complete measurement program to study indoor air quality can be both | ||
| + | time-consuming and expensive. For this reason, parameters that can be measured easily | ||
| + | and quickly are often used for an initial assessment of the situation. One of these parameters | ||
| + | is TVOC (Total Volatile Organic Compounds). | ||
| + | |||
| + | The current situation makes it necessary to subject VVOC, VOC, SVOC and TVOC | ||
| + | measurements to a critical analysis due to their widespread use in questions of indoor air | ||
| + | quality and to the evaluation of emissions of organic compounds from building and consumer | ||
| + | products. | ||
| + | |||
| + | A detailed description of the measurement methods is followed by a critical evaluation of the | ||
| + | various TVOC values, their possible applications and guide values for individual compounds. | ||
| + | The aim is to provide a deeper understanding of TVOC in order to use this parameter | ||
| + | correctly and to be able to better assess published results. The analytical definition of VOCs | ||
| + | also suggests total values TVVOC and TSVOC for very volatile and semi volatile organic | ||
| + | compounds. However, due to the physical properties of the potential target chemicals, this is | ||
| + | associated with a number of drawbacks. | ||
| + | |||
| + | Early work on TVOC was trend-setting and still influences current indoor guide values, even | ||
| + | if many VOCs are no longer representative from today's perspective. The spectrum of indoor | ||
| + | VOCs has shifted significantly: away from classic solvents and more towards reactive | ||
| + | compounds and indoor chemistry. However, within the framework of surveys TVOC can | ||
| + | make contributions to identifying statistical trends and reference values can be extracted | ||
| + | from the data collected in this way. The online measured TVOC values are at best suitable | ||
| + | for screening IAQ. TVOC does not take into account the toxicological properties of the | ||
| + | individual substances for inhalation exposure. This would not even be possible, since | ||
| + | different substances usually have different toxicological endpoints that cannot simply be | ||
| + | summed up for a reliable risk assessment. Therefore, measurements of individual | ||
| + | substances are usually evaluated using substance-specific guide values. | ||
| + | |||
| + | Consequently, TVOC cannot be used in connection with health-related and odor-related | ||
| + | issues. Nevertheless, such references are repeatedly made, either intentionally or | ||
| + | unintentionally. This happens in an analytical but also in an interpretative sense. Authorities, | ||
| + | practitioners and architects use TVOC as an acceptance criterion for buildings, but without | ||
| + | specifying the methodology. Overall, there is a great deal of uncertainty about TVOC. This | ||
| + | applies in particular to the question of whether and why a TVOC value can be helpful and | ||
| + | which statements cannot be made on the basis of the TVOC." | ||
| + | |||
| + | Reference: Salthammer, T. (2022). TVOC revisited. Environment International, 167, 107440, | ||
| + | DOI link: https://doi.org/10.1016/j.envint.2022.107440 (published under CC BY-NC-ND 4.0). | ||
| + | |||
| + | <font size="4">13 February 2023</font size="4"><br> | ||
| + | <b>Springtime Arctic ozone depletion forces northern hemisphere climate anomalies</b> | ||
| + | |||
| + | [https://iac.ethz.ch/people-iac/person-detail.html?persid=264555 Marina Friedel,]<br> | ||
| + | [https://iac.ethz.ch/ IAC, ETH Zuerich] | ||
| + | |||
| + | "Large-scale chemical depletion of ozone due to anthropogenic emissions of chlorofluorocarbons (CFCs) can occur, albeit infrequently, over the Arctic. Such ozone depletion events are consistently followed by surface temperature and precipitation anomalies over vast parts of the Northern Hemisphere. Using chemistry-climate models, it can be shown that the loss of stratospheric ozone is an important cause of these surface anomalies, as it leads to persistently cold temperatures in the lower stratosphere and alters stratospheric dynamics. Including chemical processes involving ozone in forecast models could therefore increase predictability on a sub-seasonal to seasonal scale. | ||
| + | Similarly, the long-term trend in Arctic ozone caused by the decline of CFCs also has important implications for stratospheric temperature and dynamics. The imminent recovery of the ozone layer will heat the polar stratosphere, and its fingerprint is expected to be seen all the way down to the surface, as model simulations show. Therefore, a realistic representation of ozone in climate models is required to reduce uncertainty in climate projections.” | ||
| + | |||
| + | <font size="4">6 February 2023</font size="4"><br> | ||
| + | <b>Frequency comb spectroscopy: from Nobel prize to practical application</b> | ||
| + | |||
| + | [https://www.colorado.edu/mechanical/greg-rieker Prof. Greg Rieker] & [https://www.colorado.edu/ecee/scott-diddams Prof. Scott Diddams], <br> | ||
| + | CU Engineering | ||
| + | |||
| + | "The first frequency comb laser was demonstrated on the CU Boulder campus in 1999 in Jan Hall’s lab at | ||
| + | JILA. In the two decades since, the technology has proliferated across a vast array of scientific fields | ||
| + | from timekeeping and astronomy, to low-noise frequency synthesis and precision spectroscopy for | ||
| + | fundamental physics. In recent years, it made the jump to practical applications at the forefront of | ||
| + | climate and energy, for example as the foundation of a sensor network detecting methane leaks across | ||
| + | 750 sq. miles of oil and gas infrastructure. Prof Diddams will discuss the evolution of frequency comb | ||
| + | lasers, metrology and sensing techniques developed in his laboratory at NIST and now CU, which are | ||
| + | pushing the boundaries of sensing from the UV to the THz, and from macro to micro-scale comb | ||
| + | systems. Prof. Rieker will discuss the push toward robust, fieldable frequency comb systems and | ||
| + | practical applications, ending with recent collaborative work between the laboratories on sensing in | ||
| + | high-speed chemically reacting systems." | ||
| + | |||
| + | <font size="4">30 January 2023</font size="4"><br> | ||
| + | <b>Formation and dissociation of hydrocarbons under interstellar conditions</b> | ||
| + | |||
| + | [https://sites.google.com/view/bouwmanlab Prof. Jordy Bouwman], <br> | ||
| + | CHEM & LASP, Univ. of Colorado | ||
| + | |||
| + | "Hydrocarbons of all sizes and shapes are found throughout the various stages of star and planet formation. Recently, using radio astronomical observations, a variety of cyclic and even polycyclic hydrocarbons have been detected in the very cold (10 K) Taurus molecular cloud, challenging our understanding of the chemical formation pathways under these low-temperature and low-density conditions. In photon dominated regions, on the other hand, very large Polycyclic Aromatic Hydrocarbons (PAHs) of 50 carbon atoms and larger are commonly detected as a class based on the characteristic mid-infrared emission bands that they emit after being electronically excited by ultraviolet and optical radiation. These large PAHs are exposed to a very strong radiation field that can alter their molecular structure and may even lead to dissociation. In this seminar, I will show how experimental studies using synchrotron and free electron laser radiation – in conjunction with quantum chemical computations – allow us to reveal the formation and dissociation mechanisms of interstellar (aromatic) hydrocarbons at a molecular level of detail." | ||
| + | |||
| + | <font size="4">23 January 2023</font size="4"><br> | ||
| + | <b>Secondary Organic Aerosol Mass Yields from NO3 Oxidation of α-Pinene and Δ-Carene: Effect of RO2 Radical Fate</b><br> | ||
| + | |||
| + | Doug Day,<br> | ||
| + | (ANYL ResSci, [http://cires1.colorado.edu/jimenez-group/ Jimenez group]) | ||
| + | |||
| + | "Dark chamber experiments were conducted to study the SOA formed from the oxidation of α-pinene and Δ-carene under different peroxy radical (RO<sub>2</sub>) fate regimes: RO<sub>2</sub> + NO<sub>3</sub>, RO<sub>2</sub> + RO<sub>2</sub>, and RO<sub>2</sub> + HO<sub>2</sub>. SOA mass yields from α-pinene oxidation were <1 to ∼25% and strongly dependent on available OA mass up to ∼100 μg m–3. The strong yield dependence of α-pinene oxidation is driven by absorptive partitioning to OA and not by available surface area for condensation. Yields from Δ-carene + NO<sub>3</sub> were consistently higher, ranging from ∼10–50% with some dependence on OA for <25 μg m–3. Explicit kinetic modeling including vapor wall losses was conducted to enable comparisons across VOC precursors and RO2 fate regimes and to determine atmospherically relevant yields. Furthermore, SOA yields were similar for each monoterpene across the nominal RO<sub>2</sub> + NO<sub>3</sub>, RO<sub>2</sub> + RO<sub>2</sub>, or RO<sub>2</sub> + HO<sub>2</sub> regimes; thus, the volatility basis sets (VBS) constructed were independent of the chemical regime. Elemental O/C ratios of ∼0.4–0.6 and nitrate/organic mass ratios of ∼0.15 were observed in the particle phase for both monoterpenes in all regimes, using aerosol mass spectrometer (AMS) measurements. An empirical relationship for estimating particle density using AMS-derived elemental ratios, previously reported in the literature for non-nitrate containing OA, was successfully adapted to organic nitrate-rich SOA. Observations from an NO<sub>3</sub>– chemical ionization mass spectrometer (NO<sub>3</sub>–CIMS) suggest that Δ-carene more readily forms low-volatility gas-phase highly oxygenated molecules (HOMs) than α-pinene, which primarily forms volatile and semivolatile species, when reacted with NO<sub>3</sub>, regardless of RO2 regime. The similar Δ-carene SOA yields across regimes, high O/C ratios, and presence of HOMs, suggest that unimolecular and multistep processes such as alkoxy radical isomerization and decomposition may play a role in the formation of SOA from Δ-carene + NO<sub>3</sub>. The scarcity of peroxide functional groups (on average, 14% of C10 groups carried a peroxide functional group in one test experiment in the RO<sub>2</sub> + RO<sub>2</sub> regime) appears to rule out a major role for autoxidation and organic peroxide (ROOH, ROOR) formation. The consistently substantially lower SOA yields observed for α-pinene + NO<sub>3</sub> suggest such pathways are less available for this precursor. The marked and robust regime-independent difference in SOA yield from two different precursor monoterpenes suggests that in order to accurately model SOA production in forested regions the chemical mechanism must feature some distinction among different monoterpenes." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>A study of iodine chemistry in the Indian and Southern Ocean marine boundary layer</b> | ||
| + | |||
| + | Swaleha Inamdar,<br> | ||
| + | (ANYL Professional Research Assistant, [https://volkamergroup.colorado.edu/ Volkamer Group]) | ||
| + | |||
| + | "Reactive atmospheric iodine significantly affects the tropospheric chemistry by ozone (O<sub>3</sub>) depletion and participation in various heterogeneous reaction cycles mainly involving O<sub>3</sub>, causing changes to the atmospheric oxidation capacity. There is growing experimental evidence that halogen chemistry plays a key role as part of tropospheric photochemistry. Much of the proposed halogen chemistry in literature is propagated through the reactions of a series of halogen atom and radicals. Although, some measurements of iodine oxide (IO) are reported around the globe, the remote open ocean remains undersampled. Recently, first reported observations of IO in the Indian and Southern Ocean marine boundary layer (MBL) suggest that atmospheric IO may not be well correlated with the inorganic fluxes. Further, biogenic fluxes play a significant role in active iodine chemistry in this region. This contradicts with the studies reporting that inorganic iodine emissions are responsible for 75% of the reactive iodine in the tropical Atlantic MBL. Thus, to improve our understanding of iodine chemistry in this region, field observations in the remote open ocean of the Southern Ocean are carried out in this study via several ship-based expeditions in this region. To identify the geographical emissions of reactive iodine precursors, this study compiles observations from the Indian and Southern Ocean MBL for a comprehensive region-specific parameterization for estimating the inorganic iodine fluxes via sea surface iodide concentrations. This study addresses whether the existing parameterization tools for inorganic iodine fluxes are sufficient to explain the detected IO in the remote open ocean MBL." | ||
| + | |||
| + | ==Fall 2022== | ||
| + | |||
| + | <font size="4">5 December 22</font size="4"><br> | ||
| + | <b>Vapors and Aerosols: NIST’s forensic approach to arson and cannabis intoxication</b> | ||
| + | |||
| + | [https://www.nist.gov/people/jennifer-berry Jennifer Berry], <br> | ||
| + | National Institute of Standards and Technology | ||
| + | |||
| + | "The National Institute of Standards and Technology (NIST) is world-renowned for making standards and | ||
| + | precise measurements with advanced measurement techniques. The Fluid Characterization Group, part | ||
| + | of the Boulder-based Materials Measurement Laboratory, is rooted in precise thermodynamic | ||
| + | measurements of fuels and has successfully developed technologies and methods for forensic vapor and | ||
| + | aerosol detection. The group’s Dynamic Vapor Microextraction (DVME) was originally developed for | ||
| + | vapor pressure measurements of low volatility, unstable molecules but has been expanded towards a | ||
| + | variety of forensic applications, particularly for the detection of arson from fire debris. The instrumental | ||
| + | development for this new application needs to be thoroughly planned and designed to encompass a | ||
| + | large number of factors and their interactions, breaking from a one-factor-at-a-time method. | ||
| + | Conducting a sensitivity analysis permits the exploration of multiple variables at the same time while | ||
| + | limiting the number of experiments needed. When coupling a sensitivity analysis with DVME in a fire | ||
| + | debris study, we found that both the metric and the experimental plan played a crucial role in | ||
| + | determining the importance of different instrumental and sample factors. Beyond fire debris, the group | ||
| + | has also been developing and testing breath collection techniques to detect cannabinoids in breath after | ||
| + | cannabis use. Aerosols and condensed vapors will be collected with two different devices to investigate | ||
| + | changes in breath compounds and concentrations after cannabis use. While cannabinoids have been | ||
| + | detected in breath aerosols, additional explorations into exhaled breath condensate as a part of a 128- | ||
| + | participant study will use a cannabinoid targeted and metabolomics untargeted approach to explore if | ||
| + | this type of noninvasive breath collection is a valid method as well. With the growth of forensic research | ||
| + | in NIST Boulder, numerous opportunities are available for collaborations, internships, and postdoctoral | ||
| + | fellowships at NIST Boulder." | ||
| + | |||
| + | <font size="4">28 November 22</font size="4"><br> | ||
| + | <b>Resolving The Complex Chemistry of Combustion</b> | ||
| + | |||
| + | [https://www.colorado.edu/mechanical/nicole-labbe Prof. Nicole Labbe], <br> | ||
| + | CU Mechanical Engineering | ||
| + | |||
| + | "High temperature gas reacting systems are among the most complex chemical kinetics systems to | ||
| + | model. For example, a prototypical gas phase reacting system is combustion, where fuels can | ||
| + | undergo tens of thousands of unique competing reactions via thousands of unique chemical | ||
| + | species. Further complicating the chemistry, these systems can be highly pressure dependent, and | ||
| + | even modest pressure fluctuations can drastically change both reaction rate and species | ||
| + | distributions. With such large combinatorial possibilities for reactions and product species, | ||
| + | theory alone is often unable to fully resolve the chemical mechanism due to computational | ||
| + | limitations. Furthermore, the breadth of reactions and species present limits the ability of | ||
| + | experiments to fully resolve the chemistry of may gas phase reacting systems. However, through | ||
| + | careful combination of both theoretical and experimental techniques, at least the most critical | ||
| + | reactions can often be revealed for accurate chemical model development. In this work, we | ||
| + | demonstrate how a combined approach using both semi-automated theoretical kinetics and gas | ||
| + | phase speciation experiments was used to resolve several kinetics disputes in the literature due to | ||
| + | unresolved kinetic mechanisms. First, we demonstrate how theoretical kinetics was the key to | ||
| + | clearing up a discrepancy as to the source of methyl ketene in the pyrolysis of a biodiesel | ||
| + | component, ethyl propanoate. Next, we show that a new tunable lab-scale VUV light source was | ||
| + | able to experimentally prove a theoretically predicted keto-enol tautomerization for acetone for | ||
| + | the first time. Finally, we explore how a combined experimental and theoretical approach | ||
| + | resolved how the location of the double bond in methylcyclohexene dramatically changes the | ||
| + | sooting propensity between each of the three possible isomers." | ||
| + | |||
| + | <font size="4">14 November 2022</font size="4"><br> | ||
| + | <b>ANYL Alumni Career Panel: Melissa Trainer (NASA), and Dan Bon (CDPHE)</b> | ||
| + | |||
| + | [https://science.gsfc.nasa.gov/sed/bio/melissa.trainer Dr. Melissa Trainer] is a planetary scientist at NASA's Goddard Space Flight Center (GSFC) with expertise in the composition of planetary atmospheres and the production of organic molecules and aerosols via in situ synthesis pathways. Dr. Trainer currently serves as a Deputy Principal Investigator (PI) for the Dragonfly mission to Saturn's moon Titan, part of the NASA Planetary Science New Frontiers Program. She is also the lead for the Dragonfly Mass Spectrometer (DraMS), which enables the investigation of Titan's surface composition and characterization of potential prebiotic chemistry. She has spent more than two decades characterizing the properties of Titan and early Earth aerosol analogs, with publications on the chemical, optical, and isotopic properties of organic hazes. Dr. Trainer is a member of the Mars Science Laboratory Sample Analysis at Mars (SAM) instrument team, for which she led the campaign to conduct the first in situ multi-year study of the seasonal variations of the composition of the Mars atmosphere through surface mass spectrometry measurements. She received her undergraduate degree in Chemistry from Franklin and Marshall College in 2000, and her PhD in Analytical Chemistry from the University of Colorado-Boulder in 2006. She has been a scientist at NASA since 2009. | ||
| + | |||
| + | & | ||
| + | |||
| + | Daniel Bon, PhD. Supervisor, Air Toxics Measurement and Special Projects Unit, Technical Services Program, [https://cdphe.colorado.gov/air-pollution/air-pollution-control-division-topics Air Pollution Control Division], Colorado Department of Public Health and Environment. | ||
| + | Daniel Bon completed his PhD in Atmospheric Chemistry in 2011 and the University of Colorado, Boulder and the NOAA ESRL Chemical Sciences Division, where his research focused on PTR-MS field measurements of volatile organic compounds in the atmosphere. Since 2011, Dr. Bon has worked for the Colorado Department of Public Health and Environment, Air Pollution Control Division. From 2016-2022, Dr. Bon built and operated the Colorado Air Monitoring Mobile Laboratory. In 2021, he became the Unit Supervisor for the newly created Air Toxics Measurement and Special Projects Unit created to respond to HB21-1189 and HB22-1244 Air Toxics bills passed by the Colorado State Legislature. His growing team includes 7 scientists and 3 mobile measurement labs. | ||
| + | Students could learn about what we do by reading the summaries of the new Colorado Air Toxics bills our group is working to implement and a recent press release: [https://leg.colorado.gov/bills/hb21-1189 hb21-1189], [https://leg.colorado.gov/bills/hb22-1244 hb22-1244], [https://cdphe.colorado.gov/press-release/state-health-department-investigating-an-elevated-benzene-measurement press release] | ||
| + | |||
| + | |||
| + | We have asked the speakers to present for 12-15 min on their perspective on careers outside academia for PhD chemists, and in particular for graduates of our ANYL PhD program. For example, they may address the following questions: what path took you to where you are? What strengths from the ANYL program helped you in your career? What could have the program done to make that better? What do you wish someone had told you when you were a PhD student? What advice would you give to current PhD students and postdocs? A Q&A session will follow, prioritizing questions from current students and postdocs. | ||
| + | |||
| + | <font size="4">7 November 2022</font size="4"><br> | ||
| + | <b>The Ice Nucleating Properties of Southern Great Plains Mineral Dusts</b> | ||
| + | <br> | ||
| + | Guy Symonds, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Mixed phase clouds, a collection of supercooled liquid and frozen water droplets, are potentially important regulators of the Earth’s climate. In the troposphere, water droplets are primarily frozen through the immersion-mode freezing mechanism, where a rare particle within the droplet acts as a template and catalyzes the production of the ice phase. Commonly, these ice nucleating particles in the atmosphere are some type of suspended mineral dust. The research being presented examines the ice nucleating behavior of previously unprobed dust samples in the Southern Great Plains area in Oklahoma, United States of America. This location was subject to a field campaign due to its isolation from major industrial activity on the North American continent and so it serves as a “Continental background site”. By examining the ice nucleating activity of these dusts, the contribution to ambient aerosol in the Southern Great Plains area can be determined, as well as their relative contribution to total aerosol ice nucleating activity. Furthermore, these dusts were subjected to aging processes designed to simulate environmental weathering processes to further investigate the variability of their ice nucleating activity and how their freezing properties correlate with chemical changes caused by the aging." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Formation of a Polariton Through the Creation of Micro Cavities with an Active Layer of PFO</b> | ||
| + | <br> | ||
| + | Nicole Silver, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "The presence of an exciton polariton, a quasiparticle formed through the strong coupling between a photon and an electron hole pair, creates important properties that have the potential to significantly increase the absorption efficiency of organic semiconductor materials in a solar cell. In my undergraduate research at Cornell University in Andrew Musser’s Light Matters Research Group, I focused on finding evidence of a polariton in the organic semiconductor material Perfluorooctanesulphonate (C<sub>8</sub>F<sub>17</sub>O<sub>3</sub>S), also known as PFO. This presentation will outline the intricate process of forming micro cavities, which involves the development of a spin coating technique and identification of several parameters specific to the polymer PFO. These are determined through absorption spectroscopy, profilometry, evaporation in vacuum, angle dependent reflectivity, and simulations. The process of creating a micro cavity and using an angle dependent reflectivity goniometer to discover two polariton branches in a PFO micro cavity will be discussed along with the identification of polariton branches in two other materials, TM82 and TM83." | ||
| + | |||
| + | <font size="4">31 October 2022</font size="4"><br> | ||
| + | <b>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</b> | ||
| + | <br> | ||
| + | [https://sites.google.com/site/ziemanngroup/ Paul Ziemann], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative studies of indoor air chemistry at CU. In this talk I will provide a brief overview of recent research in my lab for the purpose of informing first-year chemistry graduate students." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Sustainable Magnesium Production via Molten Salt Electrolysis and G-METS Distillation</b> | ||
| + | <br> | ||
| + | Madison Rutherford, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Current methods of magnesium production are prohibitively expensive and resource intensive with low energy efficiency and high environmental impact. Molten salt electrolysis (MSE) using a reactive liquid cathode, e.g., tin, combined with vapor compression distillation in a gravity-driven multiple effect thermal-system (G-METS VCD) can significantly reduce the energy requirement and environmental impact of both magnesium primary production and recycling. This presentation presents a techno-economic model of cost, energy consumption, and emissions associated with magnesium primary production via MSE and G-METS VCD. The model includes a mass balance with 17 elements, electrolysis process energy balance with carbon or solid oxide membrane anodes, and detailed operating and capital cost estimates. Based on the properties of magnesium and expected operating conditions, the cost of magnesium production using this process could be comparable to or lower than that of aluminum production. Initial electrolysis experiments show high current efficiencies with both carbon and SOM anodes, and future work on G-METS VCD is outlined." | ||
| + | |||
| + | <font size="4">24 October 2022</font size="4"><br> | ||
| + | <b>Quantifying particulate halogens in the atmosphere: Airborne measurements and instrumentation</b> | ||
| + | <br> | ||
| + | Dongwook Kim, <br> | ||
| + | ANYL 3rd year, [https://cires1.colorado.edu/jimenez-group/ Jimenez group] | ||
| + | |||
| + | "Halogens in the atmosphere play important roles in ozone chemistry. Halogen chemistry studies have been mainly focused on gas-phase chlorine, iodine, and bromine chemistry. Particulate halogens can be a reservoir for reactive halogens and can directly interact with ozone. However, they have not been well-constrained in chemical transport models due to a lack of measurements on a global scale. Recently, we have quantified submicron particulate halogens, as well as speciation of oxidation states, from several airborne datasets measured by the University of Colorado High-Resolution Aerodyne Aerosol Mass Spectrometer (CU-HR-AMS). We have identified sources that were previously not well recognized. We present particulate halogen measurements (i.e., I, Br, ClO<sub>4</sub><sup>-</sup>) over urban, remote, and wildfire conditions as well as instrumental development for stratospheric aerosol sampling. | ||
| + | |||
| + | (1) On aircraft platforms, AMS uses an aerodynamic lens that requires constant upstream pressure to work consistently. Airborn interfaces that provide that (pressure-controlled inlets –PCI-) have historically performed less well at high altitudes. We show the recent development of a new PCI inlet design coupled with a PM<sub>2.5</sub> aerodynamic lens towards the goal of sampling PM<sub>1</sub> aerosols up to ~15 km altitude. | ||
| + | |||
| + | (2) The first quantitative detection of iodine (both particle and gas phase) in the stratosphere has been reported (Koenig et al., 2020). It suggested that particulate iodine is a major fraction in the stratosphere. We present particulate iodine measurement up to the lower stratosphere over the Pacific Ocean during NSF TI3GER and its implications for stratospheric ozone. | ||
| + | |||
| + | (3) Reactive halogens (i.e., chlorine and bromine) can affect urban air quality by providing radical sources. We show that particulate bromine is emitted by anthropogenic sources and compare it with other ground measurements. Also, we identify particulate iodine sources and discuss the potential impact on air quality. | ||
| + | |||
| + | (4) We quantify the emission of particulate iodine from Western US wildfires during FIREX-AQ. We found that particulate fore could be the major form of iodine emission from US wildfires. | ||
| + | |||
| + | (5) Exposure to perchlorate affects the human endocrine system. While the atmospheric sources of perchlorate are highly uncertain, snow and ice core records suggest that chlorofluorocarbons (CFCs) might be important precursors for the photochemical production of perchlorate in the stratosphere. We show the first global in-situ perchlorate measurements from the ATom campaign over the remote oceans and comparison to the GEOS-Chem model with updated perchlorate-related mechanisms. Also, we show anthropogenic emissions of perchlorate that may affect the tropospheric perchlorate burden. | ||
| + | " | ||
| + | |||
| + | <font size="4">26 September 2022</font size="4"><br> | ||
| + | <b>Particle formation and growth </b> | ||
| + | <br> | ||
| + | [https://sites.google.com/view/brownelab/people Eleanor Browne], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "The Browne group uses a combination of laboratory experiments and field measurements to understand the sources and transformations of trace gases in the atmosphere with the ultimate goal of understanding the impacts of this chemistry on air quality, climate, and nutrient delivery to ecosystems. Here, I will discuss some of our recent work investigating particle formation and growth in an agricultural setting." | ||
| + | |||
| + | <font size="4">19 September 2022</font size="4"><br> | ||
| + | <B>Chemistry of Volatile Organic Compounds in the Atmosphere</b> | ||
| + | <br> | ||
| + | [https://sites.google.com/view/de-gouw-lab Joost de Gouw], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products. | ||
| + | |||
| + | In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments. | ||
| + | |||
| + | Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments to better understand their sources and fate. One particular study involves the impact of the Marshall Fire in Boulder County on indoor air in homes that were near the burnt area. Second, we are working on the emissions and chemistry of VOCs in urban air. One study that will be highlighted involved field measurements in Los Angeles in the Summer of 2022. Third, we are working on a chamber study to better understand the formation of secondary organic aerosol from biogenic VOCs. Finally, we are also using data from satellite remote sensing instruments to measure the pollutants from oil and natural gas production, and from wildfires." | ||
| + | |||
| + | and | ||
| + | |||
| + | <B>Understanding aerosols for pollution, climate change, and disease transmission</b> | ||
| + | <br> | ||
| + | [https://cires1.colorado.edu/jimenez-group/ Jose Jimenez], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly present results from different projects over the last year, as well as some future directions for our group. (1) I will introduce our aircraft research program, including results from recent projects, and how they set up scientific questions for the upcoming NASA ASIA-AQ project on multiple megacities (if politics allows: Tokyo, Seoul, Manila, Bangkok, Kolkata, Dhaka, Hanoi, Singapore, and Kuala-Lumpur). (2) I will present preliminary results from the CalNexT-2022 field study in the Los Angeles area, where we returned (along with Prof. de Gouw’s group and other colleagues) to the Caltech campus 12 years after CalNex-2010. Analysis so far suggests that ½ of the SOA potential in ambient air is due to species less volatile than sesquiterpenes and aromatics (c* < 1e4 ug m-3). (3) I will discuss recent results on COVID airborne transmission, as well as the indoor chemistry due to germicidal ultraviolet (GUV) air disinfection and other commercial “air cleaners”. | ||
| + | |||
| + | For those interested in COVID-19 aerosol transmission, you can find a summary of the evidence supporting airborne transmission of COVID-19 at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00869-2/, a review for airborne transmission of all respiratory diseases at https://doi.org/10.1126/science.abd9149, an overview of how airborne transmission really works at https://doi.org/10.1111/ina.13025, and an overview of the historical reasons for the confusion on this topic at https://doi.org/10.1111/ina.13070." | ||
| + | |||
| + | <font size="4">12 September 2022</font size="4"><br> | ||
| + | <b>From Coastal Megacities to the tropical UTLS: Iodine and Ion induced Particle Formation</b> | ||
| + | <br> | ||
| + | [https://volkamergroup.colorado.edu/ Rainer Volkamer], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "The tropical upper troposphere and lower stratosphere (UTLS) is a unique yet understudied atmospheric environment. The combination of higher flux of ion inducing cosmic radiation and lower temperatures makes ion-induced nucleation a more significant source of particles in the UTLS than in the lower troposphere. The Volkamer group develops advanced instrumentation (optical and mass spectrometric) to measure small molecules, condensable vapors and ambient ions that are relevant to particle formation, public health and climate discussions. We seek to better quantify and understand the interconnections between marine ecosystem, aerosols and their control on atmospheric chemistry. We also study the changing oxidative chemistry in coastal Megacities using research aircraft and satellites. Examples from recent field and laboratory experiments are discussed that aim to develop a molecular level understanding of the fundamental processes that affect a) new particle formation from iodine, and b) apply high-resolution mass spectrometry on high-flying aircraft to sample ambient ions in the UTLS. Opportunities for graduate research exist to 1) conduct and analyze field measurements using research aircraft (i.e., TI3GER-2022, CUPiDS-2023 projects), 2) laboratory experiments of particle formation (incl. at CLOUD/CERN) and multiphase chemistry, and 3) develop instrumentation to exploit synergies between optical spectroscopy and mass spectrometry." | ||
| + | |||
| + | ==Summer 2022== | ||
| + | <font size=4>27 May 2022</font size=4> | ||
| + | <br> | ||
| + | <b>Calibration of Iodide-CIMS for oxygenated hydrocarbons in the gas-phase: Application to measure Henry’s Law of 1,2-ISOPOOH</b> | ||
| + | |||
| + | Joey D'Alesio, <br> | ||
| + | [https://volkamergroup.colorado.edu/ Volkamer group] | ||
| + | |||
| + | "Two gas-phase quantification methods for Chemical Ionization Mass Spectrometry | ||
| + | using Iodide reagent ions (Iodide-CIMS) were developed in this work: the integration method and the flow rate method. Both methods utilize a Hamilton syringe | ||
| + | pump to relate the detected signal to the gas-phase concentration, also known as | ||
| + | a calibration coefficient, injected into a VOCUS 2R API-LToF mass spectrometer | ||
| + | configured with an AIM source and equipped with VUV light to generate iodide | ||
| + | reagent ions. These quantification methods can be combined with any chemical | ||
| + | ionization mass spectrometer utilizing a large variety of target analytes. The integration method was used to determine a calibration coefficient, which was applied | ||
| + | to partitioning experiments in order to determine the Henry’s Law coefficient for | ||
| + | 1,2-ISOPOOH. Henry’s Law describes the ratio of the presence of a compound in | ||
| + | the aqueous-phase and the gas-phase when partitioning is in equilibrium. Henry’s | ||
| + | Law applies only to dilute solutions. The 1,2-ISOPOOH Henry’s Law coefficient | ||
| + | was determined to be 2.61E+05 M/atm +/- 1.81E+04 M/atm. The reported value | ||
| + | is bracketed by available literature values, which range from 1.00E+05 M/atm to | ||
| + | 1.30E+06 M/atm. The flow rate method was used during initial calibration development to determine preliminary calibration coefficients for a suite of atmospherically | ||
| + | relevant acids. This method will continue to be optimized for future laboratory and | ||
| + | aircraft calibrations." | ||
| + | |||
| + | <font size=4>23 May 2022</font size=4><BR> | ||
| + | <b>Role of Oxidative Conditions on the Formation of Aerosol and Organic Nitrates from the Reactions of Monoterpenes with NO<sub>3</sub> and OH radicals</b> | ||
| + | |||
| + | Marla DeVault, <br> | ||
| + | [https://sites.google.com/site/ziemanngroup/ Ziemann group] | ||
| + | |||
| + | "Monoterpenes are widely emitted biogenic volatile organic compounds, which react quickly in the atmosphere and the oxidized products of which can partition into secondary organic aerosol (SOA). Understanding the types of products that are formed, how they might react in the particle-phase, and the effect of oxidative conditions on these factors are key for improving atmospheric models. Specifically, we are interested in the formation of organic nitrates and their impact on SOA. In order to study this, five monoterpenes were oxidized by NO<sub>3</sub> radicals, to simulate nighttime conditions, and OH radicals in the presence of NO<sub>x</sub>, to simulate daytime conditions. The SOA collected from these ten reactions show a prevalence of acetal and hemiacetal dimers and trimers for NO<sub>3</sub> radical oxidation for cyclic monoterpenes, which are notably absent from the OH/NO<sub>x</sub> oxidized SOA. This talk explores what mechanistic processes lead to this and other differences in particle-phase organic nitrates derived from these two reactions." | ||
| + | |||
| + | ==Spring 2022== | ||
| + | <font size=4>18 April 2022</font size=4><br> | ||
| + | <b>New approaches to ambient ion analysis reveal chemical trends</b> | ||
| + | |||
| + | Daniel Katz, ANYL 3rd year, <br> | ||
| + | [https://sites.google.com/view/brownelab/people Browne group] | ||
| + | |||
| + | "Atmospheric ions control the electrical properties of the atmosphere, influence | ||
| + | chemical composition via ion-molecule and/or ion-catalyzed reactions, and affect new particle | ||
| + | formation. Understanding the role of ions in these processes requires knowledge of ionic | ||
| + | chemical composition. However, determining the chemical composition of these ions is | ||
| + | analytically challenging owing to the low concentration of ambient ions in the atmosphere | ||
| + | (~100s-1000 ions/cm<sup>3</sup>). Here, we analyze measurements of the composition of ambient cations | ||
| + | and anions collected using an atmospheric pressure interface time-of-flight mass spectrometer | ||
| + | (APi-TOF) during the 2016 Holistic Interactions of Shallow Clouds, Aerosols, and Land- | ||
| + | Ecosystems (HI-SCALE) campaign. We utilize a newly developed technique, binned positive | ||
| + | matrix factorization (binPMF), in conjunction with Resolution-enhanced Kendrick Mass Defect | ||
| + | (REKMD) analysis. These techniques allowed for improved chemical insight into the trends in | ||
| + | ion composition with no requirement for a priori assignments of chemical composition. This | ||
| + | advancement is of particular importance for measurements with low signal-to-noise. Mass | ||
| + | spectral factors were first identified by binPMF and then analyzed using REKMD plots to | ||
| + | elucidate chemical patterns within the factors. REKMD demonstrated that otherwise unidentified | ||
| + | compounds were related by repeating units of CH<sub>2</sub> and O. Back trajectories and correlation with | ||
| + | other measurements provide insight into potential sources of the various identified factors. | ||
| + | Overall, we demonstrate that binPMF in combination with REKMD is a powerful tool to analyze | ||
| + | challenging mass spectrometric datasets." | ||
| + | |||
| + | |||
| + | <font size=4>11 April 2022</font size=4><br> | ||
| + | <b>Measurements of VOCs in Homes Impacted by Smoke from the Marshall Fire</b> | ||
| + | |||
| + | William Dresser, ANYL 3rd year,<br> | ||
| + | [https://groups.google.com/g/degouw-lab de Gouw group] | ||
| + | |||
| + | "The Marshall Fire was one of the most destructive fires in Colorado history, and burned and damaged over 1000 structures in Boulder County. In the immediate aftermath of the fire, there was an intense interest in accessing the persistent air quality effects of the fire both due to its close proximity to major population centers as well as the unique nature of the fire fuel, mostly man-made structures as opposed to biomass. While smoke emissions from traditional wildfires have been well studied and characterized, the emissions from building materials are less well understood and can vary significantly based on the structure. We are interested in how the smoke emissions from this fire infiltrated and then interacted in indoor environments, which have large surface reservoirs, leading to potential persistent indoor air quality effects. In the weeks following the fire, multiple instruments were deployed in a smoke-impacted home immediately adjacent to one of the burn areas to monitor effects for a period of roughly a month. Our measurements focused on gas phase Volatile Organic Chemicals (VOCs) and were carried out with a Vocus Proton-Transfer-Reaction Time-of-Flight Mass Spectrometer (Vocus PTR-TOF) and Aerodyne Gas Chromatograph (GC). Both indoor and outdoor levels were measured to look at long term trends and indoor enhancements. Ventilation experiments as well as mitigation tests using Corsi-Rosenthal boxes were done, and analyzed with respect to changes in indoor VOC concentration and exposure. Smoke remediation took place during the study, and the data give insight into enhancements in VOCs before, during, and after the cleaning." | ||
| + | |||
| + | |||
| + | <font size=4>4 April 2022</font size=4><br> | ||
| + | <b>Kinetics of Oligomer-Forming Reactions Involving the Major Functional Groups Present in Atmospheric Secondary Organic Aerosol Particles </b> | ||
| + | |||
| + | Hannah Maben, ANYL Master's Thesis Defense, <br> | ||
| + | [https://sites.google.com/site/ziemanngroup/ Ziemann group] | ||
| + | |||
| + | "Atmospheric organic aerosol particles impact climate as well as human and environmental health. Secondary organic aerosol (SOA), which is formed by the gas-to-particle partitioning of products of the oxidation of volatile organic compounds (VOCs) emitted from biogenic or anthropogenic sources, contributes a large fraction of this material. In the particle phase, these products can undergo accretion reactions to form oligomers that impact the formation, composition, and chemical-physical properties of aerosols. While these reactions are known to occur in the atmosphere, data and models describing their kinetics and equilibria are sparse. Here, reactions of compounds containing potentially reactive hydroperoxide, hydroxyl, carboxyl, aldehyde, and ketone groups were investigated in single and phase-separated organic/aqueous mixtures in the absence and presence of a sulfuric acid catalyst. Compounds containing these groups and a nonreactive UV-absorbing nitrate group were synthesized and their reactions and products were monitored and characterized using high-performance liquid chromatography with UV detection (HPLC-UV), electrospray ionization-mass spectrometry (ESI-MS) and attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy. Reactions were observed between hydroperoxides and aldehydes to form peroxyhemiacetals, and between carboxylic acids and alcohols to form esters, and their rate and equilibrium constants were determined. No reactions were observed in other mixtures, indicating that under the conditions of these experiments only a few reaction pathways form oligomers. Reactions were also conducted with probe compounds and SOA formed in an environmental chamber reaction of α-pinene with O<sub>3</sub> SOA. Whereas in a previous study we observed a rapid hydroperoxide reaction in this SOA, among the other compounds studied here only alcohols reacted. These results provide insight into the types of accretion reactions that are likely to occur in atmospheric aerosols, and the rate and equilibrium constants can be used to better model SOA chemistry." | ||
| + | |||
| + | |||
| + | <font size=4>1 April 2022</font size=4><br> | ||
| + | <b>Atmospheric Chemistry of Volatile Methyl Siloxanes – Kinetics and Oxidation Mechanism from Experimental and Theoretical Investigations</b> | ||
| + | |||
| + | Mitchell Alton, ANYL PhD thesis defense, <br> | ||
| + | [https://sites.google.com/view/brownelab/people Browne group] | ||
| + | |||
| + | "Volatile methyl siloxanes (VMS) are solely anthropogenic chemicals that have come under recent scrutiny for their environmental persistence and tendency to bioaccumulate. Millions of tons of these chemicals are produced every year, with an estimated 30 kilotons of decamethylcyclopentasiloxane (D5) emitted into the environment every year, with additional contributions from other VMS. A large source of VMS to the environment is from use of personal care products containing these compounds. Due to environmental concerns about the impact of these compounds, the European Union placed restrictions on the quantity of VMS in wash-off personal care products in 2018 with recommendations to increase the restrictions to include some industrial processes in 2021. Although there is significant interest in these high-production chemicals, the understanding of the environmental fate of these compounds is incomplete. It is predicted that >90% of VMS released to the environment will partition into the atmosphere. Once these compounds are in the atmosphere, they can react with hydroxyl radicals (OH) or chlorine atoms (Cl) through hydrogen-abstraction reactions, though the previously reported rate constants for reactions with OH vary by more than a factor of 3 and only one measurement exists for the rate constant with Cl atoms. Additionally, previously published works reported inconsistent oxidation products of these compounds, likely due to unconstrained oxidation chemistry in their experiments. | ||
| + | To better constrain the atmospheric chemistry and fate of these compounds, I measured the kinetics of seven VMS with OH radicals and Cl atoms in a 1 cubic meter FEP Teflon™ chamber I designed and built. Additionally, I measured the oxidation products of VMS in a variety of atmospheric conditions to better constrain the oxidation mechanism. Finally, I used quantum theoretical calculations to investigate the plausibility of the reactions proposed from our experimental results." | ||
| + | |||
| + | |||
| + | <font size=4>14 March 2022</font size=4><br> | ||
| + | <b>Spectroscopic studies of oxygen and iodine using cavity enhanced extinction spectroscopy</b> | ||
| + | |||
| + | Henning Finkenzeller, ANYL PhD thesis defense, <br> | ||
| + | [https://volkamergroup.colorado.edu/ Volkamer group] | ||
| + | |||
| + | "Atmospheric oxygen extinguishes approximately 2 W m<sup>-2</sup> solar radiation in oxygen-oxygen collision-induced absorption (O<sub>2</sub>-O<sub>2</sub> CIA). This heats the atmosphere, affects radiative transfer, and needs to be considered in atmospheric spectroscopy. The lack of O<sub>2</sub>-O<sub>2</sub> CIA spectra below 335 nm wavelength is limiting remote sensing applications. Here we report measured spectra of the O<sub>2</sub>-O<sub>2</sub> CIA cross-section between 297-500 nm using Cavity Enhanced Extinction Spectroscopy. Several heretofore unmeasured weak absorption bands (at 315, 328, 420, and 495 nm) are characterized for the first time in the gas phase under controlled laboratory conditions, i.e., at atmospheric pressure and variable temperature (293, 263, and 223 K). The spectra are optimized for use in hyperspectral remote sensing applications, provide opportunities to develop theory, and warrant application in radiative transfer calculations to re-assess O<sub>2</sub>-O<sub>2</sub> CIA heating rates in the atmosphere. | ||
| + | |||
| + | Iodine is a reactive trace element in atmospheric chemistry that destroys ozone and efficiently nucleates particles. Iodine emissions have tripled since 1950 and are projected to keep increasing. While iodic acid (HIO<sub>3</sub>) is widespread and grows particles more efficiently than sulfuric acid, its gas-phase formation mechanism is unresolved. Here, in CLOUD experiments which generate iodine radicals at atmospherically relevant rates, we show that iodooxy hypoiodite, IOIO, is efficiently converted into HIO3 via reactions (R1) IOIO + O<sub>3</sub> → IOIO<sub>4</sub> and (R2) IOIO<sub>4</sub> + H<sub>2</sub>O → HIO<sub>3</sub> + HOI + O<sub>2</sub>. The laboratory derived mechanism is corroborated by theory and shown to explain field observations of daytime HIO<sub>3</sub> in the remote lower free troposphere. The new mechanism provides a missing link between iodine sources and particle formation and - since aerosol iodate is readily reduced in the atmosphere, thereby recycling iodine back to the gas phase - suggests an important catalytic role of iodine in aerosol formation." | ||
| + | |||
| + | |||
| + | <font size=4>7 March 2022</font size=4><br> | ||
| + | <b>Polymer Sorption of VOCs for Indoor Air Quality and Atmospheric Sampling</b> | ||
| + | |||
| + | Melissa Morris, ANYL 3rd year, <br> | ||
| + | [https://cires1.colorado.edu/jimenez-group/ Jimenez group] | ||
| + | |||
| + | "Polymers comprise a substantial fraction of the surfaces in indoor environments, where we spend about 90% of our time. It is well-known that polymers absorb gas-phase volatile organic compounds (VOCs), which directly affect indoor and outdoor air quality, but few studies have investigated the interactions between polymers relevant to indoor materials and VOCs. To better understand the role of carpets on indoor air quality, we have extended recent studies from our groups on polymer-VOC interactions to include polymers relevant to carpets (nylon, polyester, polypropylene, polyethylene), as well as carpet itself. We use a Vocus proton transfer mass spectrometer to measure sorption of a series of 2-ketones by polymer or rolled-carpet tubes. Then, we use two sorption models adapted from Pagonis et. al. (2017) and Algrim et. al. (2020) to quantify sorptive capacities (and for highly-sorptive polymers, VOC diffusion coefficients) for these materials. The polymers were found to increase in sorptive capacity as: nylon, polyester, polypropylene and polyethylene. Polypropylene and polyethylene (often used in carpet backing) are more sorptive for the same geometry. However, nylon and polyester (the materials of carpet fibers), do comparable sorption when scaled up to account for the enormous surface area of carpet fibers. The sorptive capacities of carpet polymers suggest that carpet indoors is comparable in sorptive capacity to paint (Algrim et al. 2020) and wood (Ziola et al. 2022). Sorption experiments also showed that nylon carpet irreversibly sorbs C4-C9 acids. While testing carpet-relevant polymers for VOC sorption, we tested a few additional polymers relevant to atmospheric sampling, including TSI conductive silicone tubing. We found that conductive silicone tubing only transmits very high-volatility compounds (C* > 1e7 ug m-3, such as terpenes and more volatile species). We have demonstrated the use of a system with tubing of multiple polymer materials as a separator of gas-phase compounds into volatility classes. This technique could be useful e.g. for measuring chemical characteristics (i.e. OH reactivity or SOA potential) of complex mixtures of gas-phase compounds (such as ambient air) according to volatility class." | ||
| + | |||
| + | |||
| + | <font size=4>28 February 2022</font size=4><br> | ||
| + | <b>Reduction of Iodate in aqueous organic and inorganic thin films</b> | ||
| + | |||
| + | Margarita Reza, ANYL 3rd year, <br> | ||
| + | [https://volkamergroup.colorado.edu/ Volkamer group] | ||
| + | |||
| + | "Iodine species are known to catalytically destroy ozone, and in the case of Iodic acid (HIO<sub>3</sub>), efficiently forms particles. Iodic acid is a source of particulate iodate (IO<sub>3</sub><sup>-</sup>); this has been detected in aged stratospheric air alongside gas-phase IO radical, suggesting the existence of recycling mechanisms reducing IO<sub>3</sub><sup>-</sup> to form volatile iodine species. The reduction of iodate is explored through a series of photochemical coated wall flow tube (CWFT) experiments. A quartz glass tube was coated with an aqueous solution containing sodium iodate in a matrix (i.e., either ammonium bisulfate (ABS), 1,2,3,4-butanetetracarboxylic acid (BTCA), citric acid (CA), or Fe(III) citrate (Fe-Cit) and CA) at a concentration ratio iodate:matrix = 1:100. A thin aqueous film forms on the inside walls by passing a stream of humidified air through the CWFT (~80% RH). For each film, the formation of gaseous iodine (I<sub>2</sub>) was followed in three types of experiments: (1) dark reaction with H<sub>2</sub>O<sub>2</sub> involved flowing H<sub>2</sub>O<sub>2</sub> through the CWFT for several hours in the dark; (2) photochemical experiments involved irradiating the CWFT with visible lights and UVA lights, separately; and (3) dark-aging experiments involved flowing H<sub>2</sub>O<sub>2</sub> through the CWFT for several hours in the dark, followed by irradiation with visible light. The evaporation of gas-phase I<sub>2</sub> from the aqueous films was measured by cavity enhanced differential optical absorption spectroscopy (CE-DOAS) coupled to the CWFT. The cleanliness of the materials, cleaning procedures used, and the absence of uncontrolled chromophores reducing iodate, that might be intrinsic to the CWFT setup was confirmed in blank experiments. The I<sub>2</sub> released from aged films irradiated with visible light (type 3 experiments) was found to be substantially greater than that from irradiated fresh films (type 2), or fresh films exposed to H<sub>2</sub>O<sub>2</sub> in the dark (type 1). This increase of I<sub>2</sub> in H<sub>2</sub>O<sub>2</sub> aged films, independently observed in both inorganic and organic matrices, suggests that a secondary inorganic chromophore is formed from the reaction of iodate with H<sub>2</sub>O<sub>2</sub>. A photochemical pathway was discovered in which visible light is sufficient for reducing iodate to I<sub>2</sub>. This is relevant in the atmosphere because it helps to explain the co-existence of particulate IO<sub>3</sub><sup>-</sup> and gas-phase IO radicals recently observed by aircraft measurements in the stratosphere. Multiphase re-cycling of I<sub>2</sub> from particulate IO<sub>3</sub><sup>-</sup> in absence of ultraviolet light further suggests that a catalytic reaction cycle is more active than previously thought, and involves three steps: (1) gas-phase HIO<sub>3</sub> formation from I<sub>2</sub> photolysis, (2) condensation and dissociation of HIO<sub>3</sub> to form IO<sub>3</sub><sup>-</sup>, and (3) IO<sub>3</sub><sup>-</sup> reduction to re-cycle I<sub>2</sub>. This catalytic reaction cycle destroys O<sub>3</sub> by multiphase chemistry, and highlights a possible catalytic role of HIO<sub>3</sub> in particle formation. This photochemistry is currently missing in atmospheric models, seems relevant to predictions about the partitioning of iodine between the gas- and particle phases, invigorates particle formation from iodine oxoacids, and seems relevant to better understand iodine’s role in the recovery of the ozone layer." | ||
| + | |||
| + | |||
| + | <font size=4>21 February 2022</font size=4><br> | ||
| + | <b>An Investigation of Carboxylic Acid Chemistry in Indoor and Outdoor Environments</b> | ||
| + | |||
| + | Anna Ziola, ANYL 3rd year, <br> | ||
| + | [https://sites.google.com/site/ziemanngroup/ Ziemann group] | ||
| + | |||
| + | "Carboxylic acids are prominent organic molecules in indoor and outdoor environments and also have chemical properties that make them useful probes for investigating the chemistry of indoor and outdoor air. Even though the average person spends nearly 90% of their lifetime indoors, we know very little about the processes that impact volatile organic compounds (VOCs) in indoor environments, where there are a variety of surfaces that VOCs can interact with. To understand the role of wood surfaces, we have used an iodide chemical ionization mass spectrometer (I-CIMS) and an attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectrometer along with a model to quantify and better understand how carboxylic acids interact with a variety of wood species and varnishes. These experiments have provided parameters for use in models and shown that the behavior of carboxylic acid VOCs indoors relies more on the identity of the varnish and less on the identity of the wood species. We have also taken advantage of the chemical properties of carboxylic acids to study the oxidation of alkenes in low NOx environments. According to data from CalNex in 2010 and LAAQS in 2020, the concentration of mid-day NO, a prominent component in VOC oxidation mechanisms, has decreased by about 75% in Los Angeles in the past decade. There have been many studies on the oxidation of VOCs in high NOx environments, but far fewer in the absence of NOx. To gather more knowledge about low NOx chemistry, we reacted 6-heptenoic acid, a C<sub>7</sub> 1-alkene with a terminal carboxylic acid group, with OH radicals in an environmental chamber while using an I-CIMS to identify and quantify gas-phase products. We also collected aerosol particles on filters, derivatized the carboxylic acid groups, and identified and quantified the products using high performance liquid chromatography (HPLC) in tandem with an electrospray ionization mass spectrometer (ESI-MS). Therefore, by taking advantage of carboxylic acid properties, we have been able to better understand their interactions with indoor surfaces and gain insights into the oxidation of alkenes under low NOx conditions." | ||
| + | |||
| + | |||
| + | <font size=4>14 February 2022</font size=4><br> | ||
| + | <b>Longer term aging of organic aerosol: photobleaching and chemical transformations</b> | ||
| + | |||
| + | [https://www.wm.edu/as/chemistry/people/faculty/obrien_rachel.php Rachel O'Brien], <br> | ||
| + | College of William and Mary | ||
| + | |||
| + | "Organic aerosol particles can influence the climate through either directly absorbing or scattering | ||
| + | solar radiation or by acting as nuclei for cloud droplets. Some aerosol particles are dominantly | ||
| + | scattering while others contain organic molecules that can absorb solar radiation in the visible | ||
| + | region, termed brown carbon (BrC). We still have large uncertainties in the magnitude of these | ||
| + | climate effects and a better understanding of the removal rates for the particle mass and | ||
| + | absorption properties (i.e. color) is needed. One removal process is photolysis, where absorption | ||
| + | of solar radiation leads to fragmentation of the organic molecules and the loss of particle mass | ||
| + | and/or color. However, the photolysis rates and the overall extent of mass that can be removed | ||
| + | via direct photolysis in laboratory experiments does not match what is used in models and often | ||
| + | differs from ambient measurements. In this talk, I will combine results from work in our lab | ||
| + | looking at photolysis of biogenic secondary organic aerosol as well as BrC from biomass burning | ||
| + | organic aerosol to evaluate gaps in our ability to predict the observed ambient removal rates. By | ||
| + | probing complex mixtures from recent biomass burning experiments (e.g. FIREX samples), I | ||
| + | will demonstrate that our current measured rates in the laboratory are overestimated and that a | ||
| + | slower photolysis rate, as well as a potential gas-phase oxidation rate, should be used to predict | ||
| + | the role of photolysis on organic aerosol lifetime in the atmosphere." | ||
| + | |||
| + | ==Fall 2021== | ||
| + | <b>Evaluating TROPOMI CO Column Observations Using CU AirSOF During BB-FLUX</b> | ||
| + | |||
| + | Jake Rowe, | ||
| + | ANYL MS Defense, [http://volkamergroup.colorado.edu/ Volkamer group] | ||
| + | |||
| + | "The Biomass Burning Fluxes of Trace Gases and Aerosols (BB-FLUX) field campaign was carried out during the summer of 2018 with the primary goal of quantifying emission fluxes of trace gases by mass balance of actual wildfires. To characterize these fluxes, the University of Colorado Airborne Solar Occultation Flux (CU AirSOF) instrument was flown below biomass burning plumes to measure vertical trace gas columns, such as carbon monoxide (CO, with the first overtone fit from 4214 - 4254 cm-1), along the direct solar beam. The TROPOspheric Monitoring Instrument (TROPOMI) provides measurements of trace-gas maps in the Ultraviolet-Visible (UV-Vis.) and shortwave-IR (SWIR) spectral regimes (e.g. CO from 4277 - 4303 cm-1). A subset of flights during BB-FLUX were dedicated to position the aircraft below smoke plumes while the satellite measures the same scene aloft. | ||
| + | |||
| + | Radiative transfer simulations were conducted to estimate the effect of aerosol multiple scattering in smoke plumes, which is not routinely accounted for in TROPOMI CO retrievals at short-wave infrared wavelengths. Aerosol multiple scattering recovers sensitivity losses in the absence of surface albedo (by up to 80%), but the loss is greatly reduced (5-10%) if surface albedo exceeds 10%. The difference in the temporal and spatial scales, and measurement geometries sampled from the aircraft and satellite are actively addressed by 1) focusing on near coincident case studies, 2) comparing spatial integrals of CO differential vertical column densities (dVCDs) across plumes, and 3) using the FLEXible PARTicle (FLEXPART) dispersion model to correct for different sampling times. CU AirSOF revealed variations in CO dVCD integrals on the order of 24% (up to 37%) over 30 mins (consecutive transect measurements) that characterize changes in emissions on the satellite sub-pixel scale. TROPOMI CO is found to be systematically higher relative to the aircraft by +26% for the operational product (+23% scientific product) without FLEXPART correction; such high-bias is reduced to +8.7% (+5.8%) upon adding the FLEXPART correction. The small but systematic high-bias in TROPOMI CO is most likely due to unaccounted aerosol multiple scattering, atmospheric variability, or a combination thereof." | ||
| + | |||
| + | |||
| + | <font size=4>8 November 2021</font size=4><br> | ||
| + | <b>Bringing Real-World Chemical Complexity to the Laboratory – Secondary Organic Aerosol Formation from Plant Emissions Increases Particle Viscosity and Alters Composition</b> | ||
| + | |||
| + | [https://faculty.sites.uci.edu/cfaiola/ Prof. Celia Faiola], | ||
| + | Univ. of California-Irvine | ||
| + | |||
| + | "Climate change is influencing ecosystem health and plant biogeography. This affects the composition and spatiotemporal distribution of biogenic volatile organic compounds (BVOCs) across Earth’s surface. Perturbations to the types of compounds emitted could have significant impacts on secondary organic aerosol (SOA) production, but the chemistry of many of these compounds (including complex mixtures of these compounds) have not been studied comprehensively in a controlled laboratory environment. This presentation will summarize laboratory studies investigating SOA formation from complex mixtures of real plant emissions representing different plant emission types. Unexpected effects on aerosol chemistry, composition, and properties attributed to the presence of oxygenated monoterpenes and acyclic terpenes in the BVOC mixture were observed. Oxygenated monoterpenes (i.e. camphor and eucalyptol) that dominate emissions from sage and sagebrush produce SOA with more highly oxygenated molecules than SOA formed from more traditionally-studied terpenes. Acyclic terpenes (i.e. myrcene and farnesene) that are often associated with plant stress, reduce SOA yields and promote fragmentation reactions while increasing the viscosity of the resulting SOA. Aerosol chemistry of these compounds could become increasingly important with the expansion of drought-tolerant sage scrub and elevated frequency of plant stress conditions." | ||
| + | |||
| + | <font size=4>1 November 2021</font size=4><br> | ||
| + | <b>Evaluation of Halogen-Induced Ozone Depletions Near Salt Lake City</b> | ||
| + | |||
| + | Wyndom Chace, | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "An unexpected halogen-rich plume, originating from an industrial plant on the western shore of the Great Salt Lake, was sampled on nine separate flights during the NOAA 2017 Utah Winter Fine Particulate Study (UWFPS). Tropospheric ozone depletions were also measured in the halogen plume, analogous to halogen-induced ozone degradation in other regions of the atmosphere such as the arctic troposphere and the stratosphere. This study sought to quantify the halogen flux from the industrial plant and to characterize the photochemical processes that result in tropospheric ozone depletions. The calculated Cl<sub>2</sub> and HCl emission fluxes were consistent with the industrial plant’s reported EPA emissions inventory; the inventory does not report fluxes of Br<sub>2</sub> and BrCl, which also had significant calculated fluxes. The observed ozone depletions were investigated using a semi-Lagrangian plume setup in a zero-dimensional atmospheric chemistry box model (F0AM). The box model was able to reproduce the magnitude of ozone depletion observed in the daytime January 26 plume (>35 ppbv below background levels) and confirmed that chlorine and bromine radicals, generated from the photolysis of halogen species emitted by the plant, were responsible for the observed ozone depletions. These results suggest that atypical levels of industrial halogen emissions may have significant impacts on local air quality, particularly relevant in the case of this industrial plant due to its close proximity to Salt Lake City." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>An Investigation of Ozone Chemistry During KORUS-AQ Using Observations and Modeling</b> | ||
| + | |||
| + | Lindsey Anderson, | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Ground-level ozone is a toxic air pollutant that is created from reactions of nitrogen oxides (NO<sub>x</sub> = NO + NO<sub>2</sub>) and volatile organic compounds (VOCs) in the presence of sunlight. The production of ozone is a nonlinear function of its precursors, which complicates emission regulations that target ozone reduction. In this study, ozone chemistry in Seoul, South Korea was investigated using flight observations from the Korea United States Air Quality (KORUS-AQ) field study. Measurements near the city center are characteristic of a highly polluted urban region with high levels of NO<sub>x</sub> concentration, while measurements to the southwest exhibit high levels of VOC concentration from chemical production facility emissions. The ozone production regime (NO<sub>x</sub>-limited vs. VOC-limited) in the Seoul Metropolitan Area (SMA) was determined to be VOC-limited, meaning reductions in the concentration of reactive VOCs would lead to a decrease in ozone production. Possible emission reduction regulations were then modeled to understand how they would impact ozone formation in the SMA. It was determined that emission regulations targeting ozone reduction in the SMA should include reductions in both NO<sub>x</sub> and reactive VOCs because NO<sub>x</sub> reduction alone would lead to an increase in ozone formation." | ||
| + | |||
| + | <font size=4>25 October 2021</font size=4><br> | ||
| + | <b>Comparison of Common Vapor Pressure Estimation Methods through Modeling of Alkene OH·/NO<sub>x</sub> Systems</b> | ||
| + | |||
| + | Emmaline Longnecker, | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Modeling of atmospheric reactions is an important tool in understanding the current and future impacts of human activity on the environment. Vapor pressure is a key parameter in modeling these reactions, as it largely determines the ability of a species to transition from the gas to particle phase. However, the vapor pressures of many atmospherically relevant molecules are still poorly constrained. In order to aid modeling efforts, several group contribution methods have been developed for estimating compound vapor pressures. The current study evaluates how four of these methods: SIMPOL, EVAPORATION, SPARC, and Nannoolal, impact the modeled predictions of secondary organic aerosol (SOA) yields for the reactions of C8-C14 1-alkenes with OH radicals in the presence of NO<sub>x</sub>. The models were created in the program KinSim and included detailed reaction mechanisms and branching ratios determined in several previous chamber studies by our research group, as well as gas-particle and gas-wall partitioning. SOA yields predicted using each of the four estimation methods were then compared to the measured values. The results of the models were variable, with the maximum discrepancies ranging from an underestimate of ~40% to an overestimate of ~30% compared to the experimentally determined mass yields. This variability exemplifies the impact of vapor pressure in modeling atmospheric reactions and indicates the need for further research in development of estimation methods." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Redox-Active Coordination Complexes for Small Molecule Activation with Environmental Applications</b> | ||
| + | |||
| + | Hanalei Lewine, | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Nitrate and nitrite are harmful pollutants resulting from the overuse of nitrogen fertilizers in agriculture. In Nature, denitrification converts these to lower-oxidation state nitrogen species, each step being catalyzed by a different metalloenzyme such as nitrate reductase. Synthetic systems that mimic these enzymes could convert NOx- to less harmful, and potentially useful compounds like nitrogen (N2) and ammonia (NH3). The highly versatile pyridinediimine (PDI) ligand has been successful in the reduction of these species due to the potential for incorporating redox-activity, proton-responsivity, and hemilability into the ligand scaffold. A new PDI iron complex featuring a hemilabile pendant phosphine shows reactivity towards nitrate and nitrate to selectively reduce to NO on a mononitrosyl iron complex (MNIC) will be presented." | ||
| + | |||
| + | <font size=4>27 September 2021</font size=4><br> | ||
| + | |||
| + | <b>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</b> | ||
| + | |||
| + | [https://sites.google.com/site/ziemanngroup/home Paul Ziemann], | ||
| + | ANYL Faculty, CU Boulder | ||
| + | |||
| + | "Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative studies of indoor air chemistry at CU." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Chemistry of Volatile Organic Compounds in the Atmosphere</b> | ||
| + | |||
| + | [https://sites.google.com/view/de-gouw-lab Joost de Gouw], | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products. | ||
| + | |||
| + | In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments. | ||
| + | |||
| + | Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments to better understand their sources and fate. Second, we are working on the emissions and chemistry of VOCs in urban air. Third, we are working on a chamber study to better understand the formation of secondary organic aerosol from biogenic VOCs. Finally, we are also using data from satellite remote sensing instruments to measure the pollutants from oil and natural gas production, and from wildfires." | ||
| + | |||
| + | <font size=4>20 September 2021</font size=4><br> | ||
| + | |||
| + | <b>Heterogeneity in Airborne Transmission of COVID-19 by Respiratory Aerosols</b> | ||
| + | |||
| + | Prasad Kasibhatla (Duke Univ., CIRES Visiting Fellow) | ||
| + | |||
| + | "The recognition of the importance of airborne transmission of the SARS-CoV-2 virus by | ||
| + | respiratory aerosols has spurred several research groups to develop and apply simple | ||
| + | mechanistic aerosol models to investigate COVID-19 outbreaks associated with specific events. | ||
| + | These studies have highlighted the importance of layered, non-pharmaceutical intervention | ||
| + | strategies such as masking, ventilation, and air cleaning to mitigate transmission risk. To date, | ||
| + | however, there is a disconnect between these micro-scale mechanistic aerosol models and the | ||
| + | macro-scale epidemiological models that have been used to study large-scale COVID-19 disease | ||
| + | dynamics. | ||
| + | In this talk, I will discuss my recent research to try to close this gap. I will present a Monte | ||
| + | Carlo analysis of transmission risk and secondary infections arising in social settings by | ||
| + | modeling aerosol transmission of COVID-19 in thousands of indoor locations in the United | ||
| + | State. I will show that variability of viral load among index cases plays a key role in shaping the | ||
| + | variability in transmission risk at these locations. I will further demonstrate that aerosol | ||
| + | transmission is consistent with the observation that COVID-19 transmission is overdispersed | ||
| + | and will provide a mechanistic explanation for this large-scale characteristic of the pandemic. | ||
| + | Implications of this finding with regards to the effectiveness of non-pharmaceutical | ||
| + | interventions in curbing the pandemic will be discussed. Finally, I will outline my ideas for the | ||
| + | next steps in this research that I will be working on during my sabbatical at CIRES." | ||
| + | |||
| + | <font size=4>13 September 2021</font size=4><br> | ||
| + | <b>Small molecules in the Anthropocene: Oceans, Wildfires, and New Particle Formation from Iodine</b> | ||
| + | |||
| + | [https://volkamergroup.colorado.edu/ Rainer Volkamer], | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "The Volkamer group develops advanced optical instrumentation (in situ and remote sensing) to measure small molecules and aerosols that are relevant to public health discussions and climate. We seek to better quantify and understand emissions of small molecules, total carbon, and aerosols from natural and managed ecosystem (e.g., wildfires, oil & natural gas, ocean surface, UTLS), and develop a molecular level understanding of the fundamental processes that affect their chemical transformations and sinks (e.g., new particle formation). The combination of optical spectroscopy and high-resolution mass spectrometry is an emerging future theme in the group. Opportunities for graduate research exist in the areas of 1) field measurements using research aircraft (i.e., TI3GER-2022, CUPiDS-2023 projects), 2) laboratory experiments of particle formation (incl. at CLOUD/CERN) and multiphase chemistry, and 3) instrumentation to study carbon closure, and exploit synergies between optical spectroscopy and mass spectrometry." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Atmospheric chemistry of reduced nitrogen and organosilicon</b> | ||
| + | |||
| + | [https://sites.google.com/view/brownelab/home?authuser=0 Eleanor Browne], | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "The Browne group uses a combination of laboratory experiments and field measurements to understand the sources and transformations of trace gases in the atmosphere with the ultimate goal of understanding the impacts of this chemistry on air quality, climate, and nutrient delivery to ecosystems. Here, I will discuss some of our recent work on the atmospheric chemistry of cyclic volatile methyl siloxanes and of reduced nitrogen compounds. Finally, I will discuss research opportunities in our group." | ||
| + | |||
| + | <font size=4>30 August 2021</font size=4><br> | ||
| + | <b>Understanding aerosols for pollution, climate change, and disease transmission</b> | ||
| + | |||
| + | [http://cires1.colorado.edu/jimenez/ Jose-Luis Jimenez], | ||
| + | ANYL Faculty, CU Boulder | ||
| + | |||
| + | "Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly present results from different projects over the last year, as well as some future directions for our group. I will introduce our aircraft research program, including results from the recent NASA/NOAA FIREX-AQ wildfire smoke study, where we obtained near-molecular level aerosol speciation data at 1 second time resolution. We are investigating both the inorganic and organic composition of the smoke, including important properties such as pH and volatility. I will summarize modeling work with GECKO-A exploring the evolution of OH reactivity with OH exposure for different hydrocarbons. I will discuss our indoor air research, including the apportionment of gases in the CU Art Museum combining 3 different CIMS instruments. I will also briefly describe recent developments on the importance of aerosols for disease transmission, not just for COVID-19 but for all (or most) respiratory diseases. | ||
| + | |||
| + | For those interested in COVID-19 aerosol transmission, you can find a summary of the evidence supporting airborne transmission of COVID-19 at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00869-2/fulltext, a review for airborne transmission of all respiratory diseases at https://doi.org/10.1126/science.abd9149 (starting Thu 26-Aug), and an overview of the historical reasons for the confusion on this topic at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3904176." | ||
| + | |||
| + | ==Spring 2021== | ||
| + | <font size=4>24 May 2021</font size=4><br> | ||
| + | <b>Measurements related to the fate of organic compounds in outdoor and indoor environments </b> | ||
| + | |||
| + | ANYL PhD Defense, Benjamin Deming, [https://sites.google.com/site/ziemanngroup/ Ziemann group] | ||
| + | |||
| + | "A massive quantity of organic compounds is emitted into the Earth’s atmosphere every | ||
| + | day. Depending on their structure and the ambient conditions, these species may persist | ||
| + | anywhere between minutes to years in the atmosphere. This presentation discusses three studies | ||
| + | broadly related to the fate of organic species in both outdoor and indoor environments. In the | ||
| + | first project, the interactions between volatile organic compounds and various types of tubing | ||
| + | were quantitatively investigated. It was found that, depending on the material of the tubing in | ||
| + | question, organic species may either adsorb onto the tubing surface or absorb into the tubing | ||
| + | itself. Besides leading to an improved understanding of tubing used in sampling lines for field | ||
| + | campaigns or laboratory experiments, this work also provides unique insight into the interactions | ||
| + | of volatile organic compounds and solid surfaces. Oxidation of the aforementioned organic | ||
| + | species in the atmosphere often leads to the production of secondary organic aerosol. Depending | ||
| + | on the composition of the aerosol and the ambient conditions, particles may undergo liquid- | ||
| + | liquid phase separation, forming a largely aqueous layer and a predominantly organic phase. The purpose of the second study was to investigate the distribution of atmospherically common inorganic acids between these phases. In particular, the presence of significant quantities of acid | ||
| + | in the organic layer has important implications for the mechanisms and rates of acid-catalyzed | ||
| + | particle-phase reactions. We found that, depending broadly on factors such as the O:C ratio and | ||
| + | water content of the organic phases, between 0.1% and 10% of the acid in the system may be | ||
| + | found in the organic layer. These results have important implications for particle-phase | ||
| + | chemistry, which may further process and functionalize organic carbon in the atmosphere. The | ||
| + | final study investigates the presence of alkenes on indoor surfaces and the effects these species | ||
| + | have on indoor air chemistry. Upon developing a microscale derivatization-spectrophotometric | ||
| + | method for measuring non-conjugated double-bonds, we applied it to a number of small, but | ||
| + | informative, studies investigating open questions about indoor air and surfaces. The results show | ||
| + | that the reaction of gas-phase ozone with surface-bound alkenes is prevalent and drives | ||
| + | significant chemistry indoors." | ||
| + | |||
| + | |||
| + | <font size=4>Monday, 26 April 2021</font size=4><br> | ||
| + | <b>An extraterrestrial start and a volcanic end to the Cretaceous-Paleogene mass extinction?</b> | ||
| + | |||
| + | [https://www.colorado.edu/geologicalsciences/boswell-wing Prof. Boswell Wing], <br> | ||
| + | CU Boulder, Geological Sciences | ||
| + | |||
| + | "The Cretaceous–Paleogene (KPg) mass extinction is one of the “big five” mass extinctions that occurred during the last 550 million years. Although geological records of the KPg transition preserve ample evidence of an impact, there is significant controversy about whether this impact was the sole mass extinction trigger, or whether volcanism from the Deccan traps contributed significantly to the sudden onset of the biotic crisis. In this presentation I will discuss how precise measurements of the ratio of 34S-32S in KPg sediments at high-spatial resolution can trace atmospheric sulfur injections from these two events in the immediate vicinity of the KPg extinction horizon." | ||
| + | |||
| + | <font size=4>Monday, 19 April 2021</font size=4><br> | ||
| + | <b>Submicron Particle Composition and Acidity in Fire Plumes during FIREX-AQ aircraft study</b> (1/2 seminar) | ||
| + | |||
| + | Hongyu Guo, <br> | ||
| + | Postdoctoral Fellow, [https://cires1.colorado.edu/jimenez-group/ Jimenez Group] | ||
| + | |||
| + | "During the Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ) aircraft | ||
| + | study, the chemical composition of fire-emitted submicron particles was quantified with a High- | ||
| + | Resolution Aerosol Mass Spectrometer (AMS). The western wildfire-emitted particles show similar | ||
| + | composition across the plumes and are overwhelmingly dominated by organic aerosol (OA). The eastern | ||
| + | agricultural fires show larger variability in particle composition with a higher inorganic fraction, in | ||
| + | particular Cl and K. Fast (1 or 5 Hz) measurements of K in fire plumes, which show excellent correlation | ||
| + | with SAGA filter measurements, allow a quantitative closure of the particle anion/cation balance. | ||
| + | Although lab experiments suggest variable AMS relative ionization efficiency (RIE) of K for mixed K | ||
| + | inorganic salts, field observations indicate a uniform RIE for fresh fire-emitted particles dominated by | ||
| + | OA. SO<sub>4</sub> in some fresh biomass burning plumes (both wild and agricultural fires) had major contributions | ||
| + | from organosulfur species (as quantified by two separate methods), vs. typically a few percent in regional | ||
| + | background air. This is consistent with limited previous observations (DC3, FLAME-3). The AMS | ||
| + | inorganic SO<sub>4</sub> agrees better with SAGA-MC SO<sub>4</sub>, as expected from the ion chromatography detection of | ||
| + | the latter instrument. The organosulfur appears to be dominantly a primary emission and was removed on | ||
| + | a similar timescale as fresh primary biomass burning OA (BBOA). Ultrahigh-resolution analysis of | ||
| + | FIREX-AQ filter samples is used to aid in the identification of the organosulfur species. Lastly, we use | ||
| + | thermodynamic models to estimate aerosol pH, an important lever on many particulate physical and | ||
| + | chemical processes, based on AMS-quantified K, inorganic-only SO<sub>4</sub>, pNO<sub>3</sub> and collocated gas | ||
| + | measurements (NH<sub>3</sub> and HNO<sub>3</sub>). The fresh western biomass burning particles had near-neutral pH (on | ||
| + | average ~6), which was buffered by high levels of NH<sub>3</sub> and contrasts with far lower pH observed for | ||
| + | continental (~1-4) and remote oceanic (~0) submicron particles. Regional background particles during | ||
| + | FIREX-AQ show moderate acidity (pH~3), which indicates drastically different rates for H<sup>+</sup> -influenced | ||
| + | processes inside and outside of the plumes." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Emissions, Partitioning, and Aging of Biomass Burning Organic Aerosol at FIREX-AQ</b> (1/2 seminar) | ||
| + | |||
| + | Demetrios Pagonis, <br> | ||
| + | Postdoctoral Fellow, [https://cires1.colorado.edu/jimenez-group/ Jimenez Group] | ||
| + | |||
| + | "The chemical composition of biomass burning organic aerosol (OA) evolves rapidly as it is | ||
| + | transported downwind of a fire. Changes in composition are driven by dilution, evaporation, and | ||
| + | secondary OA (SOA) production. Here we present airborne OA measurements from an | ||
| + | extractive electrospray mass spectrometer (EESI) and an Aerodyne high-resolution Aerosol Mass | ||
| + | Spectrometer (AMS) during FIREX-AQ. By combining the bulk OA composition measured by | ||
| + | AMS with speciated OA composition measured by EESI, we quantify the relative contributions | ||
| + | of SOA production and primary OA (POA) evaporation to the evolution of OA composition | ||
| + | downwind of wildland fires. Evaporation rates of bulk OA and levoglucosan (a component of | ||
| + | POA) are quantified through a combination of thermodenuder measurements and sampling | ||
| + | smoke plumes at varying ambient temperatures. Rates of SOA production are quantified through | ||
| + | measurements of individual SOA components by EESI, and through positive matrix factorization | ||
| + | of AMS spectra. We observe SOA production rates exceeding 100 μg sm -3 h -1 (standard conditions | ||
| + | 273 K, 1013 mbar) and simultaneously measure evaporation of POA components, with minimal | ||
| + | change in dilution-corrected OA concentration; this provides direct evidence for balance in SOA | ||
| + | production and POA evaporation rates in near-field aging of biomass burning OA." | ||
| + | |||
| + | <font size=4>Monday, 12 April 2021</font size=4><br> | ||
| + | <b>OS and pH estimation from AMS measurements and OH oxidation of phenolic compounds in wildfire smoke</b> | ||
| + | |||
| + | Melinda Schueneman, <br> | ||
| + | ANYL 3rd year student, [https://cires1.colorado.edu/jimenez-group/ Jimenez Group] | ||
| + | |||
| + | "This talk will focus on two unrelated research projects: the first will highlight a paper recently published in AMT, and the second will introduce and show preliminary results for a biomass burning project. Shortened abstracts are included here for reference. | ||
| + | |||
| + | Part I: Sulfate can be present in aerosols as inorganic (mainly ammonium sulfate, AS) or organosulfate (OS). Although OS is thought to be a smaller fraction of total sulfate in most cases, recent literature argues that this may not be the case in more polluted environments. Two new methods have been proposed to quantify OS separately from AS with AMS data. We use observations collected during several airborne field campaigns covering a wide range of sources and air mass ages and targeted laboratory experiments to investigate the proposed OS methods. Four chemical regimes are defined. In polluted areas with high ammonium nitrate concentrations and in remote areas with high aerosol acidity, the decomposition and fragmentation of sulfate in the AMS is influenced by multiple complex effects, and estimation of OS does not seem possible with current methods. In regions with lower acidity (pH > 0) and ammonium nitrate (fraction of total mass < 0.3), the proposed OS methods might be more reliable, although application of these methods often produced nonsensical results. Under highly acidic conditions (when calculated pH < 0 and ammonium balance < 0.65), sulfate fragment ratios show a clear relationship with acidity. The measured ammonium balance is a promising indicator of rapid estimation of aerosol pH < 0, including when gas-phase NH<sub>3</sub> and HNO<sub>3</sub> are not available. These results allow an improved understanding of important intensive properties of ambient aerosols. | ||
| + | |||
| + | Part II: The intensity and frequency of fires has been sharply increasing with an expanding population, increased land clearing for agriculture, and climate change. Fire plumes introduce large amounts of diverse chemical species into the atmosphere. The abundant emissions of VOCs, particles, and NO<sub>x</sub> suggest that substantial aerosol formation should occur downwind of fires. However, typically no enhancement of total OA is observed in most field studies. To explore the relationship between POA and SOA in aging smoke, we use measurements from the Extractive Electrospray Soft Ionization Time-of-Flight Mass Spectrometer (EESI) taken during FIREX-AQ, along with EESI and Vocus measurements from chamber experiments. A suite of laboratory chamber experiments, targeting known and suspected BB SOA precursors, are being conducted to identify key tracer species in this system, for both the particle and gas phases. Key chemical species from the OH initiated oxidation of phenol, catechol, and styrene were identified from chamber studies and their formation and evolution were modeled with KinSim. Some identified products were calibrated for and identified in a FIREX-AQ research flight, and one plume was also modelled in KinSim. We present preliminary results from the analysis of these observations." | ||
| + | |||
| + | <font size=4>Monday, 5 April 2021</font size=4><br> | ||
| + | <b>Aerosol optical closure and long-term MAX-DOAS observations</b> | ||
| + | |||
| + | Christopher Lee, <br> | ||
| + | ANYL 3rd year student, [http://volkamergroup.colorado.edu/ Volkamer group] | ||
| + | |||
| + | "Aerosols remain a major source of uncertainty in the global radiative budget. Studies that combine vertically-resolved measurements of aerosol size distributions and refractive index (inferred from aerosol composition measurements) are needed to assess our understanding of multispectral aerosol optical closure. Here we use data from the NASA airborne HSRL-2 instrument, which retrieves aerosol extinction profiles at 355, 532, and 1064 nm from backscatter measurements. The dataset we use is from the Two-Column Aerosol Project (TCAP) in July 2012, which deployed two research aircraft above the DoE ARM mobile facility at Cape Cod, MA. This dataset is reanalyzed here to investigate the effects of aerosol water on dry aerosol size and composition, and our ability to constrain Mie calculations to obtain multispectral optical closure. | ||
| + | |||
| + | High-altitude ground-based remote sensing is well-suited for long-term profile measurements of trace gases and aerosols, and provides unique sensitivity to the free troposphere. An example of this is the University of Colorado Multi-AXis Differential Optical Absorption Spectroscopy (CU MAX-DOAS) instrument, which measures vertical profiles of trace gases (BrO, IO, HCHO, CHOCHO, NO<sub>2</sub>, O<sub>3</sub>, SO<sub>2</sub>, H<sub>2</sub>O, etc.) and aerosols. Since February 2017, two CU MAX-DOAS instruments have operated continuously at Mauna Loa Observatory, Hawaii (155.578 W, 19.539 N, 3397 msl) and Maido Observatory, Reunion Island (55.384 E, 21.080 S, 2160 msl), respectively. In March 2021, a third CU MAX-DOAS instrument was deployed at Storm Peak Laboratory (106.744 W, 40.455 N, 3209 msl) in Steamboat Springs, Colorado. Leveraging the data from these three measurement sites, the vertically-resolved spatiotemporal variation of trace gases and aerosols can be characterized in both hemispheres." | ||
| + | |||
| + | <font size=4>Monday, 29 March 2021</font size=4><br> | ||
| + | <b>Measurements of Volatile Organic Compounds during the COVID-19 Lockdown in Changzhou, China</b> | ||
| + | |||
| + | Andrew Jensen, <br> | ||
| + | ANYL 3rd year student, [https://sites.google.com/view/de-gouw-lab de Gouw group] | ||
| + | |||
| + | "The COVID-19 outbreak in January 2020 prompted strict lockdowns, reduced human activity, and reduced emissions of associated pollutants. These reduced emissions have been estimated via remote and in-situ methods, but detailed measurements of volatile organic compounds (VOCs) are lacking. We measured VOCs in Changzhou, a Chinese city on the Yangtze River, during the local COVID-19 lockdowns from 8 January through 27 March, including periods of pre-lockdown, strict measures (level 1), and more relaxed measures accompanied by the return to work (level 2). VOCs were measured using a new, compact model of the Vocus proton-transfer-reaction time-of-flight mass spectrometer (Vocus Elf PTR-TOF-MS). We used positive matrix factorization to attribute VOCs to sources and employ an ensemble approach to determine uncertainties in the measured emission reductions. These uncertainties were further informed by satellite remote sensing and in-situ monitoring measurements of criteria pollutants, where we had measurements from previous years. Four factors of interest were resolved: textile industrial emissions (62±10%; average reduction during level 1 relative to pre-lockdown), pharmaceutical industrial emissions (40±20%), fresh traffic emissions (69±10%), and aged traffic emissions (73±14%). The quantified changes in the factors due to the lockdowns serve to constrain emission inventories and inform models, particularly for sectors where activity data are sparse, as the effects of lockdowns on air quality are explored." | ||
| + | |||
| + | <font size=4>Monday, 8 March 2021</font size=4><br> | ||
| + | <b>Aqueous Aerosols, Aerosol Chemistry, and the Chemical Disciplines</b> | ||
| + | |||
| + | [https://sites.google.com/a/udel.edu/johnston-group/ Prof. Murray Johnston], <br> | ||
| + | University of Delaware | ||
| + | |||
| + | "Airborne particles strongly influence climate and human health. Understanding these impacts | ||
| + | requires knowledge of chemical processes associated particle formation and growth, and draws | ||
| + | upon fundamental principles in the chemical disciplines (analytical, physical, etc.). While these | ||
| + | disciplines provide insight into aerosol chemistry, the reverse is also true: studying aerosols as | ||
| + | opposed to bulk phases provides insight into disciplinary topics. This presentation will focus on | ||
| + | the unique environment of aqueous aerosol particles, which can show very different reactivity | ||
| + | from a bulk aqueous solution as well as from their dry particle counterparts. These differences | ||
| + | have important implications for how particles grow in the atmosphere, and they help us better | ||
| + | understand chemical measurements that rely on the formation of aerosol droplets." | ||
| + | |||
| + | <font size=4>Monday, 1 March 2021</font size=4><br> | ||
| + | <b>Managing Chemical Complexity in Highly Parameterized Air Quality Models</b> | ||
| + | |||
| + | [https://barsantigrp.engr.ucr.edu/people/Kelly-barsanti Kelley Barsanti], <br /> | ||
| + | University of California, Riverside | ||
| + | |||
| + | "Tropospheric chemistry and air quality are strongly influenced by source emissions. In the western US, | ||
| + | much attention has been on the air quality impacts of wildland fires; and more recently, changes in air | ||
| + | quality associated with COVID-19 shelter-in-place restrictions. Fires emit high levels of trace gases, | ||
| + | including semi-volatile and volatile organic compounds (S/VOCs); and primary (directly emitted) | ||
| + | particulate matter (PM). During plume evolution, S/VOCs react to form ozone (O<sub>3</sub>) and secondary PM, | ||
| + | thereby degrading air quality downwind. The amount of pollutants formed depends on fuel and fire | ||
| + | characteristics, and plume dynamics and chemistry. Unfortunately, model predictions of O<sub>3</sub> and PM from | ||
| + | wildland fires are characterized by significant uncertainties. While there are a number of factors that lead | ||
| + | to poor model predictions, research in our group has focused on three particular limitations: 1) incomplete | ||
| + | identification and quantification of gaseous compounds emitted from fires that may serve as pollutant | ||
| + | precursors; 2) incomplete understanding of the transformations of the precursors that lead to pollutant | ||
| + | formation in smoke plumes; and 3) over-simplified representation of emissions and processes in current | ||
| + | smoke and air quality models. Somewhat analogously, urban anthropogenic sources also emit large | ||
| + | quantities of S/VOCs that react to form O<sub>3</sub> and secondary PM. Changes in anthropogenic activity as result | ||
| + | of COVID-19 restrictions have provided an opportunity to test recent hypotheses about the changing mix | ||
| + | of VOCs emitted by urban anthropogenic sources and the relative importance of emerging anthropogenic | ||
| + | sources for secondary pollutant formation; as well as to test our abilities to represent the chemistry of | ||
| + | these sources in predictive models. In this talk, I will present an overview of our efforts in these areas: | ||
| + | applying advanced analytical techniques to characterize the S/VOCs in smoke as a function of fuel | ||
| + | species and component, and the S/VOCs over the LA Basin during COVID-19 shelter-in-place | ||
| + | restrictions; using chemically-detailed box models to develop air quality model parameterizations; and | ||
| + | developing a comprehensive emissions inventory that is broadly useful in air quality model applications." | ||
| + | |||
| + | <font size=4>Monday, 22 February 2021</font size=4><br> | ||
| + | <b>An Investigation of the Gas-Phase Products of NO3 Radical Oxidation of Δ-3-Carene</b> | ||
| + | |||
| + | Olivia Jenks, <br> | ||
| + | ANYL 3rd year, [https://sites.google.com/view/de-gouw-lab de Gouw Group] | ||
| + | |||
| + | "Organic aerosol can have an impact on the climate, visibility, and human health. Oxidation of biogenic volatile organic compounds forms secondary organic aerosol (SOA) by lowering the volatility of the molecule and partitioning to the particle phase. The NO3-initiated oxidation of Δ-3-carene is being studied because it connects the interaction of biogenic and anthropogenic emissions to form aerosol, and expands on previous work with ɑ- and β-pinene. In this work, I explore the first generation gas-phase products of the NO3-initiated oxidation of Δ-3-carene in a 8 m3 Teflon FEP chamber using a Vocus proton-transfer-reaction time-of-flight mass spectrometer (Vocus PTR-ToF) and a high-resolution time-of-flight chemical-ionization mass spectrometer (HR ToF-CIMS) using iodide adducts. The mass spectra of reaction products show the presence of many of the expected products, including hydroxynitrates, dicarbonyls, hydroxycarbonyl nitrates, hydroxy dicarbonyls, and dicarbonyl hydroxynitrates, but also show significant fragmentation of the parent ions through loss of water and/or nitric acid. Parent and fragment ions are grouped together taking advantage of gas-wall interactions in Teflon tubing, which provide some simple “poor-person’s chromatography,” while transmitting all the species. Understanding the mechanism of the oxidation of Δ-3-carene by NO3 radicals allows for a better understanding of the fate of organic nitrate in the atmosphere, which will improve interpretation of field data and global chemical models." | ||
| + | |||
| + | <font size=4>Monday, 15 February 2021</font size=4><br> | ||
| + | <b>Towards improved understanding of nitrogen dioxide emissions from forest fires</b> | ||
| + | |||
| + | Debora Griffin, <br> | ||
| + | Environment Canada, | ||
| + | |||
| + | "Smoke from wildfires are a significant source of air pollution, which can adversely impact ecosystems and the air quality in downwind populated areas. With increasing severity of wildfires over the years, these are a significant threat to air quality in densely populated areas. Emissions from wildfires are most commonly estimated by a bottom-up approach, using proxies such fuel type, burn area, and emission factors. Emissions are also commonly derived with a top-down approach, using satellite observed Fire Radiative Power. Furthermore, wildfire emissions can also be estimated directly from satellite-borne measurements. | ||
| + | |||
| + | I will present recent advancements and improvements of direct emission estimates of forest fire NOx emissions by using TROPOMI (Tropospheric Monitoring Instrument) high-resolution satellite datasets, including NO2 vertical column densities (VCDs) and information on plume height and aerosol scattering. The effect of smoke aerosols on the sensitivity of TROPOMI to NO2 (via air mass factors) is estimated with recalculated VCDs, and validated with aircraft observations. Different top-down emission estimation methods are tested on synthetic data to determine the accuracy, and the sensitivity to parameters, such as wind fields, satellite sampling, instrument noise, NO2:NOx conversion ratio, species atmosphere lifetime and plume spread. Lastly, the top-down, bottom-up and direct emission estimates of fire emissions are quantitatively compared." | ||
| + | |||
| + | <font size=4>Monday, 1 February 2021</font size=4><br> | ||
| + | <b>Understanding the Earth from a thermodynamic systems perspective</b> | ||
| + | |||
| + | [https://www.bgc-jena.mpg.de/index.php/BTM/AxelKleidon Axel Kleidon], <BR> | ||
| + | Max Planck Institute | ||
| + | |||
| + | "The Earth is a vastly complex system that converts the energy contained in sunlight into various forms, from the kinetic energy of motion to chemical energy of life and the electric energy that powers human societies. These conversions follow the laws of thermodynamics, which set the directions and fundamental limits, yet these also result in interactions and feedbacks that emphasise the need for an Earth system perspective. In this talk, I provide the background for this thermodynamic description of the Earth system and use examples to show how thermodynamic limits in combination with a formulation of the dominant interactions can be used to describe the emergent behaviour. I show how this approach can be used to provide simple, yet physically-based estimates of climate and climate change, as well as how it sets limits on different forms of renewable energy. I close with an outlook on potential future applications, highlighting the generality of the approach as energy, its conversions into other forms, and interactions are at the very core of literally every Earth system process." | ||
| + | |||
| + | <font size=4>Monday, 25 January 2021</font size=4><br> | ||
| + | <b>Peroxy radicals are getting curiouser and curiouser </b> | ||
| + | |||
| + | [https://www.cmu.edu/epp/people/faculty/neil-donahue.html Prof Neil Donahue], <br> | ||
| + | Carnegie Mellon University | ||
| + | |||
| + | "Organic peroxy radicals sit at the center of tropospheric chemistry. Essentially all oxidation processes produce peroxy radicals immediately after an oxidant attacks a stable organic molecule. They have gas-phase lifetimes ranging from 1-1000 seconds. They also have choices; peroxy radical branching defines tropospheric chemistry. In the textbook case, they can react either with hydroperoxy radicals or with nitric oxide, either terminating as hydroperoxides or propagating to dark places that only Paul Ziemann understands while producing nitrogen dioxide and ultimately ozone. However, we have recently found that organoperoxy radicals with additional oxygenated functional groups can undergo progressive “autoxidation” (oxidation in the presence of molecular oxygen only) via internal hydrogen atom transfer and subsequent oxygen addition. These oxygenated organoperoxy radicals also appear to react with each other very rapidly (though this is uncertain) and to produce covalently bound organoperoxides (though this should be spin forbidden). Some of these products can have exceptionally low vapor pressures, and when formed in the gas phase can thus drive new-particle formation. It is possible that this is a major new-particle formation process in the pristine atmosphere, and that it was the dominant particle formation process in the pre-industrial atmosphere. In this talk I will explore recent experimental findings from the CLOUD experiment at CERN as well as modeling frameworks we have been developing to represent this chemistry and to translate chemical behavior observed in chambers to real-world conditions." | ||
| + | |||
| + | ==Fall 2020== | ||
| + | <font size=4>Monday, 30 November 2020</font><BR> | ||
| + | <b>Prototype Readout System Software for The STAR Interlock Safety System at Brookhaven National Laboratory</b> | ||
| + | |||
| + | Joseph D'alesio, ANYL First Year | ||
| + | |||
| + | "RHIC, located at BNL, collides nuclei at relativistic speeds to artificially recreate the initial conditions of the universe. The STAR Collaboration studies these collisions using a detector, the Solenoidal Tracker At RHIC. The Interlock Safety System is responsible for monitoring and displaying parameters in the STAR control room. These parameters include the temperature and pressure of the TPC gas mixture, the Oxygen Deficiency Hazard status, the Uninterruptable Power Supply status and the water cooling system status. If these parameters fall outside an accepted range, alarms will sound to notify the control room. The readout system and software allow for the shift operator to adjust detector variables while the experiment is running and thus prevent circumstances in which fires and explosions are likely. This project focuses on upgrades to the Interlock monitoring system. The current Interlock Readout monitor uses a VME to communicate with various STAR systems while the upgraded monitor uses a Programmable Logic Controller interfaced to a PC. Device support for the upgraded monitor has been written and compiled using a prototype input/output controller program to communicate to the new readout PLC. Functionally, the existing and upgraded system will have the same capabilities. However, the new readout system will be easier to maintain and more easily updated to include, for example, additional safety signal outputs. In turn, this will result in a critical readout system prepared for future operations." | ||
| + | |||
| + | <font size=4>Monday, 23 November 2020</font><BR> | ||
| + | <b>First satellite mapping of nitrous acid (HONO) in wildfire plumes</b> | ||
| + | |||
| + | Dr. Nicolas Theys, <br> | ||
| + | Royal Belgian Institute for Space Aeronomy (BIRA-IASB) | ||
| + | |||
| + | "Nitrous acid (HONO) is a short-lived reactive gas and plays a key role in the atmosphere through its | ||
| + | influence on the OH budget, and contributes to secondary aerosols and ozone formation. Laboratory | ||
| + | experiments and aircraft field campaigns have revealed that biomass burning is a source of HONO. | ||
| + | However, the global importance of pyrogenic HONO is poorly constrained. | ||
| + | Here we present the first global measurements of HONO using the TROPOspheric Monitoring Instrument | ||
| + | (TROPOMI) onboard Sentinel-5 Precursor. Using Differential Optical Absorption Spectroscopy (DOAS) | ||
| + | applied in the UV, we analyzed one year of TROPOMI data and found that HONO is unambiguously | ||
| + | detected in wildfire plumes from main biomass burning regions, consistent with volume mixing ratios of | ||
| + | several ppbvs of HONO. | ||
| + | |||
| + | During July-September 2018, the University of Colorado participated to a field campaign (Biomass Burning | ||
| + | Fluxes of Trace Gases and Aerosols (BB-Flux)), performed research flights near fire plumes in the US and | ||
| + | successfully detected HONO for several of them using the CU-DOAS aircraft instrument. For some cases, | ||
| + | flights were planned around the TROPOMI overpass. We exploit this unique opportunity to validate our | ||
| + | satellite HONO retrievals. We discuss the comparison results and reasons for discrepancies. In particular, | ||
| + | viewing observations are very different and can lead to air mass sampling differences in the presence of | ||
| + | elevated aerosols. To overcome this problem, we compared the ratio HONO/NO2 (RHN), which is also a | ||
| + | proxy for HONO production. We found that satellite and aircraft RHNs are in excellent agreement, within | ||
| + | their mutual error estimates. From the global RHNs, we demonstrate that previous assessments | ||
| + | underestimate pyrogenic HONO emissions by a factor of 2–4 across all ecosystem types. | ||
| + | |||
| + | Finally, we performed model simulations to assess the impact of HONO on atmospheric oxidants, such as | ||
| + | OH and tropospheric ozone. We estimate that HONO emissions are responsible for two-thirds of the OH | ||
| + | production in fresh wildfire plumes worldwide and act to accelerate oxidative plume chemistry and ozone | ||
| + | production. Our findings suggest that pyrogenic HONO emissions have a substantial impact on | ||
| + | atmospheric composition, which enhances regional ozone levels by up to 7 ppbv." | ||
| + | |||
| + | <small>N. Theys, R. Volkamer, J.-F. Müller, K. Zarzana, N. Kille, L. Clarisse, I. De Smedt, C. Lerot, H. Finkenzeller, F. Hendrick, T. Koenig, C.F. Lee, C. Knote, H. Yu, M. Van Roozendae. </small> | ||
| + | |||
| + | <font size=4>Monday, 16 November 2020</font><BR> | ||
| + | <b>Role of the Metal Support Interface in H2 Activation on Supported Gold Nanoparticles</b> | ||
| + | |||
| + | Alexander Bradley, | ||
| + | ANYL 1st year student | ||
| + | |||
| + | "Global Hydrogen production exceeds 50 million tons per year, largely for the production of ammonia. Given its importance, a fundamental understanding of hydrogen activation is vital when designing new catalysts. Hydrogen activation on gold catalysts is poorly understood and understudied, and the weak adsorption effects allow only a few types of experimental measurements. We have developed a kinetic model for analyzing hydrogen binding parameters on gold, and a method for extracting a proposed mechanism. | ||
| + | |||
| + | Hydrogen is thought to activate homolytically on late transition metals, but recent evidence suggests that it activates heterolytically at the metal-support interface of supported gold catalysts. The thermodynamic properties of this reaction were calculated by Van’t Hoff analysis. Enthalpy and entropy terms were found to be much lower than popular supported metals (Ni, Rh, etc). Kinetic Isotope Effect (KIE) experiments were carried out to determine the nature of the hydrogen activation, and whether the mechanism includes a proton coupled electron transfer (PCET) step." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Theoretical Examination of Isoprene’s Behavior at the Air-Water Interface</b> | ||
| + | |||
| + | Kyle McMillan, | ||
| + | ANYL 1st year student | ||
| + | |||
| + | |||
| + | "Isoprene is the most widely emitted biogenic hydrocarbon in the atmosphere (approximately 500 Tg | ||
| + | emitted annually), making it a significant player in atmospheric chemistry. So far, most research | ||
| + | concerning isoprene’s role in the atmosphere has examined its gas-phase chemistry with little | ||
| + | consideration given to its potentially significant chemistry within clouds. The purpose of this study was to | ||
| + | elucidate isoprene’s behavior at the air-water interface of a water droplet. To do this, high level molecular | ||
| + | dynamics (MD) calculations were utilized to simulate isoprene’s interaction with a droplet of 10-nm | ||
| + | diameter. The data generated by these calculations were then used to describe both isoprene’s | ||
| + | conformational dynamics and chemical group orientations at the air-water interface, looking closely at the | ||
| + | torsion angles of both its s-trans and s-gauche conformers as well as the distances between each of its | ||
| + | atoms and the water molecules comprising the water droplet. Though the results of this study were not | ||
| + | wholly conclusive, due primarily to incomplete datasets generated by the MD calculations, there were | ||
| + | some indications of potentially favorable interactions of isoprene with the air-water interface, especially | ||
| + | for its s-gauche conformer. Should the air-water interface selectively alter the chemical properties of | ||
| + | either of isoprene’s two major conformers (for example, by augmenting the reactivity of either toward a | ||
| + | major atmospheric oxidant such as OH) this would have clear implications for isoprene’s chemistry in the | ||
| + | troposphere, which has been shown to vary significantly with conformational state." | ||
| + | |||
| + | <font size=4>Monday, 9 November 2020</font><BR> | ||
| + | <b>Sources of Formaldehyde in Bountiful, Utah</b> | ||
| + | |||
| + | [https://www.snow.edu/academics/science_math/chemistry/ryan_thalman.html Ryan Thalman], <br> | ||
| + | Snow College | ||
| + | |||
| + | "Starting in 2013, the mean concentration of HCHO measured in Bountiful, UT exceeded the non-cancer risk threshold and the 1 in 1 million cancer risk threshold. In addition, the measured concentrations were more than double those found at surrounding locations in Utah. A Positive Matrix Factorization (PMF) analysis using PMF-EPA v5 was done using historical data (2004-2017) to better understand the sources of formaldehyde in the region. The historical data set is composed of samples that were collected every sixth day on a 24-hour basis. Beginning in February 2019 an eight-week air sampling campaign measuring formaldehyde on a two-hour averaged basis was initiated. Formaldehyde was measured using a Broadband Cavity Enhanced Absorption Spectrometer (BBCEAS). In addition, the concentrations of NO, NO<sub>2</sub>, and O<sub>3</sub> were measured. Two-hour averaged measurements of benzene, toluene, ethylbenzene, and xylenes (BTEX) measurable by gas chromatography-flame ionization detection were also done. Corresponding back wind trajectory calculations for selected time periods were calculated to aid in the understanding of the effects of BTEX emission sources and formaldehyde formation. | ||
| + | |||
| + | |||
| + | I will also discuss career options related to teaching and research at Junior and Community Colleges." | ||
| + | |||
| + | <font size=4>Monday, 2 November 2020</font><BR> | ||
| + | |||
| + | <b>Iron polypyridyl complexes for electrocatalytic proton reduction</b> | ||
| + | |||
| + | Zachary Schiffman, ANYL 1st year student | ||
| + | |||
| + | "In recent decades the rate of human consumption has accelerated dangerously. This is especially true of energy, the usage of which is unsustainable for long-term continuation at the current rate. We should then turn to renewable energy using materials that can be used infinitely, as opposed to fossil fuels which are notably finite and are contributing to the continuously rising levels of greenhouse gas pollution. | ||
| + | |||
| + | Solar energy would be a source of clean energy. An idea for a method of harnessing and storing solar energy is inspired by Earth’s plant life. In the process of photosynthesis, plants take in sunlight and store the solar energy in the form of chemical bonds. Just as plants reduce carbon dioxide to produce sugars as fuel, we look to use the energy of the sun to reduce protons from water into hydrogen gas, which burns cleanly with no CO2 emission. Metal-based complexes have been developed which can act as catalysts for this hydrogen evolution reaction (HER). Many of these complexes are synthesized in lengthy, low-yielding processes. In terms of developing practical technology that can be used on a large scale, it is best to synthesize these complexes in a straightforward, easy synthesis using inexpensive, commercially available materials. | ||
| + | |||
| + | We produced a tridentate bismethylpyridyl amine bound to an iron (III) center. This complex is synthesized from commercially available materials in high yield, and was shown to be an active electrocatalyst for proton reduction. Cyclic voltammetric techniques were used to verify and benchmark the complex’s activity and efficiency as an electrocatalyst. It was also shown to be active as a pre-catalyst in a three-component system for photocatalytic hydrogen generation. To this end, we seek to simplify our catalyst precursors to produce a readily synthesized complex that can be obtained at low cost and in high yields. This may take us one step closer to developing a wide-spread, accessible system for artificial photosynthesis." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Synthesis of Phosphate Diesters for use as Ligands on Bismuth Catalysts</b> | ||
| + | |||
| + | Bri Dobson, | ||
| + | ANYL 1st year | ||
| + | |||
| + | "New catalysts can simplify synthetic schemes or open up new possibilities in synthetic chemistry. In particular, bismuth phosphates have shown promise as an expanding family of green catalysts. Some bismuth compounds have Lewis acid catalyst properties but need to be made more effective in order to be used industrially. Phosphate diesters with varying ester groups may be able to tune the activity of these catalysts to fit specific reactions. With the final goal of creating a tunable bismuth phosphate diester catalyst, this project attempted to find a simple, but effective method for synthesizing a variety of phosphate diesters, which could then be used as ligands on homogenous bismuth phosphate diester Lewis acid catalysts for a variety of different synthetic uses. Two schemes for phosphate diester synthesis were attempted. One of the two was successful in the synthesis of pentamethylene phosphate. Further testing with different reagents is needed to establish the applicability of this scheme to the synthesis of other phosphate diesters, but the preliminary results are promising." | ||
| + | |||
| + | <font size=4>Friday, 30 October 2020</font><BR> | ||
| + | |||
| + | <b>Speciation and Transformation of Reduced Nitrogen in the Atmosphere: A Laboratory and Field Investigation</b> | ||
| + | |||
| + | Aroob Abdelhamid, | ||
| + | [https://sites.google.com/view/brownelab/people Browne Group] | ||
| + | |||
| + | "Reduced nitrogen compounds negatively affect air quality, climate, and human health. These | ||
| + | compounds have been shown to enhance new particle formation, promote brown carbon | ||
| + | formation, and increase the toxicity of aerosol. Little is known about the impact reduced nitrogen | ||
| + | has on either the gas phase or aerosol because measurements detailing their speciation and | ||
| + | interactions with aerosol is lacking. Due to increases in food production, the concentrations of | ||
| + | reduced nitrogen compounds are expected to increase in the future, and thus it is important to | ||
| + | understand their speciation and transformations in the atmosphere. | ||
| + | In the first and second studies, I present data from the Holistic Interaction of Shallow Clouds, | ||
| + | Aerosols, and Land Ecosystem (HISCALE) field campaign conducted in August- September, | ||
| + | 2016 in the agricultural region of Lamont, OK. The purpose of the field campaign was to study | ||
| + | the true atmospheric speciation of ambient reduced nitrogen. | ||
| + | For the first study, I measured positive ambient ions, which have been shown to promote new | ||
| + | particle formation, and are largely composed of organic nitrogen (ON) compounds. Ions help | ||
| + | inform our understanding of high proton affinity trace gases because neutral compounds that are | ||
| + | below the detection limit of normal instruments are likely to get ionized and seen as ions. I | ||
| + | observed water, small organic nitrogen compounds, alkylpyridines, and compounds in the m/z | ||
| + | 250 – 400 range. The ions in the high mass region had not been identified prior to my work. | ||
| + | Once I identified the ON in the high mass region, I determined that these high mass ions have a | ||
| + | diurnal cycle and were correlated with neutral ammonia measurements. Importantly, these | ||
| + | compounds were only present in the latter half of the field campaign despite ammonia being | ||
| + | present throughout the entirety of the field campaign, indicating the complexity of these | ||
| + | compounds’ sources. | ||
| + | |||
| + | I then discuss ambient neutral compounds seen in HISCALE. These compounds were measured | ||
| + | with an ethanol Chemical Ionization Mass Spectrometry (CIMS), an instrument sensitive to | ||
| + | reduced nitrogen compounds. Ammonia, methylamine, and trimethylamine showed morning | ||
| + | signal spikes that I suggest are from evaporating dew. I measured amides which showed a | ||
| + | nighttime spike that is unexplained with current emissions of amides and indicates an unknown | ||
| + | local amide source. Finally, I observed imines, which can be partially explained through a | ||
| + | combination of aerosol uptake and amine processing, but the diurnal cycle cannot be fully | ||
| + | explained. | ||
| + | |||
| + | I finally present work that focuses on understanding the ON formed upon reactive uptake of | ||
| + | ammonia into biogenic secondary organic aerosol done in a collaboration with the University of | ||
| + | Eastern Finland. I show that increasing the ammonia concentration in aerosol promoted greater | ||
| + | production of a variety of ON, especially ON with larger carbon numbers. Ammonium in the | ||
| + | particle created low volatility ON, while increasing ammonia concentrations lead to the | ||
| + | formation of mid-volatility ON. Finally, I observed that ON volatility is up to 3 orders of | ||
| + | magnitude lower than the volatility of biogenic SOA compounds lacking nitrogen incorporation. | ||
| + | My work helps constrain the impact of ammonia uptake on aerosol." | ||
| + | |||
| + | <small>Projects: | ||
| + | <ul> | ||
| + | <li>Abdelhamid, A.; Stark, H.; Worsnop, D. R.; Nowak, J. B.; Browne, E. C. Measurement of | ||
| + | Organic Nitrogen Positive Ions as Part of the HISCALE Field Campaign. in prep</li> | ||
| + | <li>Abdelhamid, A.; Buchholz, A.; Pullinen, I.; Schobesberger, S.; Virtanen, A.; Browne, E. | ||
| + | Nitrogen Incorporation in Biogenic Aerosol and its Effect on Aerosol Volatility. in prep</li> | ||
| + | <li>Abdelhamid, A.; Stark, H.; Nowak, J. B.; Kimmel, J. R.; Smith, J.; Jayne, J. T.; Worsnop, D. R.; | ||
| + | Browne, E. C. Nitrogen Chemistry in a Rural Environment: Neutral Measurements during the | ||
| + | HISCALE Field Campaign. in prep</li> | ||
| + | </ul></small> | ||
| + | |||
| + | <font size=4>Monday, 26 October 2020</font><BR> | ||
| + | <b>Variability and diel dependence of O<sub>3</sub> – NO<sub>x</sub> – VOC chemistry in western wildfire plumes: Results from the NOAA Twin Otter during FIREX-AQ</b> | ||
| + | |||
| + | Michael Robinson, | ||
| + | ANYL 1st year | ||
| + | |||
| + | "The variability in photochemical ozone production from western wildfire plumes is important to the accurate prediction of their influence on North American air quality. A set of photochemical measurements including ozone, nitrogen oxides, photolysis rates and a suite of volatile organic compounds (VOCs), were made from the NOAA Twin Otter research aircraft as a part of the Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ) experiment. This research aircraft sampled nine unique fire complexes in five western states. Typical research flight days included flights during the afternoon, evening and night. In general, observed ozone production in the sampled plumes is rapid, reaching maximum ozone within 30 minutes downwind. A 0-D box modeling tool was developed to further probe the chemistry driving ozone production in these plumes. This tool was used to calculate afternoon and evening ozone isopleths which allows for a comparison of each individual fire complex in a common framework. The analysis shows the sensitivity of ozone production to initial NO<sub>x</sub> and VOC emissions. However ozone isopleths are not capable of describing the temporal transition of NO<sub>x</sub> and VOC sensitive chemical regimes. A radical budget approach is used for probing the temporal evolution of the chemistry. Afternoon photochemical plumes display a rapid transition from VOC sensitive chemistry to NO<sub>x</sub> sensitive chemistry, mainly driven by radical production from photolysis of HCHO and HONO emitted directly from the fire. Evening photochemical plumes exhibit a slower transition from NO<sub>x</sub> sensitive chemistry to VOC sensitive chemistry, with a larger portion radical production from alkene ozonolysis." | ||
| + | <br><small> | ||
| + | Michael A. Robinson, Zachary C.J. Decker, Kelley C. Barasanti, Matthew M. Coggon, Frank M. Flocke, Carley D. Fredrickson, Georgios I. Gkatzelis, Christopher D. Holmes, Denise D. Montzka, Brett B. Palm, R. Bradley Pierce, Rebecca H. Schwantes, Geoffrey S. Tyndall, Joel A. Thornton, Paul Van Rooy, Andrew J. Weinheimer, and Steven S. Brown. </small> | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Model Inter-comparisons of Inorganic Nitrate Formation During KORUS-AQ Campaign</b> | ||
| + | |||
| + | Seonsik Yun, | ||
| + | ANYL 1st year | ||
| + | |||
| + | "Nitrogen oxides play an important role in global tropospheric chemistry. The lifetime of nitrogen oxides is controlled by organic and inorganic nitrate formation. GEOS-Chem, a global 3-D chemical transport model, has historically had an over-estimation problem of inorganic nitrates relative to surface observation networks. This study investigated this problem by using model inter-comparisons with six different chemical transport model simulations during the KORUS-AQ field campaign period. N<sub>2</sub>O<sub>5</sub> hydrolysis reaction, a primary nocturnal inorganic nitrate formation, was investigated to compare differences in calculations of inorganic nitrate formation between the model simulations. Differences in heterogeneous loss of N<sub>2</sub>O<sub>5</sub> between the models were analyzed with the uptake coefficient, aerosol surface area density, and N<sub>2</sub>O<sub>5</sub> concentration. Results show that high N<sub>2</sub>O<sub>5</sub> concentrations at nighttime in GEOS-Chem could be attributed to the high O<sub>3</sub> concentrations at nighttime compared to the Ensemble model, which can contribute to the over-estimation of inorganic nitrates in GEOS-Chem." | ||
| + | |||
| + | <font size=4>Monday, 12 October 2020</font><BR> | ||
| + | |||
| + | <b>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</b> | ||
| + | |||
| + | [https://sites.google.com/site/ziemanngroup/home Paul Ziemann], <br> | ||
| + | ANYL Faculty, CU Boulder | ||
| + | |||
| + | "Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative studies of indoor air chemistry at CU." | ||
| + | |||
| + | <font size=4>Monday, 21 September 2020</font><BR> | ||
| + | |||
| + | <b>Organic nitrogen chemistry: Recent results and future projects</b> | ||
| + | |||
| + | [https://sites.google.com/view/brownelab/home?authuser=0 Eleanor Browne], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Organic nitrogen is a ubiquitous atmospheric component that affects biogeochemistry, air quality, and climate. Assessing the impact of organic nitrogen on these processes remains challenging because traditional measurement techniques have lacked the sensitivity and chemical resolution to characterize the speciation and chemistry of organic nitrogen. Here, I will discuss measurements made with protonated ethanol cluster chemical ionization time-of-flight mass spectrometry during the Holistic Interactions of Shallow Clouds, Aerosols, and Land-Ecosystems (HI-SCALE) campaign at the Southern Great Plains research station in Lamont, Oklahoma. As the site is located in an agricultural region, reduced nitrogen compounds are prevalent. I will present measurements of novel compounds including imines and urea and will discuss the sources and sinks of compounds at this site. Finally, I will discuss research opportunities in our group." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Small molecules in the Anthropocene: Wildfires, Oceans and Iodine</b> | ||
| + | |||
| + | [https://volkamergroup.colorado.edu/ Rainer Volkamer], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "The Volkamer group develops advanced optical instrumentation (in situ and remote sensing) to measure small molecules and aerosols that are relevant to public health and climate. We seek to better quantify and understand emissions of small molecules, total carbon, and aerosols from natural and managed ecosystem (e.g., wildfires, oil & natural gas, ocean surface, UTLS), and develop a molecular level understanding of the fundamental processes that affect their chemical transformations and sinks (e.g., new particle formation). Opportunities for graduate research exist in the areas of 1) field measurements using research aircraft (i.e., BB-FLUX, TI3GER projects), 2) laboratory experiments of nanoparticle formation and growth (incl. at CLOUD/CERN), and 3) instrumentation to study carbon closure, and retrieve aerosol optical properties." | ||
| + | |||
| + | <font size=4>Monday, 14 September 2020</font><BR> | ||
| + | |||
| + | <b>Chemistry of Volatile Organic Compounds in the Atmosphere</b> | ||
| + | |||
| + | [https://sites.google.com/view/de-gouw-lab Joost de Gouw], | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products. | ||
| + | |||
| + | In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments. | ||
| + | |||
| + | Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments. From the results we learn about the sources of VOCs from people, chemical products and building materials, the chemical transformations of these VOCs and other loss process such as surface uptake and ventilation to the atmosphere. Second, we are working on the emissions and chemistry of VOCs released from volatile chemical product (VCP) use to the atmosphere, which was recently discovered to be the dominant source of VOCs in urban air. In this research we make measurements of VOCs in urban air, separate the different emission sources and describe the chemical transformations of VOCs after emission. Finally, we are working on a chamber study to better understand the formation of secondary organic aerosol from biogenic VOCs." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Laboratory Studies of Contact Efflorescence in a Long Working Distance Optical Trap</b> | ||
| + | |||
| + | [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Maggie Tolbert], | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Because homogeneous salt efflorescence typically requires low relative humidity (RH), atmospheric salt particles are often assumed to be aqueous throughout much of their atmospheric lifetime. Here we use a long working distance optical trap to examine heterogeneous efflorescence that occurs when a supersaturated salt droplet comes into contact with a solid particle. We compare our findings for pure salt droplets with those obtained for salt/organic droplets where the organic may be found either in a core shell structure or as an amorphous/glassy solid. We find that in many cases, single collisions with a range of nuclei promote efflorescence at relatively high RH, causing salt particles to be solid for more of their atmospheric lifetime. Similar experiments are proposed in the future to examine the role of contact in ice cloud nucleation." | ||
| + | |||
| + | <font size=4>Monday, 31 August 2020</font><BR> | ||
| + | |||
| + | <b>Understanding aerosols for pollution, climate change, and disease transmission</b> | ||
| + | |||
| + | [http://cires1.colorado.edu/jimenez/ Jose-Luis Jimenez], <br> | ||
| + | ANYL Faculty | ||
| + | |||
| + | "Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly present results from different projects over the last year, especially those most relevant to first year graduate student opportunities. I will introduce our aircraft research program, including results from the recent NASA/NOAA FIREX-AQ wildfire smoke study, where we obtained near-molecular level aerosol speciation data at 1 second time resolution. I will discuss our indoor air research, and hopefully convince you that you should never use black conductive tubing except for non-volatile aerosols. I will also describe the importance of aerosols for disease transmission, a topic in which I have been working with many of the world leaders since March 2020. I will summarize the reasons why I think COVID-19 transmission is driven by aerosols, with a smaller fraction of surface transmission, and with a minor contribution of ballistic “WHO” droplets from coughing and sneezing. I will present some ideas about how to protect ourselves better from COVID-19 in the coming months and from other respiratory diseases in the future. | ||
| + | |||
| + | For people interested in COVID-19 aerosol transmission, you can find some selected resources linked here: https://twitter.com/jljcolorado/status/1298246684539940866 and a draft summary of the evidence here: https://docs.google.com/presentation/d/11rY9tQtkFaV_M4N-hf5qp1_Xtuw8JYb4Qvv5e7BEmcc/edit# | ||
| + | " | ||
| + | |||
| + | ==Summer 2020== | ||
| + | <font size=4>Friday, 10 July 2020</font><BR> | ||
| + | |||
| + | <B>Laboratory Studies of Secondary Organic Aerosol Formation and Heterogeneous/Multiphase Chemistry</b> | ||
| + | |||
| + | ANYL PhD Defense, Julia Bakker-Arkema, <br> | ||
| + | [https://sites.google.com/site/ziemanngroup/home Ziemann Group] | ||
| + | |||
| + | "The oxidation of volatile organic compounds (VOCs) in the atmosphere to form secondary organic aerosol (SOA) is known to influence climate by directly scattering and absorbing incoming radiation, and by participating in cloud formation and processing. Furthermore, SOA decreases visibility, negatively impacts human health, and provides surfaces for heterogeneous and multiphase chemistry to occur, reactions that can alter the composition and properties of the aerosol. However, mechanisms of SOA formation and the chemistry that can occur within and on the surface of the SOA remain poorly understood. This thesis presents three laboratory studies of VOC oxidation and SOA formation, and heterogeneous/multiphase chemistry, which can provide insights and improve our understanding of the complex chemistry of the atmosphere. The first study investigates the oxidation of linear 1-alkenes by OH radicals as a model system for the oxidation of alkenes in the atmosphere. Due to discrepancies in past measurements, the molar yields and branching ratios for the formation of β-hydroxynitrates, important alkene oxidation products in the atmosphere, were measured in a series of environmental chamber experiments. The measured β-hydroxynitrate branching ratios indicate that the yields of organic nitrates are lower for alkenes than for alkanes. This work also presents an extensive inventory of the inherent sources of uncertainty when performing quantitative measurements of reaction products in environmental chamber studies, which can be applied to other quantitative studies of VOC oxidation and SOA formation. The second study presents a full quantitation of the major gas- and particle-phase products for the same model alkene system, 1-alkenes + OH radicals in the presence of NOx, using a suite of sampling methods and analytical techniques. This work achieves mass closure for the products of the OH addition pathway, and when combined with past measurements, demonstrates that the important oxidation pathways are captured in the reaction mechanism. The measured product yields are used to calculate branching ratios for key reaction pathways, including for the isomerization and decomposition of β-hydroxyalkoxy radicals, important reaction intermediates. The final study determines kinetics and equilibria for the condensed phase reactions of hydroperoxides and aldehydes to form peroxyhemiacetals, a model system for the analogous heterogeneous/multiphase reactions in atmospheric SOA. The synthesis of a hydroperoxide probe molecule allows for the measurements of rate constants and equilibrium constants even in complex matrices, such as SOA formed from the ozonolysis of α-pinene. The results demonstrate that timescales of peroxyhemiacetal can be short in SOA, and thus may be important in the atmosphere." | ||
| + | |||
| + | <font size=4>Thursday, 4 June 2020</font><BR> | ||
| + | |||
| + | <b>Development of Solar Occultation Flux Spectroscopy for Ground and Airborne Applications</b> | ||
| + | |||
| + | ANYL PhD Defense, Natalie Kille, <br> | ||
| + | [http://volkamergroup.colorado.edu/ Volkamer Group] | ||
| + | |||
| + | Air quality is a major issue that directly affects human health, ecosystems, and climate. Area sources such as natural gas production, agriculture, and wildfires emit primary pollutants that undergo transport and chemical transformation in the atmosphere that leads to the formation of secondary pollutants. Signatures of air quality impacts are being observed on local, regional, and global scales. However, there is a need for better measurement techniques to quantify trace gas emissions from area sources. Better understanding of emissions directly relates to lowering the uncertainty in model predictions of the impacts of pollution on human health, ecosystem, and climate. | ||
| + | |||
| + | I present the development and scientific results of the University of Colorado Solar Occultation Flux instrument (CU SOF) from ground and airborne applications to address the need for better emission quantification of trace gases from area sources. Specifically, the CU SOF instrument consists of a digital mobile solar tracker coupled to a Fourier transform spectrometer that can simultaneously be coupled to a UV-Visible spectrometer. The direct-sun observations made by CU SOF provide high photon flux, are independent of boundary layer height, and measure the vertical column density of trace gases directly in the open atmosphere without any inlets. These characteristics allow the CU SOF instrument to measure trace gas emission fluxes from area sources using the mass balance method. | ||
| + | |||
| + | Scientific results from the CU SOF instrument are described from four field campaigns. During the FRAPPE campaign, ammonia fluxes from concentrated animal feeding operations in Colorado are quantified, and found to be a factor of 2-10 higher than expected. Furthermore, feedlot soils are found to be a significant source of NOx, which is a missing NOx source in national inventories, and relevant in semi-polluted and remote locations where ozone formation is NOx sensitive. Next, in collaboration with the Karlsruhe Institute of Technology, CU SOF and three COCCON type instruments were used to source apportion methane emissions in the Colorado Front Range. A tracer method is employed finding that natural gas sources dominate over biogenic sources. Finally, the CU SOF instrument was deployed from a research aircraft during the Pre-BB-FLUX and BB-FLUX campaigns. Emission fluxes from nine trace gases relevant to wildfires are measured. Carbon monoxide emissions spanning over four orders of magnitude are observed for a variety of wildfire sizes. CU SOF measurements are compared with pyrogenic carbon monoxide emission predictions from seven different satellite-based emission inventories, and are shown to reduce the uncertainty in emissions from a factor 35 to about a factor of 2. These improvements in understanding of emissions directly scale the better predictions of air quality impacts in the form of ozone and particulate matter. | ||
| + | |||
| + | ==Spring 2020== | ||
| + | <font size=4>Monday, 27 April 2020</font><BR> | ||
| + | |||
| + | <b>Fires, fuels, and fluxes: Using airborne remote sensing to understand fire emissions</b> | ||
| + | |||
| + | Kyle Zarzana, postdoc, <br> | ||
| + | [http://volkamergroup.colorado.edu/ Volkamer Group] (1/2 seminar) | ||
| + | |||
| + | Biomass burning emits a complex mixture of gases and particles that can vary rapidly over short | ||
| + | spatial and temporal scales. Secondary chemistry driven in part by gas-phase radicals leads to | ||
| + | downwind formation of ozone and particles which affect climate and adversely impact public | ||
| + | health. Quantifying the emissions of radical precursors such as NO<sub>2</sub>, HONO, and carbonyls such | ||
| + | as HCHO is an important first step to constraining their role in smoke chemistry, but this can be | ||
| + | difficult due to plume inhomogeneities. Column measurements along the direct solar beam | ||
| + | integrate over the vertical variability, and when made from an airborne platform can be used to | ||
| + | determine mass fluxes on the scale of a wildfire. In this talk I will be presenting measurements | ||
| + | from the Biomass Burning Flux Measurements of Trace Gases and Aerosols (BB-FLUX) | ||
| + | campaign that was conducted in the northwest United States during the summer of 2018. The | ||
| + | University of Colorado Solar Occultation Flux (SOF) and the Zenith Sky Differential Optical | ||
| + | Absorption Spectroscopy (ZS-DOAS) instruments were deployed on the University of Wyoming | ||
| + | King Air research aircraft, and these instruments measured column densities of numerous gases, | ||
| + | including but not limited to CO, NH<sub>3</sub>, C<sub>2</sub>H<sub>6</sub>, HCN, PAN, HONO, NO<sub>2</sub>, and HCHO. Using a | ||
| + | combination of data from these two instruments I will present estimates of radical fluxes from | ||
| + | wildfires. Additionally, I will briefly touch on several other aspects of our BB-FLUX work, such | ||
| + | as determining carbon fluxes from fires. | ||
| + | |||
| + | and | ||
| + | |||
| + | <B>Constraining wildfire emission inventories using airborne flux measurements</b> | ||
| + | |||
| + | Johana Romero-Alvarez, postdoc, <br> | ||
| + | [http://volkamergroup.colorado.edu/ Volkamer Group] (1/2 seminar) | ||
| + | |||
| + | Wildfire emit significant amounts of trace gases and particulate matter that impact atmospheric | ||
| + | processes and human health, and accurate emissions are required to quantify determine the | ||
| + | impacts of fires. Numerous inventories have been developed to estimate emissions using either | ||
| + | measurements of fire radiative energy or of the burned area. These inventories often vary by | ||
| + | several orders of magnitude, even for inert species such as CO, and until recently opportunities | ||
| + | to valid these models have been limited. The University of Colorado Solar Occultation Flux (CU | ||
| + | SOF) instrument can determine highly time resolved fluxes for many species from wildfires, and | ||
| + | during the summer of 2018 was deployed as part of the Biomass Burning Flux Measurements of | ||
| + | Trace Gases and Aerosols (BB-FLUX) campaign in the northwest United States. Over 100 | ||
| + | fluxes measurements of a variety of species including but not limited to CO, NH<sub>3</sub>, small alkanes, | ||
| + | and HCN, were performed on 18 different fires spanning different fuel types, fuel loadings, and | ||
| + | fire intensities. Additionally, highly detailed measurements of the fire areas were made post | ||
| + | campaign by the National Ecological Observatory Network and the US Forest Service. This rich | ||
| + | dataset provides a unique opportunity to evaluate and constrain emission inventories. | ||
| + | Comparisons between the measured fluxes and the FRP-based emissions inventory used in | ||
| + | HRRR-Smoke will be presented as well as experimentally derived conversion factors of FRP to | ||
| + | pyrogenic CO emissions. | ||
| + | |||
| + | <font size=4>Monday, 20 April 2020</font><BR> | ||
| + | <b>Atmospheric Aerosol Analysis by Online Extractive Electrospray Ionization Mass Spectrometry</b> | ||
| + | |||
| + | [https://www.psi.ch/en/lac/people/jay-gates-slowik Dr. Jay Slowik], <br> | ||
| + | Paul Scherrer Institute | ||
| + | |||
| + | Mass spectrometry is a powerful tool for the analysis of aerosol composition. However, tradeoffs | ||
| + | typically exist between the loss of chemical information due to thermal decomposition and/or | ||
| + | ionization-induced fragmentation on the one hand, and lower time resolution and/or separated | ||
| + | collection/analysis stages on the other. We address these issues through the development of an | ||
| + | extractive electrospray ionization time-of-flight mass spectrometer (EESI-TOF), which provides | ||
| + | online, highly time-resolved measurements of aerosol composition without significant | ||
| + | decomposition or fragmentation. Further, the EESI-TOF provides a versatile sampling/ionization | ||
| + | framework, as by simply changing the composition of the primary spray and mass spectrometer | ||
| + | polarity, the instrument can be configured to optimize detection of different organic fractions or | ||
| + | water-soluble metals, while the sampling inlet can be configured to allow separate detection of the | ||
| + | gas and particle phase. Two applications of the EESI-TOF are presented. First, we demonstrate rapid | ||
| + | intra-particle decomposition reactions in secondary organic aerosol generated from the dark | ||
| + | ozonolysis of α-pinene, as well as further reaction on the exposure of the aerosol to visible light. | ||
| + | Second, we explore the sources and processes governing SOA composition in complex urban | ||
| + | environments. | ||
| + | |||
| + | <font size=4>Monday, 6 April 2020</font><BR> | ||
| + | |||
| + | <b>Tweezing Out the Interfacial Properties that Control the Chemistry of Atmospheric Aerosol Particles</b> | ||
| + | |||
| + | [https://www.cmu.edu/me/people/sullivan.html Ryan Sullivan], Associate Professor<br> | ||
| + | Department of Chemistry, Department of Mechanical Engineering, Carnegie Mellon University | ||
| + | |||
| + | Atmospheric aerosol particles have important yet poorly understood effects on air quality, health, atmospheric chemistry, cloud microphysics, and climate change. All these impacts of aerosols are governed by their composition and chemical mixing state – how components are distributed amongst individual particles – and by their morphology or internal structure. Together these two properties determine what lies at each particle’s interface, and this in turn controls how the particle interacts with reactant gases, condensable vapors, radiation, water vapor, and clouds. We advanced the aerosol optical tweezers (AOT) technique to determine the morphology and chemical properties of phase-separated individual droplets that contain complex secondary organic aerosol (SOA). We can now perform AOT experiments on realistic mimics of mixed atmospheric particles to understand their interfacial properties and how these evolve. Our finding of the prevalence of a phase-separated core–shell morphology and the existence of a stable emulsified state of SOA have important new implications for the reactivity and impacts of atmospheric aerosol. Our recent high-accuracy measurements of droplet acidity have enabled novel explorations of the interplay between gas reactive uptake and the resulting changes in pH that can then drive phase separations and alter morphology, which in turn can impede further reactivity. | ||
| + | |||
| + | Biomass burning is a major global source of atmospheric pollutants and much research has focused on the carbonaceous emissions from wildfires. Biomass-burning aerosol is complex and highly heterogeneous, often containing appreciable amounts of inorganic solutes including chloride salts with unknown reactivity and implications for oxidant budgets and atmospheric chemistry. The natural production of a key nighttime reservoir of nitrogen oxides, N<sub>2</sub>O<sub>5</sub>(g), in biomass-burning smoke was identified through chamber experiments on authentic biomass-burning aerosol. Our discovery that N<sub>2</sub>O<sub>5</sub>(g) often reacts with the chloride salts in smoke aerosol from tall grasses to produce ClNO<sub>2</sub>(g) instead of HNO<sub>3</sub>(g) has important implications for the lifetime of nitrogen oxides and the impacts of biomass burning on atmospheric oxidants and photochemical smog production. The surprisingly low reaction probability of N<sub>2</sub>O<sub>5</sub>(g) was determined to be due to organic carbon coatings that protect the salt phases from the gaseous reactants but can be removed as the aerosol ages further. Salt deliquescence at high relative humidity can greatly increase the reaction probability as the hydrolysis of N<sub>2</sub>O<sub>5</sub> is driven by chemistry in aqueous phases. | ||
| + | |||
| + | |||
| + | <font size=4>Tuesday, 10 March 2020</font><BR> | ||
| + | <b>ANYL Dissertation Defense: Measurements of the Emissions and Reactions of Anthropogenic and Biomass Burning VOCs</b> | ||
| + | |||
| + | Zachary Finewax, <br> | ||
| + | CU Boulder, [http://sites.google.com/view/de-gouw-lab de Gouw Group] / [https://sites.google.com/site/ziemanngroup/home Ziemann Group] | ||
| + | |||
| + | "Organic carbon is ubiquitous in the atmosphere, present in the gas phase (volatile organic | ||
| + | compounds, VOCs) or as a suspension of liquid or solid particles in air (organic aerosol, OA). | ||
| + | Oxidation of VOCs leads to the production of OA, impacting climate, health and visibility. | ||
| + | Emissions of VOCs occur from a variety of sources, both outdoors and indoors, requiring | ||
| + | measurement to determine the sources, and laboratory studies to investigate the ability of a given | ||
| + | emission to produce OA. | ||
| + | |||
| + | First, laboratory studies investigated the oxidation of catechol, an important biomass | ||
| + | burning emission, under both daytime and nighttime conditions. The organic aerosol yield, yield | ||
| + | of its primary product, 4-nitrocatechol, and mechanism provide evidence that catechol is an | ||
| + | important precursor to biomass burning SOA. Vapor pressure and absorption spectra of 4- | ||
| + | nitrocatechol elucidate its fate in the atmosphere, and its role in potentially suppressing ozone | ||
| + | formation in biomass burning plumes. Second, laboratory studies were developed to study the | ||
| + | oxidation of resorcinol, an isomer of catechol with much lower volatility, under daytime and | ||
| + | nighttime conditions. The differences in organic aerosol yields, and products highlights the | ||
| + | difference in chemistry between the two isomers. Third, the development of a vaporizer for gas- | ||
| + | wall partitioning and particle generation is described. The procedure was designed to measure | ||
| + | gas-wall timescales and the extent of gas-wall partitioning using instrumentation commonly | ||
| + | available in an atmospheric chemistry laboratory. Quantitative particle generation is achieved, | ||
| + | allowing for the calibration of particle detection instruments. Lastly, this thesis presents results | ||
| + | from a field study at a university athletic center. Emissions of VOCs from individuals exercising | ||
| + | are quantified, sources of VOCs are characterized and chemistry of bleach with amino acids is | ||
| + | observed from products in the gas-phase. | ||
| + | |||
| + | This thesis advances our understanding of the chemistry governing OA production in biomass | ||
| + | burning plumes, improves the ability to study low volatility compounds in the laboratory, and | ||
| + | provides new insight on the impacts of exercise on indoor air quality. | ||
| + | |||
| + | <small> | ||
| + | <b>List of Publications</b><br> | ||
| + | 1. Finewax, Z.; Pagonis, D.; Claflin, M.; Handschy, A.; Brown, W.; Jenks, O.; Day, D. A.; | ||
| + | Lerner, B.; Jimenez, J. -L.; Ziemann, P. J.; de Gouw, J. A. Quantification and source | ||
| + | characterization of volatile organic compounds from exercising and application of | ||
| + | chlorine-based cleaning products in a university athletic center. In preparation for | ||
| + | submission to Indoor Air.<br> | ||
| + | 2. Claflin, M.; Pagonis, D.; Finewax, Z.; Handschy, A.; Day, D. A.; Brown, W.; Jayne, J. | ||
| + | T.; Worsnop, D. R.; de Gouw, J. A.; Lerner, B. Isomer-resolved measurements of indoor | ||
| + | air using an in situ gas chromatograph with automatic detector switching between Vocus | ||
| + | PTR-TOF-MS and EI-TOF-MS. In preparation for submission to Environmental Science | ||
| + | & Technology.<br> | ||
| + | 3. Finewax, Z.; Jimenez, J. -L.; Ziemann, P. J. Development and Application of a Low- | ||
| + | Cost Vaporizer for Rapid, Quantitative Addition of Organic Gases and Particles to an | ||
| + | Environmental Chamber. In preparation for submission to Aerosol Science and | ||
| + | Technology.<br> | ||
| + | 4. Finewax, Z.; de Gouw, J. A.; Ziemann, P. J. 2019, Products and secondary organic | ||
| + | aerosol yields from the OH and NO 3 radical-initiated oxidation of resorcinol. Earth and | ||
| + | Space Chemistry, 3: 1248 – 1259.<br> | ||
| + | 5. Finewax, Z.; de Gouw, J. A.; Ziemann, P. J. 2018, Identification, quantification, and | ||
| + | volatility of 4-nitrocatechol formed from reactions of catechol with OH and NO 3 radicals. | ||
| + | Environmental Science and Technology. 52: 1981 - 1989. </small> | ||
| + | |||
| + | |||
| + | <b>ANYL Dissertation Defense: Development and Application of Chemical Ionization Mass Spectrometry to for Measuring Terrestrial and Exoplanetary Organic Nitrogen</b> | ||
| + | |||
| + | Jennifer Berry, <br> | ||
| + | CU Boulder, [https://sites.google.com/view/brownelab Browne Lab] | ||
| + | |||
| + | "Nitrogen is one of the key elements that is essential to life, but it is mostly trapped in | ||
| + | unreactive molecular nitrogen. The form of nitrogen that is biologically, photochemically, and | ||
| + | radiatively active is called reactive nitrogen, of which organic nitrogen accounts for around | ||
| + | 30%. Organic nitrogen has remained relatively unstudied until the development of high | ||
| + | resolution mass spectrometric techniques. In this dissertation, I discuss the application of | ||
| + | chemical ionization mass spectrometry (CIMS) and atmospheric pressure interface time-of- | ||
| + | flight mass spectrometry (APi-ToF) in new experimental conditions, from pharmaceuticals to | ||
| + | exoplanetary haze formation, to study organic nitrogen. I also introduce new modifications of | ||
| + | the CIMS that makes it more applicable to high importance organic nitrogen studies that | ||
| + | require even higher time resolution. Through developing CIMS and APi-ToF for new | ||
| + | applications, the role of organic nitrogen in terrestrial and exoplanetary environments may | ||
| + | become more clear. | ||
| + | |||
| + | I first introduce the novel mass spectrometric imaging technique that combines laser | ||
| + | ablation with aerosol mass spectrometry and CIMS (LA-AMS-CIMS). Both mass spectrometric | ||
| + | methods provide the fast response, rapid data acquisition, low detection limits, and high- | ||
| + | resolution peak separation desirable for imaging complex samples. Additionally, the two | ||
| + | techniques provide complementary information with AMS providing near universal detection of | ||
| + | all aerosol constituents and CIMS with a heated inlet providing molecular-level detail of both | ||
| + | gases and aerosols. | ||
| + | |||
| + | Then I introduce the study of organic nitrogen in ambiently charged ions and neutral | ||
| + | gases during photochemical haze production in planetary atmospheres. Ions are known to play | ||
| + | an important role in haze formation chemistry; however, the role of ions in laboratory | ||
| + | simulations of haze formation is poorly characterized. By using the APi-ToF, I find that the | ||
| + | chemical composition of the cations during haze formation is complex. High molecular weight | ||
| + | ions up to m/z 400 are observed, with organic nitrogen ions, including ions with multiple | ||
| + | nitrogen atoms, accounting for the majority of the identified ion peaks. With the mechanism of | ||
| + | nitrogen incorporation being still unclear, I use CIMS to make in-situ measurements of gas- | ||
| + | phase products up to m/z 400 and find organic nitrogen species dominate the mass spectra | ||
| + | with smaller contributions from unsaturated hydrocarbons. Through the use of structural group | ||
| + | relationships, I investigate the partitioning of haze formation products between the gas- and | ||
| + | aerosol-phase. I also leverage the sensitivity and fast time response of the CIMS measurements | ||
| + | to investigate how the gas-phase chemistry evolved over the course of the experiment, | ||
| + | identifying a general mechanism for nitrogen incorporation with far ultraviolet light. | ||
| + | |||
| + | Finally, I introduce the ongoing modification of CIMS with the crossflow ion molecular | ||
| + | reactor (IMR). Through the development of the crossflow IMR, measurements of ON | ||
| + | compounds with a faster time response may be possible. I discuss a few experiments optimizing | ||
| + | the clustering distribution and total ion signal with the crossflow IMR. With further | ||
| + | modifications and calibrations, the crossflow IMR may be exceptionally important to studying | ||
| + | ON in the field." | ||
| + | |||
| + | <small> | ||
| + | <b>Publication list</b>:<br> | ||
| + | Berry, J., Day, D., Elseberg, T., Palm, B., Hu, W., Abdelhamid, A., Schroder, J., Karst, W., Jimenez, J., Browne, E. Laser Ablation-Aerosol Mass Spectrometry-Chemical Ionization Mass Spectrometry for | ||
| + | Ambient Surface Imaging, Anal. Chem, 2018, doi: 10.1021/acs.analchem.7b05255. <br> | ||
| + | Berry, J., Ugelow, M., Tolbert, M., Browne, E. Chemical Composition of Positive Ions During Laboratory Simulations of Titan’s Haze Formation, ACS Earth Space Chem., 2019, doi:10.1021/acsearthspacechem.8b00139q. <br> | ||
| + | Berry, J., Ugelow, M., Tolbert, M., Browne, E. The Influence of Gas-phase Chemistry on Organic Haze | ||
| + | Formation, Astrophys. J. Lett., 885(1), L6 (7pp), 2019, doi: 10.1080/02786826.2019.1599321. <br> | ||
| + | Ugelow, M., Berry, J., Tolbert, M., Browne, E. The Impact of Molecular Oxygen on Ion Chemistry in a | ||
| + | Hazy Archean Earth Atmosphere, [in press by Astrobiology]. </small> | ||
| + | |||
| + | <font size=4>Monday, 9 March 2020</font><BR> | ||
| + | <b>Development of a “Lab-on-a-chip” platform</b> | ||
| + | |||
| + | Victoria Arau, <br> | ||
| + | CU Boulder ANYL 1st year student, [https://sites.google.com/view/brownelab Browne group] | ||
| + | |||
| + | "Current detection methods for environmental contaminants (which include but are not limited to HPLC, GC-MS, LC-MS, and LC-MS/MS) are generally time-consuming, expensive, and lack portability, limiting field deployment. Herein we evaluate a microfluidic electrochemical cell with viability for an in situ field deployment. The microfluidic channel consists of an interdigitated microelectrode and granulated activated carbon electrodes for optimized sensitivity. Electrochemical response is monitored using cyclic voltammetry with a known redox standard, with consistency in this response between macroelectrode systems and this microelectrode system. The linearity of the peak-current versus square root of scan rate demonstrates chemical reversibility, suggesting operability of this device. Results here suggest feasibility for further development of this technology as a sensor with real-time in situ capabilities. " | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Very Remote Field Studies of Frost Formation on Mars Using the Curiosity Rover</b> | ||
| + | |||
| + | Raina Gough, <br> | ||
| + | CU ANYL Research Scientist, [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Tolbert Group] | ||
| + | |||
| + | "For the last 3 years, I have been a Participating Scientist on the Mars Science Laboratory (aka: Curiosity) mission. This rover has traversed the Martian surface since 2012, totaling 4 full Mars years of environmental and geological observations. In this talk, I will summarize some of the most interesting results from the mission and talk about my role in planning the rover's science operations. I will also discuss a series of frost-detection experiments performed on Mars that I helped to lead. Although there is little water vapor on Mars, it is expected that water exchanges seasonally and diurnally with the surface. The rover’s weather station had predicted that frost should indeed form on the soil in the early morning in the winter. However, no condensed phase water had been observed along the rover’s traverse, until recently. Last November, we successfully used the rover’s laser-induced breakdown spectroscopy (LIBS) instrument to detect enhanced hydrogen on the soil just before dawn. We believe that this indicates the presence of diurnally exchanged water, potentially frost. I will discuss these results, and also some of the unexpected hurdles to performing science on Mars." | ||
| + | |||
| + | <font size=4>Monday, 2 March 2020</font><br> | ||
| + | <b>Multiphase Atmospheric Chemistry: From the molecular to the regional and global scales</b> | ||
| + | |||
| + | [http://mcneill-lab.org/ Prof. Faye McNeill], <br> | ||
| + | Department of Chemical Engineering, Columbia University, NY | ||
| + | |||
| + | "Multiphase chemistry in the atmosphere is a major source of organic and inorganic atmospheric | ||
| + | particulate matter, and also has a profound influence on gas-phase composition and precipitation | ||
| + | chemistry. Despite considerable progress, mechanistic understanding of some key aqueous | ||
| + | atmospheric processes is still lacking, and their representation is incomplete in most regional and | ||
| + | global models. I will present an overview of aqueous chemical processes in the atmosphere, | ||
| + | highlighting recent developments and critical uncertainties. I will also discuss my group’s efforts in | ||
| + | characterizing these processes in the laboratory and improving their representation in atmospheric | ||
| + | chemistry models." | ||
| + | |||
| + | <font size=4>Monday, 24 February 2020</font><br> | ||
| + | <b>Physics and chemistry of new particle formation in the atmosphere</b> | ||
| + | |||
| + | [https://www.psi.ch/en/lac/people/urs-baltensperger Dr. Urs Baltensperger], <br> | ||
| + | Laboratory of Atmospheric Chemistry, Paul Scherrer Institute, Villigen, Switzerland | ||
| + | |||
| + | "Globally, a significant source of cloud condensation nuclei for cloud formation is thought to originate | ||
| + | from new particle formation (aerosol nucleation). Despite extensive research, many questions remain | ||
| + | about the dominant nucleation mechanisms. Specifically, a quantitative understanding of the | ||
| + | dependence of the nucleation rate on the concentration of the nucleating substances such as gaseous | ||
| + | sulfuric acid, ammonia, water vapor and others has not been reached. This is of relevance for climate | ||
| + | as the atmospheric concentrations of sulfuric acid, ammonia and other nucleating agents are strongly | ||
| + | influenced by anthropogenic emissions. Ions, produced e.g. by galactic cosmic rays, are also known to | ||
| + | influence nucleation rates, however their importance is still debated. | ||
| + | By providing extremely well controlled and essentially contaminant free conditions in the CLOUD | ||
| + | chamber, we were able to show that indeed sulfuric acid is often an important component for such | ||
| + | new particle formation, however, for the typical temperatures encountered in the planetary boundary | ||
| + | layer the concentrations of sulfuric acid are not high enough to explain the atmospheric observations | ||
| + | [1]. Under these conditions, ammonia [1], amines [2] or oxidized organic molecules [3] are needed for | ||
| + | nucleation with sulfuric acid to occur. The relevant organic molecules are highly oxygenated | ||
| + | molecules (HOMs). HOMs produced by the oxidation of biogenic precursors are able to trigger new | ||
| + | particle formation on their own, even in the absence of sulfuric acid [4,5]. We confirmed that this | ||
| + | mechanism does occur in today’s lower free troposphere [6]. Moreover, the latest CLOUD results | ||
| + | from new particle formation in urban environments, a topic of extensive current research, will be | ||
| + | presented." | ||
| + | |||
| + | <small> | ||
| + | References: | ||
| + | [1] Kirkby, J. et al., Nature, 476, 42-433, 2011 | ||
| + | [2] Almeida, J. et al., Nature, 502, 359-363, 2013. | ||
| + | [3] Riccobono F. et al., Science, 344, 717-721, 2014. | ||
| + | [4] Kirkby, J. et al., Nature, 533, 521-526, 2016. | ||
| + | [5] Tröstl, J. et al., Nature, 533, 527-531, 2016. | ||
| + | [6] Bianchi, F. et al., Science, 352, 1109-1112, 2016. </small> | ||
| + | |||
| + | <font size=4>Monday, 17 February 2020</font><br> | ||
| + | <b>The Role of H<sub>2</sub>S in Photochemical Methane Haze Experiments</b> | ||
| + | |||
| + | Nathan Reed, <br> | ||
| + | ANYL 3rd year student, [https://sites.google.com/view/brownelab Browne] / [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Tolbert] Groups), CU Boulder | ||
| + | |||
| + | "Although sulfur gases and organic haze from methane photochemistry are thought to be present in numerous planetary atmospheres, including the atmosphere of the Archean Earth (2.5-3.5 bya), direct interactions between their chemistries have historically been neglected. This assumption may limit the understanding of atmospheric sulfur and organic haze chemistry, and it would therefore be beneficial to examine photochemical haze production with sulfur gases present. Organic haze laboratory studies including H<sub>2</sub>S, an atmospheric sulfur gas with both biogenic and abiogenic (e.g. volcanic) sources, have until now been non-existent. To better understand the role of H<sub>2</sub>S in organic haze chemistry, I have conducted UV photochemistry experiments of CH<sub>4</sub> gas mixtures in N<sub>2</sub> with the inclusion of trace amounts of H<sub>2</sub>S. I investigated the product aerosol composition, number, and size distribution as a function of H<sub>2</sub>S mixing ratio. It was found that increasing the H<sub>2</sub>S mixing ratio increased the organic aerosol effective density, number, and mass loading. Further, organic sulfur compounds were found to form in the aerosol phase. I will discuss the importance of these findings in the context of organic haze and sulfur chemistry, possible mechanisms, and future steps for the project." | ||
| + | |||
| + | <font size=4>Monday, 10 February 2020</font><br> | ||
| + | <b>Revisiting dry deposition of trace gases and particles in the atmosphere</b> | ||
| + | |||
| + | [https://sites.google.com/site/delphinefarmer/ Delphine K. Farmer]<br> | ||
| + | Associate Professor, Department of Chemistry, Colorado State University | ||
| + | |||
| + | "Dry deposition is a key process that removes trace gases and particles from the atmosphere, and thus one factor that controls the atmospheric lifetime of pollutants and short-lived climate forcers. In fact, dry deposition is the single largest component of uncertainty in our understanding of aerosol effects on climate. Despite its importance, dry deposition of trace gases and particles is poorly constrained by observations due to the instrumental challenge in measuring surface-atmosphere exchange. Instruments must be adequately fast, sensitive and selective to measure low concentrations on the rapid (<1 s) timescale of turbulence. We have developed several measurement techniques that use the eddy covariance approach to flux measurements over terrestrial surfaces incorporating both spectroscopy and mass spectrometry. This talk will be divided into two parts – the first considering the mechanisms that control forest-atmosphere exchange of acidic organic molecules, and the second revisiting our understanding of size-resolved particle deposition in the atmosphere. We contrast these observations with previous measurements in the literature, and with commonly used resistance models, highlighting several model-measurement discrepancies. To further investigate the mechanisms of particle deposition, we use black carbon deposition as an inert tracer for particle wet and dry deposition. We show that wet deposition dominates in an agricultural environment in Oklahoma, and provide observational constraints on black carbon lifetime in this region. Our suite of observations inform a revised model parameterization, which we incorporate in a global chemical transport model to investigate how dry deposition can impact particle loading and the radiative balance of the atmosphere. Together, these new measurements highlight the importance of observational constraints in developing, validating, and revising models of fundamental chemical and physical processes in the atmosphere – and in reducing uncertainties in our understanding of climate." | ||
| + | |||
| + | <font size=4>Monday, 3 February 2020</font><br> | ||
| + | <b>Experimental Investigation of the Particle-Phase Products and Reaction Mechanism of Δ-3-Carene with NO<sub>3</sub> Radicals</b> | ||
| + | |||
| + | Marla DeVault, <br> | ||
| + | ANYL 3rd year, [https://sites.google.com/site/ziemanngroup/ Ziemann Group] | ||
| + | |||
| + | "Oxidation products of monoterpenes have been shown to contribute to secondary organic aerosol (SOA) formation in the atmosphere. However, the nighttime oxidative processes, which are dominated by nitrate radical (NO<sub>3</sub>) addition to alkenes, are not well understood. In order to fill this gap, I am working to quantify the yields of particle-phase products of the nitrate radical-initiated oxidation of Δ-3-carene, a monoterpene that is primarily emitted by coniferous plants. Using functional groups analysis methods, liquid chromatography with UV-Vis detection, and electrospray and electron ionization mass spectrometry to characterize SOA formed in environmental chamber reactions, I have identified a series of acetal dimers. The mechanism derived from these measured particle-phase products will help to inform chemical transport models on the contribution of nighttime oxidation of monoterpenes to SOA. In addition, these acetal dimers are analogous to those that have been identified and quantified in the β-pinene + NO<sub>3</sub> radical system, and appear to form from reactions of a variety of monoterpenes." | ||
| + | |||
| + | ==Fall 2019== | ||
| + | <font size=4>Monday, 2 December 2019</font><br> | ||
| + | <b>Soot: So Ubiquitous, So Destructive, So Important, Yet So Elusive: Exploring the Mysteries of Soot Formation</b> | ||
| + | |||
| + | [https://thesootlab.com/ Prof. Hope Michelsen], <br> | ||
| + | Mech. Eng., CU Boulder | ||
| + | |||
| + | "There are substantial gaps in our understanding of the mechanisms controlling soot inception, | ||
| + | particle growth, and chemical evolution during combustion. The first steps in soot formation | ||
| + | involve the transition of gas-phase hydrocarbon precursors to physically or covalently bound | ||
| + | complexes. These complexes are known as “incipient particles”, and the search for their | ||
| + | formation and growth mechanisms is a subject of active research [1-3]. These incipient particles | ||
| + | undergo further particle growth, generating liquid-like hydrocarbon particles, which eventually | ||
| + | reach sizes in the range of 10-50 nm, known as “primary particles” [1-5]. As these particles | ||
| + | grow, they also lose hydrogen, solidify, and agglomerate into loosely bound clusters. Under | ||
| + | high-temperature conditions, they become graphitic, covalently bound aggregates with a | ||
| + | dendritic structure. Soot aggregate sizes, primary-particle sizes, and volume fractions grow as | ||
| + | particles age in the flame [4,5]. At high temperatures in the presence of oxygen, the aggregates | ||
| + | fragment [6,7], and the primary-particle sizes and volume fractions decrease through oxidation | ||
| + | [4,8]. There is a poor understanding of the mechanisms by which particles undergo these | ||
| + | transitions and the parameters that influence them. | ||
| + | |||
| + | This talk will describe our current understanding of soot formation and the scientific evidence | ||
| + | that supports this understanding. This talk will also cover the gaps in our understanding of soot | ||
| + | chemistry, some reasons for these gaps, and what we may need to do in order to bridge these | ||
| + | gaps and develop more insight into soot formation and evolution." | ||
| + | |||
| + | <small>[1] H.A. Michelsen, Proc. Combust. Inst. 36, 717 (2017). | ||
| + | [2] H. Wang, Proc. Combust. Inst. 33, 41 (2011). | ||
| + | [3] K.O. Johansson, M.P. Head-Gordon, P. E. Schrader, K. R. Wilson, H. A. Michelsen, Science 361, 997 (2018). | ||
| + | [4] R.A. Dobbins, C.M. Megaridis, Langmuir 3, 254 (1987). | ||
| + | [5] R. Puri, T. F. Richardson, R.J. Santoro, R.A. Dobbins, Combust. Flame 92, 320 (1993). | ||
| + | [6] K.G. Neoh, J.B. Howard, A.F. Sarofim, Proc. Combust. Inst. 20, 951 (1985). | ||
| + | [7] C.A. Echavarria, I.C. Jaramillo, A.F. Sarofim, J.S. Lighty, Proc. Combust. Inst. 33, 659 (2011). | ||
| + | [8] K.O. Johansson, F. El Gabaly, P.E. Schrader, M.F. Campbell, H.A. Michelsen, Aerosol Sci. Technol. 51 (12), 1333 (2017). </small> | ||
| + | |||
| + | <font size=4>Monday, 18 November 2019</font><br> | ||
| + | <b>Rapid Wastewater Analysis Platform Based on Surface-enhanced Raman Scattering </b> | ||
| + | |||
| + | Ya-Chu Chan, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Wastewater discharged from electroplating factories contains cyanide and chromium (VI), which could pose a threat to the environment and humans if in high concentration. The standard methods used by the Environmental Protection Agency to detect both species require tedious sample pre-treatments and sophisticated instruments. Owing to these pitfalls, an alternate method that can achieve rapid and on-site detection is important to timely deal with illegal discharge. Surface-enhanced Raman Scattering (SERS) is a vibrational spectroscopic technique that provides “fingerprints” of different entities. It has the potential to apply to wastewater detection because of its high sensitivity, accuracy, and portability. However, several problems have been hindering it from becoming a quantitative analysis method. In this project, a protocol to achieve quantitative SERS is developed. Then, how the SERS signal reflects cyanide and chromium (VI) concentration is studied, and the effect of other ions in wastewater is discussed. These results build the foundation to apply SERS to real wastewater samples. To make it more relevant to atmospheric studies, this talk will end with some comments on recent applications of SERS to aerosol pH measurement." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>An evaluation of the sources of tropospheric ozone in the UK</b> | ||
| + | |||
| + | Johana Romero, <br> | ||
| + | ANYL Postdoc, [http://volkamergroup.colorado.edu/ Volkamer Group] | ||
| + | |||
| + | "Ground-level ozone (O<sub>3</sub>) is a pollutant of concern for policy-makers because of its | ||
| + | detrimental effect on human health, agriculture and ecosystems (Fuhrer, 2009; WHO, | ||
| + | 2016)(Fuhrer, 2009; WHO, 2016). Near the surface, O<sub>3</sub> has an atmospheric lifetime | ||
| + | typically of hours. However, in the free troposphere, its lifetime could range from days | ||
| + | up to several weeks (Stevenson et al., 2006) allowing O<sub>3</sub> to be transported from its | ||
| + | point of production downwind over long distances easily crossing countries and even | ||
| + | continents (Monks et al., 2015). This trans-boundary nature of O<sub>3</sub> and its precursors | ||
| + | complicates compliance of air quality standards within the territories (HTAP, 2007). | ||
| + | To develop effective emissions policies, it is, therefore, necessary to understand the | ||
| + | extent to which domestic emissions and trans-boundary inflow contribute to the O<sub>3</sub> | ||
| + | distributions across the UK. | ||
| + | |||
| + | An online O<sub>3</sub> tagging method based on the procedures described in (Emmons et al., | ||
| + | 2012) and (Butler et al., 2018) was implemented into the Weather Research and | ||
| + | Forecasting model coupled with Chemistry (WRF-Chem) to explore the contributions | ||
| + | of foreign anthropogenic NOx emissions to the surface O<sub>3</sub> in 12 receptor regions in the | ||
| + | UK in summertime 2015. Simulations represent well the observed O3 from 20 EMEP | ||
| + | ground sites within the UK and Western Europe. | ||
| + | Results confirm the importance of short-range transport of O<sub>3</sub> from continental | ||
| + | Europe to the UK as well as from no-controllable O<sub>3</sub> sources, such as the hemispheric | ||
| + | ozone, which account for 71% of the total modelled O3 from May to August. The | ||
| + | contribution of O<sub>3</sub> from European NO<sub>x</sub> emissions is principally due to the transport of | ||
| + | O<sub>3</sub> rather than NO<sub>x</sub> reservoir. | ||
| + | |||
| + | It is shown that emission controls would be required in different source regions for | ||
| + | compliance of ozone standards such as maximum daily 8 hours average MDA8 O<sub>3</sub> of | ||
| + | 50 and 60 ppbv. As an instance, emissions controls in France are important for the | ||
| + | south and southeast of the UK, while domestic emissions controls are more relevant | ||
| + | for the Midlands and the north of the UK. By contrast, attainment of lower exposure | ||
| + | thresholds, e.g., accumulated ozone over 40 ppb AOT40 metric would primarily | ||
| + | require the regulation of the hemispheric ozone levels. | ||
| + | |||
| + | The O<sub>3</sub> tagged simulation further show that O<sub>3</sub> from Germany, the Benelux, France | ||
| + | and ship emissions in the North Sea are responsible for the build-up of O<sub>3</sub> in the | ||
| + | southeast UK during a summertime 2015 pollution episode. Furthermore, process | ||
| + | analysis diagnostics demonstrates that vertical mixing in the morning can bring O<sub>3</sub> and precursors from the residual layer to the ground, which contributes to the build- | ||
| + | up of ozone during pollution episodes." | ||
| + | |||
| + | <small>References | ||
| + | |||
| + | Butler, T., Lupascu, A., Coates, J., & Zhu, S. (2018). TOAST 1.0: Tropospheric ozone | ||
| + | attribution of sources with tagging for CESM 1.2.2. Geoscientific Model | ||
| + | Development, 11(7), 2825–2840. Retrieved from https://doi.org/10.5194/gmd-11- | ||
| + | 2825-2018 | ||
| + | |||
| + | Emmons, L. K., Hess, P. G., Lamarque, J. F., & Pfister, G. G. (2012). Tagged ozone | ||
| + | mechanism for MOZART-4, CAM-chem and other chemical transport models. | ||
| + | Geoscientific Model Development, 5(6), 1531–1542. Retrieved from | ||
| + | https://doi.org/10.5194/gmd-5-1531-2012 | ||
| + | |||
| + | Fuhrer, J. (2009). Ozone risk for crops and pastures in present and future climates. | ||
| + | Naturwissenschaften, 96(2), 173–194. Retrieved from | ||
| + | https://doi.org/10.1007/s00114-008-0468-7 | ||
| + | HTAP. (2007). Hemispheric Transport of Air Pollution 2007. Retrieved from | ||
| + | 10.18356/2c908168-en | ||
| + | |||
| + | Monks, P. S., Archibald, a. T., Colette, a., Cooper, O., Coyle, M., Derwent, R., ... | ||
| + | Williams, M. L. (2015). Tropospheric ozone and its precursors from the urban to | ||
| + | the global scale from air quality to short-lived climate forcer. Atmospheric | ||
| + | Chemistry and Physics, 15(15), 8889–8973. Retrieved from | ||
| + | https://doi.org/10.5194/acp-15-8889-2015 | ||
| + | |||
| + | Stevenson, D. S., Dentener, F. J., Schultz, M. G., Ellingsen, K., van Noije, T. P. C., Wild, | ||
| + | O., ... Szopa, S. (2006). Multimodel ensemble simulations of present-day and | ||
| + | near-future tropospheric ozone. Geophysical Research Letters, 111(8). Retrieved | ||
| + | from https://doi.org/10.1029/2005JD006338 | ||
| + | |||
| + | World Health Organization. (2016). Ambient Air Pollution: A global assessment of | ||
| + | exposure and burden of disease. World Health Organization. Retrieved from | ||
| + | 9789241511353</small> | ||
| + | |||
| + | <font size=4>Monday, 11 November 2019</font><br> | ||
| + | <b>Ozone Production in Los Angeles: Investigating Spatial Differences in the Impacts of NO<sub>x</sub> Emission Control</b> | ||
| + | |||
| + | Margarita Reza, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Los Angeles has historically experienced the highest ozone in the U.S. Strategies to reduce high ozone | ||
| + | have included reduction of emissions of both nitrogen oxides (NOx) and volatile organic compounds | ||
| + | (VOC), the two main precursors to ozone. Observations of ozone on weekdays and weekends, as well as | ||
| + | from many intensive atmospheric experiments in LA, have indicated that for decades ozone production | ||
| + | chemistry in LA has remained sensitive to VOC rather than NOx. However, very recent literature has | ||
| + | suggested that LA ozone chemistry may be transitioning to a chemical regime that is sensitive to NOx, | ||
| + | which would mean that LA air quality is positioned to benefit from NOx emission control on diesel truck | ||
| + | traffic that are currently in the implementation phase. This study investigates this possible chemical- | ||
| + | regime transition by first looking at the temporal trends in NOx concentrations at nine monitoring | ||
| + | stations throughout LA. Spatial variability is found in the observed rate of decrease of NOx, suggesting | ||
| + | that ozone has been affected differently throughout the LA basin. To investigate this potential spatial | ||
| + | variability in the impacts of NOx changes, and, potentially, the change in ozone chemistry sensitivity to | ||
| + | NOx, data gathered from the Geostationary Trace Gas and Aerosol Sensor Optimization (GeoTASO) | ||
| + | airborne instrument on board the NASA UC12-B King Air on June 26 th and 27 th , 2017 was used. An | ||
| + | analytical model was used to simulate ozone production in LA to identify the sensitivity of ozone | ||
| + | production to NOx. Understanding the dependence of ozone production on NOx is critical for developing | ||
| + | effective control strategies in this area." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Nanopore Sensing for Single-Molecule Glycomics</b> | ||
| + | |||
| + | Melissa Morris, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Single-molecule sensing represents the ultimate in chemical sensitivity, but is tremendously challenging | ||
| + | to achieve. Because imaging at the nanoscale is difficult and expensive, developing techniques to | ||
| + | measure single molecules requires ingenuity, and often relies on the interpretation of electrical signals. | ||
| + | So how do nanopores help us measure at the single-molecule level? A nanopore is simply a nano-sized | ||
| + | hole in a membrane or material. In the early stages of nanopore science, biological nanopores were | ||
| + | isolated from nature, and now the field has turned to solid state nanopores. The Dwyer research group | ||
| + | uses an electric field to create solid state nanopores in silicon nitride (SiNx) membranes, using a | ||
| + | technique adopted from Vincent Tabard-Cossa.[1] Once the SiNx membrane has a hole, we mount it in a | ||
| + | holder, so that it sits between two wells of electrolyte. We then monitor the electrical current to detect | ||
| + | single molecules as they pass through the nanopore. In this study, we explored how solid state | ||
| + | nanopores can be used to detect and characterize sugar molecules. Sugars have complex branching | ||
| + | structures, and significant molecule to molecule variability. However, sugars are easily absorbed by the | ||
| + | body, and their potential to be used as a new drug delivery system depends on their characterization." | ||
| + | |||
| + | [1] Kwok, H., Briggs, K., Tabard-Cossa, V. Nanopore Fabrication by Controlled Dielectric Breakdown. | ||
| + | PLOS ONE. 2014, 9, e92880. | ||
| + | |||
| + | <font size=4>Monday, 4 November 2019</font><br> | ||
| + | <b>Impact of relative humidity on reactive uptake of glyoxal (CHOCHO) onto aerosol over the Korean peninsula during the KORUS-AQ 2016</b> | ||
| + | |||
| + | Dongwook Kim, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Glyoxal (CHOCHO) plays an important role in secondary organic aerosol (SOA) formation through aqueous phase chemistry. However, the heterogeneous processes and resulting glyoxal contribution to total SOA in various ambient conditions are largely unknown. In this study, the impact of humidity and irradiation on uptake of gaseous glyoxal onto particulate matter in has been investigated based on the glyoxal measurement acquired from airborne Cavity Enhanced Absorption Spectrometer (CEAS), during KORUS-AQ field campaign, with the help of 0-dimensional box model where the parameters were constrained by the observations from DC-8. The reactive uptake coefficient of glyoxal (γ) was derived by reconciling the gap between the observation of glyoxal and the expected abundance of glyoxal estimated from the steady-state box model without the uptake process. In the presence of > 0.1 ppb of glyoxal, the OA growth rate from glyoxal uptake was estimated to be 0.48 μg/m3/hour and γ varied from 0-20x10-3 having median and mean values of 5.89x10-3 and 8.10x10-3, respectively. The typical lifetime of glyoxal during the campaign was estimated to be ~37 minutes. γ decreased at 25-35% RH range which can be explained partially by the salting-in effect. On the other hand, γ increased at 35-75% RH range which remains uncertain. Also, γ showed an increasing trend with increasing irradiation indicating that the irreversible uptake process is dominant at the highly irradiated conditions." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>The Synthesis of Two Novel p-38α Inhibitors</b> | ||
| + | |||
| + | Hannah Maben, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Current p-38α inhibitors are in clinical trials to treat diseases such as cancer, Rheumatoid arthritis, and | ||
| + | Crohn’s disease. The synthesis of two potential p-38α inhibitors, bis-1,3-(4,6-difluoro-4-{4-[quinoxalin-2- | ||
| + | ylmethoxy]methyl}-1H-1,2,3-triazol-1-yl]pyridin-2-yl) 5-methyl-6-phenylpiperazine-2,3-dicarbonitrile (I) | ||
| + | and 2,6-di(5,6-Dimethyl-6,7-dihydro-5H-[1,2,5]oxadiazolo[3,4-b]pyrazin-4-yl)-3,5-difluoro-4-(4-phenyl- | ||
| + | [1,2,3]triazol-1-yl)-pyridine (II) were conducted. The syntheses involved several nucleophilic aromatic | ||
| + | substitution (S N Ar) reactions, a Sharpless “click” reaction, and a mild reduction via sodium borohydride. | ||
| + | All products and intermediates were sent for elemental and biological testing, and two intermediates | ||
| + | were determined to have biological activity against colon cancer." | ||
| + | |||
| + | <font size=4>Monday, 28 October 2019</font> | ||
| + | <b>Gas Phase Oxidation of Campholenic Aldehyde and Lactones as an Intermediate | ||
| + | to SOA formation</b> | ||
| + | |||
| + | William Dresser, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "This study investigated the oxidation of campholenic aldehyde(CA) and a series of lactone | ||
| + | compounds and their derivatives using flow tube chemical ionization mass spectrometry (FT- | ||
| + | CIMS) to try and understand the reaction mechanisms and identify potential intermediates | ||
| + | between the gas and secondary organic aerosol (SOA) phase. Oxidation was induced with both | ||
| + | hydroxide and chlorine radicals and proton transfer reaction (PTR) detection as well as Iodide | ||
| + | detection were used in the spectra analysis. Oxidation pathways were determined for both | ||
| + | studies at low pressures and product percentages were found for the major species. In the case | ||
| + | of CA, the expected epoxide intermediate was identified at 5-20% abundance, which was the | ||
| + | major intermediate of interest. The lactone pathways were used to predict the reactivity of a | ||
| + | previously identified intermediate, hydroxymethyl-methyl-α-lactone (HMML), between isoprene | ||
| + | and SOA." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Determination of the Active Agents in Commercial Pygeum Products Sold for the Treatment of | ||
| + | Benign Prostatic Hyperplasia</b> | ||
| + | |||
| + | Daniel Katz, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "Benign Prostatic Hyperplasia (BPH) or enlarged prostate is a common condition in older men | ||
| + | and may be a precursor to prostate cancer. Dietary supplements made from the powdered bark of | ||
| + | Prunus africana are marketed as pygeum to treat BPH, but they are only loosely regulated by the | ||
| + | U.S. FDA. Commercial pygeum products were tested for N-butylbenzenesulfonamide (NBBSA), | ||
| + | ferulic acid, atraric acid, atranorin, and β-sitosterol (BSST), components of pygeum that are | ||
| + | thought to be effective in treating BPH. Two parallel liquid-solid extractions were conducted for | ||
| + | each product. A direct extraction used acetone:hexane to extract NBBSA, atraric acid, and | ||
| + | atranorin. The other extraction followed saponification that released ferulic acid and BSST from | ||
| + | their natural esters and used dichloromethane solvent. After evaporation and reconstitution, the | ||
| + | extracts were analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS). The | ||
| + | amount of ferulic acid, atraric acid, atranorin, and BSST varied widely among the products, but | ||
| + | no NBBSA was detected in any product. The levels of each active compound found in the | ||
| + | products can be used to evaluate their possible effectiveness for the treatment of BPH." | ||
| + | |||
| + | <font size=4>Monday, 14 October 2019</font><br> | ||
| + | <b>Update on OFR185 Characterization of the Aerodyne Potential Aerosol Mass Chamber</b> | ||
| + | |||
| + | Jake Rowe, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "One disadvantage of conventional Oxidation Flow Reactors (OFRs) is the difficulty in achieving controlled, shorter (<1 day) oxidative aging processes that are also relevant to atmospheric SOA formation processes. To investigate this issue, this study characterizes two alternative mercury lamp configurations designed to attenuate 185 and/or 254 nm irradiance relative to standard low-pressure UVC mercury lamps used in the Aerodyne Potential Aerosol Mass (PAM) OFR. In the first configuration, the irradiance at 185 and 254 nm was attenuated by applying segments of Viton heat shrink tubing along standard UVC lamps. In another configuration, the 185 nm lamp output was attenuated relative to the 254 nm output by splicing segments of lamp glass that transmit either 185 and 254 nm or only 254 nm radiation. OH exposures attained by photolysis of O2/O3/H2O with these lamps were calculated from the reactive loss of CO and SO2 input to the reactor and measured under steady-state conditions as a function of photon flux and [H2O]. At a midrange relative humidity ~ 50% and τ ~ 2 min, the attainable lower-range photochemical age decreased from ~4-5 to ~0.3-0.4 days of equivalent atmospheric exposure. Thus, the combined usage of attenuated and standard UVC mercury lamps -- as demonstrated here with the PAM OFR -- significantly extends the range of attainable photochemical aging timescales in OFRs with no additional modification of OFR conditions." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Identification and Characterization of a Red Blood Cell Stabilizer</b> | ||
| + | |||
| + | Anna Ziola, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "When creating controls for hematology instruments, the volume of red blood cells (RBCs) must be held constant. Each component in control samples must retain its respective volume throughout the shelf life since the size of the cells is one method of blood component characterization in hematology instruments. The plant extract mixture currently used to stabilize the volume of RBCs is losing its effectiveness, requiring hospitals to purchase and replace these controls more often to ensure accurate patient hematology reports. To identify the active ingredient responsible for RBC stabilization in the plant extract, we used high performance liquid chromatography, mass spectrometry, SDS-PAGE gel electrophoresis, the Sysmex XN-20 and Sysmex XE-5000 to identify and characterize the active ingredient originally responsible for stabilizing the volume of RBCs." | ||
| + | |||
| + | <font size=4>Monday, 30 September 2019</font><br> | ||
| + | <b>Why should environmental chemists care about theory?</b> | ||
| + | |||
| + | [https://www.colorado.edu/lab/sharmagroup/ Sandeep Sharma], <br> | ||
| + | PChem faculty, CU Boulder | ||
| + | |||
| + | "I will begin by discussing why learning about theory might be of interest to students doing experimental research in analytic chemistry. I will then proceed to give a birds-eye view of my research program. How questions are formulated, how one goes about trying to answer them and where are the challenges. I will also discuss how this line of research might help answer some of the questions environmental chemists ask. The presentation will end with some specific problems that Paul Ziemann and I are planning to collaborate on in the near future." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</b> | ||
| + | |||
| + | [https://sites.google.com/site/ziemanngroup/home Paul Ziemann], <br> | ||
| + | ANYL Faculty, CU Boulder | ||
| + | |||
| + | "Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative | ||
| + | studies of indoor air chemistry at CU." | ||
| + | |||
| + | <font size=4>Monday, 23 September 2019</font><br> | ||
| + | <b>Laboratory Studies of Contact Efflorescence in a Long Working Distance Optical Trap</b> | ||
| + | |||
| + | [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Maggie Tolbert], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Because homogeneous salt efflorescence typically requires low relative humidity (RH), atmospheric salt particles are often assumed to be aqueous throughout much of their atmospheric lifetime. Here we use a long working distance optical trap to examine heterogeneous efflorescence that occurs when a supersaturated salt droplet comes into contact with a solid particle. We compare our findings for pure salt droplets with those obtained for salt/organic droplets where the organic may be found either in a core shell structure or as an amorphous/glassy solid. We find that in many cases, single collisions with a range of nuclei promote efflorescence at relatively high RH, causing salt particles to be solid for more of their atmospheric lifetime. Similar experiments are proposed in the future to examine the role of contact in ice cloud nucleation." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Small molecules in the Anthropocene: Wildfires, Oceans and Iodine</b> | ||
| + | |||
| + | [http://ciresgroups.colorado.edu/volkamergroup/ Rainer Volkamer], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "The Volkamer group develops advanced optical instrumentation (in situ and remote sensing) to measure small molecules and aerosols that are relevant to public health and climate. We seek to better quantify and understand emissions of small molecules, total carbon, and aerosols from natural and managed ecosystem (e.g., wildfires, oil & natural gas, ocean surface), and develop a molecular level understanding of the fundamental processes that affect their chemical transformations and sinks (e.g., new particle formation). Opportunities for graduate research exist in the areas of 1) field measurements (i.e., BB-FLUX, TI3GER project), 2) laboratory experiments to better understand air-sea exchange, and anthropogenic enhancements of biogenic organic aerosol (incl. at CLOUD/CERN), and 3) instrumentation to develop small light weight sensors for mobile air-quality networks (e.g., RTD transit)." | ||
| + | |||
| + | <font size=4>Monday, 16 September 2019</font><br> | ||
| + | <b>Chemistry of Volatile Organic Compounds in the Atmosphere</b> | ||
| + | |||
| + | [https://sites.google.com/view/de-gouw-lab Joost de Gouw], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products. | ||
| + | |||
| + | In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments. | ||
| + | |||
| + | Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments. From the results we learn about the sources of VOCs from people, chemical products and building materials, the chemical transformations of these VOCs and other loss process such as surface uptake and ventilation to the atmosphere. Second, we are working on the emissions and chemistry of VOCs released from volatile chemical product (VCP) use to the atmosphere, which was recently discovered to be the dominant source of VOCs in urban air. In this research we make measurements of VOCs in urban air, separate the different emission sources and describe the chemical transformations of VOCs after emission. Finally, we are working on a chamber study to better understand the formation of secondary organic aerosol from biogenic VOCs." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Organic nitrogen chemistry: Recent results and future projects</b> | ||
| + | |||
| + | [https://sites.google.com/a/colorado.edu/brownelab/ Eleanor Browne], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Organic nitrogen is a ubiquitous atmospheric component that affects biogeochemistry, air quality, and climate. Assessing the impact of organic nitrogen on these processes remains challenging because traditional measurement techniques have lacked the sensitivity and chemical resolution to characterize the speciation and chemistry of organic nitrogen. Here, I will discuss measurements made with protonated ethanol cluster chemical ionization time-of-flight mass spectrometry during the Holistic Interactions of Shallow Clouds, Aerosols, and Land-Ecosystems (HI-SCALE) campaign at the Southern Great Plains research station in Lamont, Oklahoma. As the site is located in an agricultural region, reduced nitrogen compounds are prevalent. I will present measurements of novel compounds including imines and urea and will discuss the sources and sinks of compounds at this site. Finally, I will discuss research opportunities in our group. These include further measurements at SGP in Spring 2020 and Fall 2021 as well as instrument development and chemical transport modeling." | ||
| + | |||
| + | <font size=4>Monday, 9 September 2019</font><br> | ||
| + | <b>Atmospheric nanoaerosols: An instrument for chemical analysis of freshly nucleated particles and their formation from biogenic organic precursors </b> | ||
| + | |||
| + | Andrea Wagner, <br> | ||
| + | ANYL Postdoctoral Researcher, CU Boulder, [https://volkamergroup.colorado.edu/ Volkamer Group] | ||
| + | |||
| + | "A large fraction of atmospheric aerosol particles are formed via new | ||
| + | particle formation from the gas phase. But the chemical analysis of | ||
| + | freshly nucleated particles smaller than 30 nm is challenging due to | ||
| + | their tiny mass. In this talk I present a new instrument that, in | ||
| + | combination with a chemical ionization time-of-flight mass | ||
| + | spectrometer, analyzes these nucleation mode particles. The | ||
| + | measurements are size-resolved and parallel to gas-phase analysis. | ||
| + | Additionally, mechanisms for the formation of particles from purely | ||
| + | biogenic organic precursors in chamber measurements are presented." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Recent Results and Upcoming Projects Investigating Aerosol Sources, Properties, Processes, and Fate</b> | ||
| + | |||
| + | [http://cires1.colorado.edu/jimenez-group/ Jose Jimenez], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "This fall ½ seminar provides an opportunity to introduce our group and its upcoming opportunities to the starting graduate student class, as well as to provide a summary of key results to the ANYL division as a whole. Our group’s research focuses on understanding the sources, properties, transformations, and sinks of submicron aerosols (and of the gases that interact with them), which have major effects on human health and climate. | ||
| + | |||
| + | In this talk I will briefly present results from different projects over the last year, especially those most relevant to incoming student opportunities. I will introduce our aircraft research program, where we are currently participating in the FIREX-AQ campaign sampling biomass burning smoke with the NASA DC8 aircraft. We are deploying an aerosol mass spectrometer (AMS) and, for the first time, an extractive electrospray (EESI) MS, with excellent results. I will also summarize results from the ATOM campaign, the first to sample the global remote troposphere systematically with vertical coverage. In particular global models are found to predict organic aerosols (OA) ok, but for a combination of wrong reasons as primary OA (POA) is greatly overestimated. On the other hand, models tend to predict pH which is substantially higher than the observations, with a pH ~ 0 being typical of the remote troposphere. A meta-analysis of large urban studies shows that urban secondary OA (SOA) leads to ~320,000 excess deaths per year globally, and should be the target of future regulations. The budget of organic carbon of indoor air is presented and compared to that of outdoor studies. Finally, fundamental measurements of the organic accommodation coefficient are presented using a new chamber technique. | ||
| + | |||
| + | Potential opportunities in our group involve the CalNexT campaign studying urban SOA in Los Angeles, studies of indoor air chemistry and surface interactions, analysis of aircraft campaign data and participation in future campaigns, and chamber experiments targeting both fundamental processes and signatures of urban SOA sources. Modeling tools such as KinSim and GECKO-A can be applied to the different problems as needed." | ||
| + | |||
| + | ==Summer 2019== | ||
| + | <font size=4>Monday, 19 August 2019</font> <br> | ||
| + | <b>Fates of Oxygenated Organics in Outdoor and Indoor Environments, Chemical Reactions and Partitioning</b> <br> | ||
| + | Lucas Algrim, <br> | ||
| + | ANYL Student, CU Boulder, [https://sites.google.com/site/ziemanngroup/ Ziemann Group] | ||
| + | |||
| + | "Volatile organics are emitted in indoor and outdoor environments where they are then subject to various losses such as deposition, or chemical transformation. In outdoor environments chemical reaction dominates, which will create products of higher volatility that stay in the gas phase or products of lower volatility that can partition to the particle phase to generate SOA. In indoor environments, which have lower concentrations of oxidants and greater surface area, deposition to and partitioning with surfaces are of increased relevance. | ||
| + | |||
| + | In the first section of this thesis, Chapters 2-4, we probe SOA forming potential of functionalized precursors. SOA yields were measured for OH radical-initiated reactions of the 2-6 dodecanone, 1-5 decanol, and 1-5 decyl nitrate positional isomers and also n-decane, n-dodecane and n-tetradecane in the presence of NO<sub>x</sub>. Yields decreased in the order n-tetradecane > dodecanone isomer average > n-dodecane, and the functionalized isomer yields decreased as the functional group moved toward the center of the molecule, with 6-dodecanone being an exception. Trends in the yields can be explained by the effect of carbon number and functional group presence and position on product vapor pressures, and by the isomer-specific effects of the functional group on branching ratios for the various alkoxy radical isomerizations, decompositions, and reactions with O<sub>2</sub>. Analysis of particle composition indicates within each isomer series, the SOA products are similar for each isomer. The results demonstrate that the presence of a functional group on a precursor alters gas and particle phase chemistry and provide new insights into the potential effects of molecular structure on the products of the atmospheric oxidation of volatile organic compounds. | ||
| + | |||
| + | In the second part of the thesis, Chapter 5, we parameterize the partitioning of VOCs to indoor paint films. Diffusion coefficients, Dc, of organics in a commercial paint were measured and found to correlate well with compound vapor pressures, C*. Experiments were performed by monitoring VOC partitioning in a painted flow tube. A model was constructed to replicate the VOC time traces observed during passivation and depassivation as a function of two parameters, C<sub>w</sub>, and D<sub>c</sub>. Modeled and measured D<sub>c</sub> values agreed reasonably well, and modeled C<sub>w</sub> values aligned with those calculated from the mass of paint in the flow tube. The relationship between D<sub>c</sub> and C*, and the determination of Cw from mass of paint allow for modeling of VOC partitioning to paint films in indoor environments that indicate >50% of the VOCs with C* of 10<sup>8</sup> μg m<sup>-3</sup> or less that contact a paint film of typical thickness, will fully permeate the paint film, regardless of emission duration." | ||
| + | |||
| + | |||
| + | <font size=4>Tuesday, 23 July 2019</font> <br> | ||
| + | <b>Aircraft measurements of bromine and iodine from the sea surface to the lower stratosphere</b> <br> | ||
| + | Theodore Koenig, <br> | ||
| + | ANYL Student, CU Boulder, [https://volkamergroup.colorado.edu/ Volkamer Group] | ||
| + | |||
| + | "Bromine and iodine change atmospheric oxidative capacity, deplete ozone, modify NO<sub>x</sub> = (NO<sub>2</sub> + NO) and HO<sub>x</sub> = (OH + HO<sub>2</sub>), and in turn impact air quality and human health as well as radiative forcing and climate. The radical monoxides, BrO and IO, have large structured rovibronic absorptions in the Ultraviolet-Visible (UV-Vis) spectrum which results in rapid and active photochemistry as key intermediates in the impacts listed above, and also makes them detectable to differential optical absorption spectroscopy (DOAS). I present measurements of BrO and IO using DOAS, particularly from aircraft, from which I infer total gas-phase bromine and iodine, Br<sub>y</sub> and I<sub>y</sub>, using chemical box-models and examine the implications of these measurements. We find active multiphase chemistry relevant from the marine boundary layer MBL where gas-phase coupling to organics modulates sea-salt aerosol debromination, to the free troposphere where we find dust initiates iodine chemistry leading to miniature ozone holes west of South America, to the upper troposphere and lower stratosphere (UTLS) where multiphase chemistry and phase partitioning around the tropopause destroy ozone and change radiative forcing in the climate relevant region." | ||
| + | |||
| + | ==Spring 2019== | ||
| + | |||
| + | <font size="4">Monday, 22 April 19</font> | ||
| + | |||
| + | <b>Methods for the quantification and identification of alkenes on indoor surfaces</b> | ||
| + | |||
| + | Benjamin Deming | ||
| + | ANYL 3rd year, [https://sites.google.com/site/ziemanngroup/home Ziemann group] | ||
| + | |||
| + | "Indoor surfaces can support organic films, which can act as sinks for semi-volatile organic compounds (SVOCs), reactors for condensed-phase reactions, sites of heterogeneous gas-condensed-phase reactions, and exposure routes for human health impacts. Although there have been some studies on indoor surface films, their size, composition, and chemistry are still | ||
| + | uncertain. Additionally, the majority of studies investigating these films have been performed on | ||
| + | impermeable surfaces, typically window glass. Painted surfaces, which tend to dominate by | ||
| + | surface area, potentially differ from glass in several important ways, and are therefore an | ||
| + | understudied aspect of indoor environments. Interior wall paint is normally composed of an | ||
| + | organic binder and an inorganic filler, which may change the way films initially form as well as | ||
| + | their subsequent growth. Film components may also absorb into paint and away from the surface, | ||
| + | changing the composition of the film that remains. Oxidation indoors is largely driven by ozone | ||
| + | and reactions with alkenes are therefore of particular interest. There are a number of sources | ||
| + | unique to indoor environments which may contribute alkenes to surface films: cooking oils can | ||
| + | have a high degree of unsaturation, many fragrances and cleaners contain terpene derivatives, | ||
| + | and human beings constantly produce skin oil, which contains squalene and unsaturated fatty | ||
| + | acids. To explore these areas of interest we characterized a method for quantitatively sampling | ||
| + | the low-volatility, organic portion of a surface film using a surface wipe and developed a | ||
| + | spectrophotometric method for quantifying nanomole quantities of alkenes. Samples were also | ||
| + | derivatized, adding a readily ionizable group to non-conjugated double bonds, allowing for | ||
| + | identification by positive-mode ESI-MS. Samples from neighboring glass and painted surfaces | ||
| + | were collected from a variety of locations, including a classroom, graduate student offices, a | ||
| + | bowling alley, a gym, and more. To investigate the effect of cooking on nearby surface | ||
| + | concentrations we pan-fried three different cooking oils at high temperatures and analyzed the | ||
| + | double bond content of the raw oil, cooked oil, and nearby surfaces. The effect of ongoing | ||
| + | exposure to ozone on the alkene concentrations on the human envelope was explored by | ||
| + | obtaining samples from the foreheads of volunteers at different times of the day. In a final | ||
| + | experiment we studied the effect of using a terpene cleaner on measured surface concentrations. | ||
| + | The results from this work should be useful to the modeling of indoor environments and help | ||
| + | assess the importance of surfaces to indoor chemistry." | ||
| + | |||
| + | <font size="4">Monday, 15 April 19</font> | ||
| + | |||
| + | <b>Dropping Acid in the Atmosphere: Is It Just a Phase?</b> | ||
| + | |||
| + | [https://sites.google.com/a/umich.edu/ault_lab/home Prof. Andrew Ault], <br> | ||
| + | Univ. of Michigan | ||
| + | |||
| + | "Atmospheric aerosols are incredibly complex chemical systems with thousands of species | ||
| + | present in yoctoliter to attoliter volumes, which makes measuring their chemical and physical | ||
| + | properties an analytical challenge. Despite these instrumental demands, measuring aerosol | ||
| + | properties is essential, as air pollution leads to 10% of global deaths annually, primarily due to | ||
| + | the effects of atmospheric particles. These aerosols are also the most uncertain aspect of radiative | ||
| + | balance leading to climate change. The Ault Laboratory is focused on understanding the complex | ||
| + | heterogeneous and multiphase chemistry occurring within aerosols through systematic physical | ||
| + | chemistry studies, the development of new analytical methods and sensors, and measurements of | ||
| + | complex systems in that atmosphere. We conduct these studies this through a combination of | ||
| + | spectroscopy, microscopy, and mass spectrometry techniques. This seminar will focus on the | ||
| + | acidity, phase, and morphology of mixed organic-inorganic atmospheric particles. Specifically, | ||
| + | we will focus on the acid-catalyzed ring opening reaction of isoprene epoxydiols (IEPOX), | ||
| + | formation of organosulfates and polyols, and subsequent changes to diffusion in viscous | ||
| + | materials. From this we can predict future properties and amounts of secondary organic aerosol | ||
| + | (SOA). With our novel analytical methodologies and physical chemistry studies, the Ault | ||
| + | Laboratory is providing fundamental molecular insights into the chemistry occurring within | ||
| + | atmospheric aerosols that have significant consequences for human health and global climate." | ||
| + | |||
| + | <font size="4">Monday, 8 April 19</font> | ||
| + | |||
| + | <b>Deliquescence and Efflorescence of Chlorate Salts under Mars-relevant Conditions</b> | ||
| + | |||
| + | Marium Fernanders, <br> | ||
| + | 3rd year ANYL student, [https://cires.colorado.edu/research/research-groups/margaret-tolbert-group Tolbert lab] | ||
| + | |||
| + | <p>"When searching for life elsewhere in the universe, scientists have mainly focused on | ||
| + | finding liquid water. However, in addition to liquid water, the presence of certain types of salts | ||
| + | could also be a marker for water and perhaps life. The presence of chlorate salts, on the surface or | ||
| + | in the sub-surface, could be an indicator of where to find life and water on present day Mars. | ||
| + | Recent research has found that certain types of terrestrial bacteria can survive in per/chlorate-rich | ||
| + | salt environments by using these salts as an energy source. Because of the salts’ low eutectic | ||
| + | temperatures and ability to deliquesce (liquefy), these salts may be able to provide the ideal | ||
| + | conditions (liquid water and energy) where life may be found in either the surface or sub-surface.</p> | ||
| + | |||
| + | <p>In the search for life on other planetary bodies, one of the places where scientists are | ||
| + | looking is our closest neighbor: Mars. Mars, like Earth, exists in the “Goldilocks” zone a set area | ||
| + | around a star where planetary bodies are not too hot or too cold for liquid water to occur. However, | ||
| + | liquid water has not yet been detected on present day Mars. Mars with a little help from salts, could | ||
| + | support liquid water on the surface or in the sub-surface through the process of deliquescence, | ||
| + | where a crystal salt absorbs water vapor from the atmosphere, like a sponge, and when the | ||
| + | conditions are just right turn into a briny droplet of water. This research proposal will study the | ||
| + | low temperature deliquescence and efflorescence (when a briny droplet loses water through | ||
| + | evaporation and turns back into a salt crystal) of chlorate salts and chlorate salt mixtures under | ||
| + | Mars-relevant pressures and temperatures. By doing this, I will improve our knowledge of how | ||
| + | chlorate salts behave under conditions where liquid water may be formed on Mars. In order to | ||
| + | determine the conditions under which chlorates will deliquesce and eventually effloresce I will use | ||
| + | a Raman microscope attached to an environmental cell where the pressure, temperature, and | ||
| + | humidity can be controlled to mimic Mars-like conditions. The Raman microscope will allow me | ||
| + | to see a salt particle turn into a liquid and back into a crystal using visual and spectroscopic | ||
| + | identification. The Raman uses a green laser to probe a specific salt crystal or briny droplet. The | ||
| + | light from the laser gets scattered back into a detector and I can then use the light scattering to | ||
| + | characterize and identify the physical state, and chemical composition of crystal or droplet. The | ||
| + | information will allow me to determine the deliquescence relative humidity (DRH), and the | ||
| + | efflorescence relative humidity (ERH) of the chlorate salts and salt mixtures. By determining these | ||
| + | fundamental properties as a function of temperature and pressure, we can then use NASA’s Mars | ||
| + | rover and satellite data to determine if there are conditions on Mars where aqueous salt solutions | ||
| + | can exist, and possibly flow, on the surface or sub-surface."</p> | ||
| + | |||
| + | <font size="4">Monday, 18 Mar 19</font> | ||
| + | |||
| + | <b>Multiphase chemistry of volatile-organic-compound oxidation products under pre-industrial | ||
| + | conditions</b> | ||
| + | |||
| + | Prof [https://projects.iq.harvard.edu/keutschgroup Frank Keutsch],<br> | ||
| + | Harvard | ||
| + | |||
| + | "Oxidation of volatile organic compounds (VOCs) is an important atmospheric process tied to the | ||
| + | formation of secondary pollutants such as ozone and secondary aerosol. Research in the | ||
| + | Keutsch Group has been focusing on VOC oxidation chemistry under preindustrial conditions, | ||
| + | which primarily produces organic hydroperoxides. I will discuss recent results on the multi- | ||
| + | phase chemistry of some of the most abundant multifunctional organic hydroperoxides, which | ||
| + | result from isoprene oxidation. The results have implications for our understanding of gas- and | ||
| + | aerosol aspects of the reactive carbon cycle as well as sulfate formation via cloud processing." | ||
| + | |||
| + | <font size="4">Monday, 11 March 2019</font> | ||
| + | |||
| + | <b>Chemical intuition on the oxidation mechanism of Hg(0) in the gaseous atmosphere</b> | ||
| + | |||
| + | Prof. [http://www.esf.edu/faculty/dibble/ Theodore Dibble], <br> | ||
| + | SUNY-ESF | ||
| + | |||
| + | "Mercury harms ecosystems and harms human health on account of consumption of fish near the top of the food chain. The | ||
| + | atmosphere transports mercury around the globe from emission sources (e.g., power plants, re- | ||
| + | emission from ecosystems). These emissions are mostly in the form of Hg(0) (atomic mercury), | ||
| + | but deposition of Hg(II) compounds dominates transfer from the atmosphere to the Earth. | ||
| + | |||
| + | Investigations of the kinetics and mechanism of Hg(0) oxidation in the atmosphere present | ||
| + | many challenges to experiment. Field studies are still plagued by interferences and issues of | ||
| + | quantification. In the past several years my group has made great strides in understanding gas- | ||
| + | phase oxidation of Hg(0). These advances have come from chemical intuition and quantum | ||
| + | chemistry calculations. The first critical advance came in recognizing that BrHg radical was | ||
| + | much more likely to react with atmospherically abundant radicals, •Y, such as NO<sub>2</sub> and HOO, | ||
| + | (see Figure 1) than with OH or Br, which had been the only two reactants included in models of | ||
| + | BrHg• chemistry. Theory suggests very large rate constants for reactions of BrHg with Y. | ||
| + | Experiments on BrHg• chemistry and kinetics are starting, but have yet to produce results. | ||
| + | |||
| + | The second major point was to realize that BrHgONO, like HONO, would photolyze at | ||
| + | tropospherically relevant wavelengths to yield BrHgO•. Subsequent studies revealed that | ||
| + | BrHgO• behaved a lot like OH (left half of Figure 1), although BrHgO• is better at hydrogen | ||
| + | abstraction than OH. | ||
| + | [[File:Dibble_figure.png]]<br> | ||
| + | Finally, we have used theory to quantify the equilibrium constant for OH + Hg <-> HOHg and show that bond energies for HOHg-Y are essentially the same as those for BrHg-Y. With a few assumptions, this lets us build a mechanism and set of rate constants for atmospheric modeling of OH-initiated oxidation of Hg(0) to Hg(II)." | ||
| + | |||
| + | <font size="4">Monday, 4 March 2019</font> | ||
| + | |||
| + | <b>Understanding the Fate of Amines: Reactions with Oxygenates</b> | ||
| + | |||
| + | Mitch Alton, <br> | ||
| + | CU ANYL 3rd year, [https://sites.google.com/a/colorado.edu/brownelab/ Browne group] | ||
| + | |||
| + | "Atmospheric aliphatic amines and ammonia have been previously reported to participate in various chemical reactions including brown carbon formation, accretion reactions forming amides, imine and enamine formation, and acid-base cluster stabilization reactions that can enhance new particle formation. As anthropogenic reactive nitrogen emissions continue to increase to keep pace with world population and food demands, the interaction of these amines and ammonia with organic compounds in the atmosphere needs to be further investigated to better understand the impacts of these emissions on air quality and the environment. I will discuss a series of chamber experiments that investigated the effects of different aliphatic amines and ammonia on secondary aerosol formation from the ozonolysis of α-pinene without the use of seed aerosol. Using hierarchical clustering analysis, different fates of α-pinene ozonolysis products with amines/ammonia were identified. Various observed reactions between amines/ammonia and α-pinene ozonolysis products to form enamines, imines, amides, and acid-base clusters will be explored to show the complex chemistry that can occur during aerosol formation and growth. Finally, I will discuss how acid-base stabilization reactions are the most important contributions to particle nucleation in this system. " | ||
| + | |||
| + | <font size="4">Monday, 25 February 2019</font> | ||
| + | |||
| + | <b>Chemistry on Ice: Shedding Light on Arctic Halogen Photochemistry</b> | ||
| + | |||
| + | Prof. [https://lsa.umich.edu/chem/people/faculty/prattka.html Kerri Pratt], <br> | ||
| + | University of Michigan | ||
| + | |||
| + | "With rapid sea ice loss and warming, there is an urgent need to understand the unique chemistry involving multiphase reactions of atmospheric aerosols and the snow-covered sea ice surface in the Arctic. Yet, the harsh environment and low analyte concentrations pose analytical challenges. The Pratt Lab utilizes novel mass spectrometry techniques to measure the complex chemistry of trace gases, aerosols, and snow in the Arctic. Using chemical ionization mass spectrometry, we are advancing understanding of Arctic halogen photochemistry through measurements of trace gas species at ppq to ppt levels, including observations of trace gases species for the first time in the ambient atmosphere. Bromine, chlorine, and iodine chemistry, and the coupling of the cycles involving these halogen species, have significant impacts on the fate of greenhouse gases and pollutants, including ozone, methane, and mercury. Sunlit and artificial light experiments conducted in the Alaskan Arctic, combined with atmospheric measurements and numerical modeling, were utilized to elucidate chemical mechanisms driving the unique multiphase processes. The new chemical insights obtained are providing crucial scientific detail needed to understand and predict changing atmospheric composition in the Arctic." | ||
| + | |||
| + | <font size="4">Monday, 11 February 2019</font> | ||
| + | |||
| + | <b>Grills and grilles: cooking and traffic as drivers of spatial variations in exposure to particulate matter</b> | ||
| + | |||
| + | Prof. Albert Presto, <br> | ||
| + | Carnegie Mellon | ||
| + | |||
| + | "Sharp spatial gradients of particulate matter (PM), organic aerosol (OA), and black carbon (BC) concentrations exist at intra-city scales (<1 km) due to intense emissions from sources like traffic and cooking activities. Typical stationary deployment of samplers is not capable of resolving these spatial gradients. By deploying an Aerodyne Aerosol Mass Spectrometer (AMS) and other high temporal resolution measurements on a mobile sampling platform, we are able to investigate the spatial variation of PM mass concentration, PM composition, and particle number concentrations within cities. Source apportionment with Positive Matrix Factorization (PMF) enables identification of contributions of traffic and other sources to the observed PM mass. Cooking and traffic sources dominate PM spatial variability. | ||
| + | |||
| + | This presentation will show results for two cities: Pittsburgh and Oakland. In locations with high local source impact in Pittsburgh, the PM1 concentration is 2 mg m-3 (40%) higher than urban background locations. Traffic emissions are the largest source contributing to population-weighted exposures to primary PM. The concentration of both cooking and traffic PM are positively correlated to their respective geographical covariates: vehicle-miles travelled (VMT) and restaurant count. VMT is a reliable predictor for traffic PM concentrations for use in air pollutant spatial models. Restaurant count is an imperfect predictor for cooking PM concentration, likely due to the highly variable emissions of individual restaurants. Cooking PM is also positively correlated to VMT, which suggests that near-road cooking emissions can be misattributed to traffic sources in the absence of PM source apportionment. In Pittsburgh, 27.7% and 8.9% of the total population are exposed to >1 mg m-3 of traffic- and cooking-related primary emissions, with some populations exposed to high concentrations from both sources. Results for Oakland show similar spatial patterns and concentration trends. While these data were collected for two cities, the source mix in many U.S. cities is similar. We therefore expect similar PM spatial patterns and increased exposures in high-source areas nationwide." | ||
| + | |||
| + | <font size="4">Monday, 4 February 2019</font> | ||
| + | |||
| + | <b>The Synthesis and Reactions of 3-Hydroxymethylphthalimides</b> | ||
| + | |||
| + | Olivia Jenks, <br> | ||
| + | CU ANYL Chem 1st Year, [http://sites.google.com/view/de-gouw-lab de Gouw group] | ||
| + | |||
| + | "Phthalimides possess a wide range of physiological properties, including anti-inflammatory and immunomodulatory activities, and have been found to be useful in the preparation of specialized polymers and macrocycles. While a vast number of N-substituted phthalimides have been reported, the number of phenyl-substituted compounds is fairly limited. We have recently developed a convenient method for the synthesis of 3-hydroxymethylphthalimides, which have the potential for conversion into a number of other phenyl-substituted compounds. In this presentation, the transformation of the hydroxymethyl group to the corresponding chloride and the reactions of the benzylic halides with a variety of amines as nucleophiles will be described. With alkyl amines, nucleophilic acyl substitution at the imide ring competes with the desired alkyl substitution to yield diamides. Studies with substituted anilines show the rate of substitution varies markedly with the basicity of the nucleophile." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Fundamental investigation of substituent effects on threshold energy and electron density for the [1,3] thioallylic rearrangment</b> | ||
| + | |||
| + | Mindy Schueneman, <br> | ||
| + | CU ANYL Chem 1st Year, [http://cires1.colorado.edu/jimenez-group/ Jimenez group] | ||
| + | |||
| + | "Computational chemistry provides information of reaction energetics, thermodynamics, and kinetics by integrating computer modelling and and chemical theory. Computational chemistry was used to examine the effect of electron withdrawing and donating groups on both the threshold energy and electron density for the [1,3] thioallylic rearrangement mechanism. Thioallyl compounds contain a 3 carbon allyl chain with a sulfur in the C1 position. Through the combination of DFT and QTAIM, two computational methods, it was found that the thioallylic mechanism undergoes a concerted transition state, with most of the electron density contained on the sulfur atom. In addition, the activation energy barrier was found to decrease with the addition of electron donating groups located at various parts on the allyl backbone." | ||
| + | |||
| + | <font size ="4">Monday, 28 January 2019</font> | ||
| + | |||
| + | <b>Global Observations of Ammonium Balance and pH Indicate More Liquid Aerosol and Acidic Conditions than Current Models Predict</b> | ||
| + | |||
| + | Benjamin Nault, <br> | ||
| + | CU ANYL Chem Postdoc, [http://cires1.colorado.edu/jimenez-group/ Jimenez group] | ||
| + | |||
| + | "The inorganic composition of aerosol impacts numerous chemical and physical processes and | ||
| + | properties. However, many chemical transport models show large variability in both the | ||
| + | concentration of the inorganic aerosols and their precursors (up to 3 orders of magnitude | ||
| + | differences) and the composition of the inorganic aerosols. Different models would predict very | ||
| + | different properties such as aerosol liquid water concentration, aerosol acidity (but most models | ||
| + | do not calculate this property), heterogeneous uptake of gases, aerosols direct and indirect impact | ||
| + | on climate, et cetera. Here, I use airborne observations from campaigns conducted around the | ||
| + | world to investigate how the inorganic composition, and one of its key parameters, aerosol | ||
| + | acidity, changes from the polluted regions (Mexico City, Los Angeles, Northeastern US, and | ||
| + | Seoul) to the most remote regions (the Atmospheric Tomography campaigns 1 and 2), to provide | ||
| + | constraints for the chemical transport models. I find that the empirical ammonium balance | ||
| + | (ammonium balance = mol NH<sub>4</sub> / (2×mol SO<sub>4</sub> + mol NO<sub>3</sub>)) rapidly decreases from 0.85 in | ||
| + | polluted regions to less than 0.2 in remote regions, contradictory to some modeling studies that | ||
| + | suggest most of the has a balance near 1. The data imply very low NH<sub>3</sub> in the upper troposphere, | ||
| + | contrary to predicts of some models. Real-world aerosols are less likely to be in the solid phase | ||
| + | and more likely to be in a metastable liquid state. Next, I explore the aerosol acidity with the E- | ||
| + | AIM model, constrained by observations, and find that the acidity increases from the most | ||
| + | polluted (median = 2.3) to most remote regions (median = –0.5). The chemical transport models | ||
| + | have difficulty reproducing the aerosol acidity, showing both over and underestimation in pH. | ||
| + | Several causes likely lead to these measurement vs model differences in aerosol acidity, | ||
| + | including the mixing state of sea salt (internal vs. external) and total amount of NH x present in | ||
| + | the atmosphere (NH x = NH<sub>3</sub> (g) + NH<sub>4</sub> + (p)), which are currently being investigated and will be | ||
| + | briefly discussed during this talk." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Towards an improved representation of organic aerosol (OA) in the remote troposphere: Overall abundance, sources and physical and chemical removal</b> | ||
| + | |||
| + | Pedro Campuzano Jost, <br> | ||
| + | CU ANYL Chem Research Scientist, [http://cires1.colorado.edu/jimenez-group/ Jimenez group] | ||
| + | |||
| + | "Organic aerosol (OA) is one of the major contributors to the PM2.5 burden in the continental Northern Hemisphere (NH); understanding its sources and aging is central to current air quality control strategies. For the remote troposphere, sparse in-situ data to date results in highly under constrained OA prediction models, with model diversity of up to three orders of magnitude. | ||
| + | As part of the recently concluded NASA Atmospheric Tomography (ATom) aircraft mission, we have acquired four unique global datasets of submicron aerosol concentration and composition over the remote Atlantic and Pacific Oceans. Overall, OA concentrations except for the cleanest regions were comparable to sulfate, as in the Northern Hemisphere, with OA, sulfate and seasalt being the main contributors to both CCN and submicron AOD. | ||
| + | An evaluation of state-of-the-art models (CESM, GEOS-Chem) with ATom meteorology fields shows that while overall models reproduce remote OA concentrations fairly well, they mostly fail to reproduce the large ratio of secondary to primary observed in the measurements and use unrealistic OA/OC ratios for tracking OA. Improved model parameterizations that account for these factors overestimate OA in most remote regions, suggesting that an additional, slow loss channel for OA is needed. Based on a photochemical clock analysis of the Atom data, we find an OA lifetime of about 10 days for this process, consistent with recent estimations of the OA removal rate due to OH oxidation and photolysis." | ||
| + | |||
| + | ==Fall 2018== | ||
| + | |||
| + | <font size="4">Friday, 7 December 2018</font> | ||
| + | |||
| + | <b>TROPOMI on-board the Sentinel-5 Precursor mission: a game changer for tropospheric composition | ||
| + | monitoring from space</b> | ||
| + | |||
| + | Michel van Roozendael, <br> | ||
| + | Royal Belgian Institute for Space Aeronomy (BIRA-IASB) | ||
| + | |||
| + | "The Sentinel-5 Precursor (S5P) is the first atmospheric composition monitoring satellite in the | ||
| + | Copernicus Sentinel series operated by EU. Successfully launched on 13 October 2017, it carries the | ||
| + | Tropospheric Monitoring Instrument (TROPOMI), which provides daily global observations of the nadir | ||
| + | backscattered earthshine radiance in 3 spectral channels covering the UV, VIS, NIR and SWIR regions at | ||
| + | the unprecedented horizontal resolution of 7x3.5 km2. | ||
| + | BIRA-IASB has been involved in the mission preparation since early 2010 and is responsible for the | ||
| + | algorithm baseline definition and maintenance of the tropospheric HCHO, SO2 and total ozone, as well | ||
| + | as the operational validation as part of the S5P Mission Performance Center (MPC). | ||
| + | After a brief introduction on the mission, instrument characteristics and retrieval methods, I focus on | ||
| + | results obtained after one year of measurements with particular attention to species retrieved in the | ||
| + | UV-Visible spectral range. Observations reveal the distribution of tropospheric species in unprecedented | ||
| + | detail, allowing for a much more accurate identification of pollution sources at the level of cities, and | ||
| + | various local emission sources both of natural and anthropogenic origin. In many cases, the sensitivity of | ||
| + | the instrument exceed the expectations of the involved scientists." | ||
| + | |||
| + | <font size="4">Monday, 3 December 2018</font> | ||
| + | |||
| + | <b>Hydroxyl radical reactivity: Results from field campaigns in Europe and China</b> | ||
| + | |||
| + | Hendrik Fuchs, Forschungszentrum Jülich, <br> | ||
| + | Institute of Energy and Climate Research, Troposphere (IEK-8) | ||
| + | |||
| + | "OH reactivity is the total loss rate coefficient of the hydroxyl radical (OH), the main responsible agent for | ||
| + | the oxidation of pollutants in the atmosphere. The direct measurement of this quantity is of great value | ||
| + | as it constrains the removal rate of OH in the analysis of the atmospheric budget of OH radicals. In | ||
| + | addition, measured OH reactivity can be compared to calculations from individual OH reactant | ||
| + | concentration measurements in order to quantify the importance of unmeasured OH reactants. OH | ||
| + | reactivity measurements were done on a Zeppelin airship during flights over Europe showing the vertical | ||
| + | and horizontal distribution of OH reactants. Ground-based field campaigns in the North China Plain and | ||
| + | the Yangtze River Delta in summer- and wintertime were performed between 2006 and 2016 giving an | ||
| + | overview of the major OH reactants that contribute to air pollution in China." | ||
| + | |||
| + | <font size="4">Monday, 5 November 2018</font> | ||
| + | |||
| + | <b>OMI and TROPOMI: towards high resolution Air Quality and Emission monitoring</b> | ||
| + | |||
| + | [https://www.knmi.nl/over-het-knmi/onze-mensen/pieternel-levelt Pieternel Levelt], <br> | ||
| + | KNMI and University of Technology Delft | ||
| + | |||
| + | "The Ozone Monitoring Instrument (OMI), launched on board of NASA’s EOS-Aura spacecraft on July | ||
| + | 15, 2004, provides unique contributions to air quality monitoring from Space. The combination of urban | ||
| + | scale resolution (13 x 24 km 2 in nadir) and daily global coverage proved to be key features for the air | ||
| + | quality community. The OMI data is currently used operationally for improving the air quality forecasts, | ||
| + | for inverting high-resolution emission maps, UV forecast and volcanic plume warning systems for | ||
| + | aviation. Due to its almost 14 year continuous operation OMI provides the longest NO<sub>2</sub> and SO<sub>2</sub> | ||
| + | record from space, which is essential to understand the changes to emissions globally. | ||
| + | |||
| + | In 2017 Tropospheric Monitoring Instrument (TROPOMI), was launched on board ESA’s Sentinel 5 | ||
| + | Precursor satellite in October 2017. TROPOMI has a spatial resolution of 3,5x7 km<sup>2</sup> in nadir; a more | ||
| + | than 12 times improvement over OMI. The high spatial resolution serves two goals: (1) emissions | ||
| + | sources can be detected with even better accuracy and (2) the number of cloud-free ground pixels will | ||
| + | increase substantially. TROPOMI will continue OMI’s ozone and air quality trace gas records. Added | ||
| + | to that TROPOMI will measure the O<sub>2</sub> A band for better cloud detection, as well as CO and the | ||
| + | second most important greenhouse gas CH<sub>4</sub>. TROPOMI will therefore be an important satellite | ||
| + | mission for the EU Copernicus atmosphere service and will be followed by ESA’s sentinel 4 and 5 | ||
| + | satellites. The first measurements of TROPOMI turn out to be above expectation. | ||
| + | |||
| + | In the coming decades air pollution in megacities will continue to be a major area of concern and the | ||
| + | need for timely, high resolution information on emissions will increase, preferably to a level where | ||
| + | sources can be isolated on the < 1 x 1 km<sup>2</sup> scale. Currently we are working on new follow-on satellite | ||
| + | instrumentation with which we envisage to improve emission monitoring to the < 1 x 1 km<sup>2</sup> spatial | ||
| + | resolution level (TROPOLITE). | ||
| + | |||
| + | An overview of air quality applications, emission inventories, and trend analyses will be given, based | ||
| + | on the excellent OMI data record, followed by first measurements and results of the TROPOMI | ||
| + | instrument. An outlook will be presented on the potentials of the TROPOMI and what new satellite | ||
| + | instrumentation with a 1 x 1 km<sup>2</sup> spatial resolution can bring in the air quality and climate domain." | ||
| + | |||
| + | <font size="4">Wednesday, 31 October 2018</font> | ||
| + | |||
| + | <b>Dissertation Defense: Influence of multiphase processes on the chemistry and measurement of organic compounds in | ||
| + | indoor and outdoor environments</b> | ||
| + | |||
| + | Demetrios Pagonis, <br> | ||
| + | [https://sites.google.com/site/ziemanngroup/home Ziemann Lab], CU Boulder | ||
| + | |||
| + | "Organic compounds are ubiquitous in indoor and outdoor environments, with organic | ||
| + | aerosols and gases impacting air quality, global climate, and human health. The life cycle of | ||
| + | volatile organic compounds (VOCs) in the atmosphere includes emission from indoor and | ||
| + | outdoor sources, oxidation in the atmosphere to form secondary organic aerosol (SOA), and | ||
| + | eventual deposition to a surface. Understanding each of these processes is necessary to predict | ||
| + | the impact of organic compounds on indoor and outdoor environments, and this thesis presents | ||
| + | the results of a series of studies across the life cycle of VOCs, examining the chemical and | ||
| + | physical processes that transform organic compounds in the atmosphere. <br> | ||
| + | |||
| + | First, the chemistry of multifunctional hydroperoxides in SOA is studied by a series of | ||
| + | laboratory studies utilizing a model hydroperoxyaldehyde designed to represent the highly | ||
| + | oxidized multifunctional compounds that impact SOA growth in pristine environments. | ||
| + | Measurements of reaction rates, equilibrium constants, and decomposition mechanisms provide | ||
| + | insight into how chemical structure and aerosol properties affect the chemistry of multifunctional | ||
| + | hydroperoxides in SOA. Second, emission rates, deposition velocities, reaction rates, and | ||
| + | reaction products from a field study in a university art museum are presented. This study | ||
| + | quantifies the significant impact of human activities on indoor VOC emissions, as well as the | ||
| + | effect of indoor surfaces and indoor oxidants on the fate of those emissions. Lastly, this thesis | ||
| + | presents a study aimed to improve researchers’ ability to make time-resolved measurements of | ||
| + | gas-phase organic compounds that partition to instrument surfaces and to Teflon tubing | ||
| + | commonly used for sampling lines. The simple chromatography model presented here accurately | ||
| + | predicts the delay in instrument response caused by gas-surface partitioning across all the tubing | ||
| + | lengths, diameters, flow rates, and analytes tested. Together, the studies presented in this thesis | ||
| + | advance the understanding of, and the ability to measure, the fate of organic compounds in | ||
| + | indoor and outdoor environments." | ||
| + | |||
| + | <font size="4">Monday, 29 October 2018</font> | ||
| + | |||
| + | <b>Non-Targeted Chemical Characterization of Waterpipe Tobacco Smoke by GC-MS and LC-MS</b> | ||
| + | |||
| + | Andrew Jensen, <br> | ||
| + | ANYL 1st year student | ||
| + | |||
| + | "As smoking waterpipes becomes more popular, it becomes necessary to understand the chemical composition of waterpipe tobacco smoke (WTS) and its impacts on human health. Current studies target known components of cigarette smoke, but no current studies acknowledge the possible differences due to the tobacco product and heating profile. The tobacco product, shisha, is made up of tobacco leaves coated in syrup which itself is about one-third glycerol. The shisha is then heated using charcoal, producing larger pyrolysis products. The resulting smoke generated from these constituents can be divided into particulate matter (PM) and vapor. Each fraction may contribute differently to smoke toxicity, making it important to understand the chemical contributions of each constituent. These constituents were studied using gas and liquid chromatography tandem mass spectrometry with a non-targeted approach. | ||
| + | |||
| + | A semi-quantitative comparison of the chromatograms allows for the identification of the sources and sinks of certain compounds in WTS. The GC-MS chromatograms of the shisha, bowl water, PM, and vapor suggest no significant portion of the WTS is trapped by the bowl-water, contrary to popular belief that the water acts as a significant filter. In the LC-MS chromatograms, charcoal alone contributes 10 chromatographic peaks while glycerol, syrup, and shisha each contribute 5, 11, and 32 peaks respectively. A similar comparison of the shisha PM and vapor chromatograms shows that the vapor phase contributes 2 unique peaks while the PM contributes 43 peaks and 11 peaks are shared between the two phases. This study has begun the process of identifying the unique chemical characteristics of the individual sources and sinks of WTS. Toxicity studies can identify WTS fractions of interest, then the unique compounds in these fractions can be isolated by the methods presented in this study." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Experimental and Theoretical Studies in the Gas Phase Kinetics of Atomic Halides with Methacrolein and Alkyl Bromides</b> | ||
| + | |||
| + | Kyle Mackey, <br> | ||
| + | ANYL 1st year student | ||
| + | |||
| + | "In the studies conducted by the Wine Group the rate coefficients of the reactions of bromine radicals with methacrolein and chlorine radicals with several alkyl bromides were determined using the laser flash photolysis - resonance fluorescence technique as a function of temperature and pressure for use in atmospheric modeling and calculations." | ||
| + | |||
| + | <font size="4">Monday, 22 October 2018</font> | ||
| + | |||
| + | <b>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</b> | ||
| + | |||
| + | [https://sites.google.com/site/ziemanngroup/home Prof. Paul Ziemann], <br> | ||
| + | ANYL Chem Faculty, CU-Boulder | ||
| + | |||
| + | "Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have recently conducted a number of studies of indoor air chemistry at CU. In this talk I will describe how we conduct the studies by using a diverse array of measurement techniques." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Recent Results and Upcoming Projects Investigating Aerosol Sources, Properties, Processes, and Fate</b> | ||
| + | |||
| + | [http://cires1.colorado.edu/jimenez-group/ Prof. Jose L. Jimenez], <br> | ||
| + | ANYL Chem Faculty, CU-Boulder | ||
| + | |||
| + | "Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, properties, and evolution remain poorly understood. In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students. | ||
| + | |||
| + | Ongoing projects include global aerosol measurements and analysis as part of the NASA ATom project, which recently sampled (almost) pole-to-pole across the vertical profile. Model comparisons suggest the importance of fast OA removal channels, and a strong overestimation of primary OA in some models. Remote aerosols are very acidic with a typical pH ~ 0, which is significantly lower than predicted by global models. We are also performing a meta-analysis of urban SOA at megacities worldwide, which shows remarkably consistent results and allows us to more accurately estimate the global number of deaths due to this source. Other topics that we are working on, and that I will touch on as time permits, are RO2 chemistry in Oxidation Flow Reactors (OFR) and how it compares with large chambers and the atmosphere; gas/particle partitioning in the laboratory for different types of seed particles; the impact of different types of tubing and instruments on the measurement of intermediate volatility and semivolatile species; the sources and budget of organic carbon in indoor air; and the development of fast SOA parameterizations for global and climate models. | ||
| + | |||
| + | Some upcoming projects include include the study of emissions and chemical evolution of smoke from real fires in the western US with the NASA DC8 (NASA FIREX-AQ) with AMS and soft-ionization EESI-TOF; an upcoming indoor campaign at a weight room at the CU Athletic Dept; and potentially the CalNexT ground-based study of urban chemistry in Los Angeles (which follows up on the highly successful CalNex-2010 study)." | ||
| + | |||
| + | <font size="4">Monday, 8 October 18</font> | ||
| + | |||
| + | <b>Small molecules in the Anthropocene: Opportunities in remote pristine and polluted air</b> | ||
| + | |||
| + | [http://ciresgroups.colorado.edu/volkamergroup/ Prof. Rainer Volkamer], <br> | ||
| + | ANYL Faculty, CU Boulder | ||
| + | |||
| + | "The Volkamer group develops advanced optical instrumentation (in situ and remote sensing) to measure | ||
| + | small molecules and aerosols that are relevant to public health and climate. We seek to develop a | ||
| + | molecular level understanding of the fundamental physical chemistry affecting their sources, | ||
| + | transformations and sinks using a combination of field observations, laboratory experiments and | ||
| + | modeling. Opportunities for graduate study exist as part of funded projects to 1) better understand | ||
| + | anthropogenic enhancements of biogenic organic aerosol and new particle formation (laboratory | ||
| + | studies), 2) to develop innovative retrievals for CU SOF, apply them to aircraft datasets that characterize | ||
| + | wildfires quantitatively for the first time, and test and develop atmospheric models, and 3) to develop | ||
| + | long-term datasets of halogen oxide radicals and oxygenated VOC in the remote marine atmosphere." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>In situ production of methyl bromide, methyl chloride, and carbon disulfide in the GISP2D ice core</b> | ||
| + | |||
| + | Christopher Lee, <br> | ||
| + | ANYL 1st year, CU Boulder | ||
| + | |||
| + | "The mixing ratios of methyl bromide, methyl chloride, carbon disulfide, and carbonyl sulfide were measured via gas chromatography-mass spectrometry | ||
| + | (GC-MS) in eleven samples from the GISP2D ice core from Summit, Greenland. The samples range in depth from 1826 meters to 2020 meters and in age from 15000 years BP to 25000 years BP. Correlations between the trace gas mixing ratios and the concentrations of major ions (Na<sup>+</sup>, NH<sub>4</sub><sup>+</sup>, K<sup>+</sup>, Mg<sup>2+</sup>, Ca<sup>2+</sup>, Cl<sup>-</sup>, NO<sub>3</sub><sup>-</sup>, and SO<sub>4</sub><sup>2-</sup>) in the ice at the same depth are examined. Additionally, correlations between the trace gas mixing ratios and the concentrations of sea salt and non-sea salt components of major ions in the ice at the same depth are examined. Finally, correlations between the trace gas mixing ratios and the concentrations of major ions in the ice at the same age are examined. Due to the significant difference in age (~619 years) between the air trapped in the ice and the ice itself, the same-depth correlations provide evidence for the in situ production of methyl bromide, methyl chloride, and carbon disulfide within the examined depth range. The same-age correlations provide evidence for a contemporaneous connection between carbonyl sulfide and sodium chloride." | ||
| + | |||
| + | <font size="4">Monday, 1 October 18</font> | ||
| + | |||
| + | <b>Investigating the pH of atmospheric fine particles and implications for atmospheric chemistry</b> | ||
| + | |||
| + | Hongyu Guo, <br> | ||
| + | Postdoctoral Researcher, [http://cires1.colorado.edu/jimenez-group/ Jimenez lab], CU Boulder | ||
| + | |||
| + | "Particle acidity is a critical but poorly understood quantity that affects | ||
| + | many aerosol processes and properties. In this talk, I will introduce a popular pH | ||
| + | prediction method in recent years since pH detection technique is limited. Particle pH | ||
| + | and water (which affects pH) are predicted using a thermodynamic model and | ||
| + | measurements of RH, T, and inorganic gas and particle species. The method was first | ||
| + | developed during the SOAS field campaign conducted in the SE US in summer (fine | ||
| + | particle pH = 0.94 ± 0.59), and then extended to aircraft observations in the NE US in | ||
| + | winter (WINTER study; pH = 0.77 ± 0.96). The results are validated by reproducing | ||
| + | particle water and gas-particle partitioning of NH<sub>4</sub> + and NO<sub>3</sub> - (sensitive to pH). I will | ||
| + | show why commonly used pH proxy, ion balance or molar ratio, doesn’t necessarily | ||
| + | represent pH. Some impacts of low particle pH were investigated, including the | ||
| + | effects on aerosol nitrate trends and the role of acidity in heterogeneous chemistry. | ||
| + | Despite a ~70% sulfate reduction in the southeastern US in the last 15 years, the fine | ||
| + | particles remained highly acidic due to buffering by semivolatile NH<sub>3</sub>. Importantly, | ||
| + | pH is not highly sensitive to NH<sub>3</sub>, a 10-fold increase in NH<sub>3</sub> only increases pH by one | ||
| + | unit in various locations and seasons, which has implications for use of NH<sub>3</sub> controls | ||
| + | to reduce PM 2.5 concentrations." | ||
| + | |||
| + | <font size="4">Monday, 24 September 2018</font> | ||
| + | |||
| + | <b>Organic nitrogen chemistry: Recent results and future projects</b> | ||
| + | |||
| + | [https://sites.google.com/a/colorado.edu/brownelab/ Prof. Ellie Browne], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Organic nitrogen is a ubiquitous atmospheric component typically accounting for between one-quarter and one- | ||
| + | third of reactive nitrogen deposition. The chemical complexity and reactivity of organic nitrogen, however, has | ||
| + | made it challenging to study. Consequently, little is known about the atmospheric processing of organic | ||
| + | nitrogen and the resulting implications for biogeochemistry, air quality, and climate. Research in my group uses | ||
| + | mass spectrometry to identify the organic nitrogen compounds present in the atmosphere and to investigate the | ||
| + | atmospheric processing of these compounds. In this talk, I will discuss our recent work on organic nitrogen | ||
| + | chemistry and describe new projects on aerosol measurement and chemical transport modeling." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Chemistry of Volatile Organic Compounds in the Atmosphere</b> | ||
| + | |||
| + | [http://cires.colorado.edu/council-fellows/joost-de-gouw Prof Joost de Gouw], <br> | ||
| + | ANYL faculty, CU Boulder | ||
| + | |||
| + | "Volatile organic compounds (VOCs) are released from many different natural and man-made | ||
| + | sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes | ||
| + | to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air | ||
| + | quality and climate and are a challenge to understand due to the large number of different | ||
| + | VOCs that are released to the atmosphere and the analytical difficulties in measuring all of | ||
| + | these compounds as well as their oxidation products. | ||
| + | |||
| + | In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight | ||
| + | mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF | ||
| + | allows measurements of many different VOCs with high time resolution and without the need | ||
| + | for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of | ||
| + | time resolution. We also combine these methods to better understand the compounds that are | ||
| + | detected by PTR-TOF in different environments. | ||
| + | |||
| + | Several different ongoing and future projects will be presented in this seminar. First, we | ||
| + | recently acquired a new Vocus PTR-TOF and are characterizing and preparing this instrument | ||
| + | for measurements of indoor air in the CU Athletic Center. This will allow quantification of VOCs | ||
| + | released from student athletes as well as during pre-game, indoor tail-gate parties. Second, we | ||
| + | are working on the emissions and chemistry of VOCs released from volatile chemical product | ||
| + | (VCP) use to the atmosphere, which was recently discovered to be the dominant source of | ||
| + | VOCs in urban air. This research will involve the development of new analytical capabilities, | ||
| + | field measurements in Boulder and in Los Angeles, and laboratory work to better understand | ||
| + | the chemistry of VCPs. Finally, we are working on a chamber study to better understand the | ||
| + | formation of secondary organic aerosol from biogenic VOCs." | ||
| + | |||
| + | <font size="4">Monday, 17 September 2018</font> | ||
| + | |||
| + | <b>Photochemical and Dark Ageing of Organic Aerosols</b> | ||
| + | |||
| + | [http://aerosol.chem.uci.edu/ Prof. Sergey Nizkorodov]<br> | ||
| + | Department of Chemistry, University of California, Irvine<br> | ||
| + | |||
| + | "Atmospheric aerosols significantly affect air quality, visibility, and global climate. Organic | ||
| + | compounds make up a significant, and often dominant, fraction of the atmospheric particulate matter. | ||
| + | Primary organic aerosol is emitted in the atmosphere by various sources, and secondary organic aerosol is | ||
| + | produced directly in the atmosphere as a result of a complex sequence of reactions that start with the | ||
| + | oxidation of volatile organic compounds and end with the condensation of the low-volatility products into | ||
| + | particles. What makes the representation of organic aerosols in climate and air quality models challenging | ||
| + | is their astonishingly high degree of chemical complexity. Furthermore, the chemical composition of | ||
| + | organic aerosols continuously changes as a result of various “ageing” processes, such as photolysis, | ||
| + | hydrolysis, oligomerization, oxidation, and other reactions involving aerosol constituents and atmospheric | ||
| + | gases. This presentation will examine the role of condensed-phase photochemical processes in the aerosol | ||
| + | ageing, i.e., processes initiated by absorption of solar radiation by an organic compound within a particle | ||
| + | or cloud/fog droplet. If time permits, we will also discuss “dark” ageing processes, which occur without | ||
| + | any involvement of solar radiation and free radicals, and result in the formation of compounds with | ||
| + | unusual properties, such as organic compounds capable of absorbing visible radiation (so called “brown | ||
| + | carbon”)." | ||
| + | |||
| + | <font size="4">Monday, 10 September 2018</font> | ||
| + | |||
| + | <b>Molecules to particles – Experiments and simulations relevant to new particle formation in the marine atmosphere</b> | ||
| + | |||
| + | Henning Finkenzeller<br> | ||
| + | ANYL 4th year, [http://ciresgroups.colorado.edu/volkamergroup/ Volkamer lab]<br> | ||
| + | |||
| + | "In this talk, I present my projects within the CLOUD consortium. CLOUD is an atmospheric simulation chamber at CERN, Geneva, Switzerland, to study the formation and growth of new particles in urban, rural, marine, and free tropospheric environments. In particular, iodic acid (HIO<sub>3</sub>) is thought to be a key precursor for new particle formation in the marine atmosphere, but the sources of HIO<sub>3</sub> are currently unknown. Condensation of HIO<sub>3</sub> is thought to be the primary mechanism by which iodine forms new particles at Mace Head, Ireland, and possibly other marine environments (Sipilä et al., 2016). | ||
| + | |||
| + | I have adapted the Volkamer group iodine chemistry box model to investigate known sources of HIO<sub>3</sub> (e.g., OIO + OH) and inform missing formation pathways. In combination with theory, I have incorporated new gas-phase reactions into the model, and compared the predictions with CLOUD observations. As pre-requisite for modeling CLOUD experiments, I have developed the photolysis module (irradiation by different lamps), temperature and humidity control, the injection of precursor gases, dilution and losses to the wall. The experimental conditions (precursor and intermediate species concentrations) can be prescribed, or taken as those observed in the chamber. | ||
| + | |||
| + | I have further developed the Model for Acid Base Chemistry and Nanoparticle Growth (MABNAG) to represent reactive uptake due to Setschenow salting-in of glyoxal (CHOCHO). Glyoxal is a volatile, ubiquitous, and putatively simple gas that forms from the oxidation of aromatic hydrocarbons, isoprene, and also non-traditional precursors, e.g., fatty acids. Upon contact with wet and sulfate containing particles glyoxal-hydrate-sulfate complexes form that have extremely low volatility. I am examining the contributions to the later stages of particle growth, and am developing a glyoxal source for future experiments at CLOUD." | ||
| + | |||
| + | ==Summer 2018== | ||
| + | |||
| + | <font size="4">Thursday, 19 July 2018 (Supergroup)</font> | ||
| + | |||
| + | <b>Secondary Organic Aerosol Production from Local Emissions Dominates OA Budget over Seoul, South Korea, during KORUS-AQ</b> | ||
| + | |||
| + | Ben Nault, <br> | ||
| + | ANYL Postdoc, Jimenez lab<br> | ||
| + | |||
| + | "Organic aerosol (OA) is an important fraction of submicron aerosols. However, it is still challenging to predict and attribute which organic compounds and sources lead to the observed OA, including over megacities. This can especially be true for megacities surrounded by numerous other regional sources that create an OA background. Here, we utilize in-situ gas and aerosol observations collected on-board the NASA DC-8 during the NASA/NIER KORUS-AQ (KORea United States-Air Quality) campaign to investigate the sources and hydrocarbon precursors that led to the SOA production observed over Seoul. First, we investigate the role of background and transport of OA into Seoul, using observations over the West Sea and WRF-Chem FLEXPART simulations. During KORUS-AQ, we conclude that the average OA advected into Seoul was ~1-3 µg sm–3. Then, taking this background into account, the dilution-corrected SOA concentration observed over Seoul was ~140 µg sm–3 ppmv–1 at 0.5 equivalent photochemical days. This value is comparable, though higher, than what has been observed in other megacities around the world (20–70 µg sm–3 ppmv–1 at 0.5 equivalent days). For the average OA observed over Seoul (13 µg sm–3), it was found that the local production of secondary OA (SOA) overwhelmed the transport/background of OA from foreign sources. The role of local SOA production was further supported by the following methods: (1) WRF-Chem FLEXPART source contribution calculations indicate any hydrocarbons with a lifetime less than 1 day, which are shown to dominate the observed SOA production, mainly originate from South Korea. (2) SOA correlated strongly with other secondary photochemical species, including short-lived species (formaldehyde, peroxy acetyl nitrate, sum of acyl peroxy nitrates, and dihydroxy toluene). (3) Results from an airborne oxidation flow reactor (OFR), flown for the first time, show a factor of 4.5 increase in potential SOA concentrations over Seoul versus over the West Sea, a region where background air masses that are advected into Seoul can be measured. (4) Results from a box model suggest that short-lived hydrocarbons (i.e., xylenes, trimethylbenzenes, semi- and intermediate volatility compounds) were the main SOA precursors over Seoul. Toluene contributes 9% of the modeled SOA over Seoul. Along with these results, we introduce using ΔOA/ΔCO2, which provides an insight into the amount of OA produced per fuel consumption in a megacity, and which shows less variability across the world than ΔOA/ΔCO. In summary, although long-distance transport was found to influence OA in Seoul, local emissions and rapid SOA production was found to dominate the total OA concentrations during KORUS-AQ. Seoul appears to have has larger relative emissions of SOA precursors compared to other megacities, which could be targeted for air quality improvement. This study provides important insight and constraints on the SOA production over large megacities." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Measurements of Positive Ambient Ions in Lamont OK as Part of the Holistic Interaction of Shallow Clouds Aerosols and Land Ecosystems (HISCALE II) Field Campaign</b> | ||
| + | |||
| + | Aroob Abdelhamid, <br> | ||
| + | ANYL Graduate Student, Browne lab<br> | ||
| + | |||
| + | "Atmospheric ions control the electrical properties of the atmosphere, influence chemical composition via ion-molecule and/or ion-catalyzed reactions, and affect new particle formation. Understanding the role of ions in these processes requires knowledge of ionic chemical composition. Due to the low concentration of ions, chemical composition measurements have historically been challenging. Recent advances in mass spectrometry, such as the atmospheric pressure interface time-of- flight mass spectrometer (APi-TOF), are now making these measurements more feasible. Here, we present measurements of ambient cations during the HISCALE II field campaign (August- September 2016) in Lamont, OK. We discuss how the chemical composition of cations varies over the course of the campaign and what patterns from this data tell us about the role of organic nitrogen. Furthermore, preliminary results of the anion data will be discussed." | ||
| + | |||
| + | <font size="4">Thursday, 19 July 2018</font> | ||
| + | |||
| + | <b>Role of Multiphase Chemistry on the Formation of Aerosol from the Reactions of Monoterpenes with NO<sub>3</sub> Radicals and O<sub>3</sub></b> | ||
| + | |||
| + | Megan Claflin, <br> | ||
| + | ANYL Student, CU Boulder, [https://sites.google.com/site/ziemanngroup/home Ziemann lab] | ||
| + | |||
| + | "Secondary organic aerosols (SOA) have been shown to influence regional and global air | ||
| + | quality, climate, and human health. To predict and mitigate the impacts of SOA formation, it is | ||
| + | essential to better understand the detailed mechanisms of formation, atmospheric processing, and | ||
| + | physical properties of SOA. In this thesis, the gas-and particle-phase reaction products and | ||
| + | mechanisms involved in the formation of SOA from the oxidation of monoterpenes in an | ||
| + | environmental chamber were studied in an extensive series of laboratory experiments that | ||
| + | employed a variety of online and offline analytical methods. In addition, a limited selection of | ||
| + | laboratory-generated samples and ambient aerosol samples collected from the Southeast US were | ||
| + | analyzed to compare the utility two different methods of functional group analysis. | ||
| + | The research included four major studies. 1) Identification and quantification of the | ||
| + | products that form SOA from the reaction of β-pinene with NO<sub>3</sub> radicals. The results highlight | ||
| + | the importance of ring-opening, alkoxy radical decomposition, and oligomerization reactions, | ||
| + | and were used to develop gas- and particle-phase reaction mechanisms. 2) A detailed study of the | ||
| + | thermal desorption characteristics of hemiacetal and acetal oligomers for use in understanding | ||
| + | mass spectra obtained from the analysis of SOA containing these components. Oligomers formed | ||
| + | by a single hemiacetal linkage, when subjected to thermal desorption analysis, will decompose to | ||
| + | their precursor aldehyde and alcohol monomers prior to desorption and detection. Oligomers | ||
| + | formed by more than one of these linkages, or an acetal or ester linkage, will desorb and be | ||
| + | detected intact. 3) Quantification of the functional group composition of SOA formed from α- | ||
| + | pinene ozonolysis over a range of α-pinene concentrations and humidities, including | ||
| + | autoxidation conditions. The SOA analyses, when combined with results of modeling, provide | ||
| + | insight into the effects of RO<sub>2</sub>• radical reaction regime, humidity, and particle-phase reactions in | ||
| + | determining SOA composition. 4) Comparison of two methods for quantifying the functional | ||
| + | group composition of organic aerosol: the Fourier Transform Infrared spectroscopy method | ||
| + | developed by the Russell group at the Scripps Institute of Oceanography and a derivatization- | ||
| + | spectrophotometric method developed by the Ziemann group at the University of Colorado, | ||
| + | Boulder. Results for laboratory-generated SOA and ambient aerosol samples show that the two | ||
| + | methods agree quite well when results for certain functional groups are combined, and that either | ||
| + | is adequate for measuring SOA functional group composition. This study also demonstrates that | ||
| + | the functional group composition of SOA can help in elucidating the sources and environmental | ||
| + | conditions under which the SOA was formed." | ||
| + | |||
| + | |||
| + | <font size="4">Thursday, 21 June 2018</font> | ||
| + | |||
| + | <b>Vapor-Liquid Equilibria Pertaining to the Study of Alternative Fuels and the Forensic Analysis of Chemical Evidence </b> | ||
| + | |||
| + | Megan Harries, <br> | ||
| + | ANYL Student, CU Boulder / NIST | ||
| + | |||
| + | "Measurement of the vapor-liquid equilibrium (VLE) of fluid mixtures with many components presents a challenge. Data describing such mixtures, like fuels, are important for the development of alternative energy sources and to support forensic science, but there is a lack of suitable instrumentation to provide data with reasonable uncertainty for mixtures with many components. In this thesis, three different techniques for fluid characterization are explored: the Advanced Distillation Curve (ADC), the Advanced Distillation Curve with Reflux (ADCR), and PLOT-cryoadsorption. | ||
| + | |||
| + | Two pyrolysis fuels similar to gasoline and diesel fuel made from polypropylene were studied with respect to volatility, composition, and energy content using the Advanced Distillation Curve. The diesel fuel demonstrated volatility very similar to existing petroleum-derived diesel fuels. The gasoline was less volatile than existing counterparts and did not meet specifications. Two pyrolysis crude oils made from ponderosa pine and dairy manure were assessed using the ADC coupled to an approach for characterizing fluids with multiple, immiscible liquid phases. Both oils contained high water levels and require further refinement. The organic phases of each oil contained components indicative of the feedstock used. | ||
| + | |||
| + | A modification of the ADC method, the Advanced Distillation Curve with Reflux, was introduced as an approach to measuring the VLE of fluids with many components. ADCR additionally approximates the weathering of an ignitable liquid that occurs during an arson fire and measures VLE across a range of weathered conditions. The method was demonstrated using two simple mixtures. The measurements agreed well with models, indicating that ADCR is a suitable VLE metrology. Vapor-liquid equilibrium data are crucial for interpreting the results of headspace characterization used often in forensic science. One headspace method, portable PLOT-cryoadsorption, was tested in a series of experiments in the laboratory, then deployed for the first time in a field environment that simulated a cargo container. The technology was found to be rapid and sensitive to a variety of compounds of interest to forensic science. Each of the three techniques described in this thesis contribute valuable property data for multicomponent mixtures, towards the development of high-quality predictive models." | ||
| + | |||
| + | <font size="4">Thursday, 10 May 2018</font> | ||
| + | |||
| + | <b>Measurements and Modeling of Nitrogen Oxides: Tropospheric Transformations During Summer and Winter in Polluted Regions Across the U.S.</b> | ||
| + | |||
| + | Erin McDuffie <br> | ||
| + | ANYL Student, CU Boulder | ||
| + | |||
| + | "Atmospheric reactions of inorganic nitrogen oxides critically influence the composition of the | ||
| + | troposphere, the lowest layer of the atmosphere that supports all terrestrial life on Earth. From | ||
| + | controlling the global budget and distribution of tropospheric oxidants, to degrading local air | ||
| + | quality through the production of ozone (O<sub>3</sub>) and secondary particulate matter (PM), | ||
| + | understanding the underlying chemistry of reactive nitrogen oxides is vital to both improving our | ||
| + | predictive capabilities of global tropospheric chemistry and to developing effective mitigation | ||
| + | strategies in regions with persistently poor air quality. Despite decades of research into their | ||
| + | chemical mechanisms, significant uncertainties remain in the seasonally dependent lifetime and | ||
| + | distribution of nitrogen oxides. Key remaining questions include: 1) the sensitivity of | ||
| + | photochemical pollutant production to location-specific emission sectors, 2) factors influencing | ||
| + | nocturnal inter-conversion processes, which involve multiphase reactions, and 3) the quantitative | ||
| + | contribution of these heterogeneous reactions to wintertime air pollution. | ||
| + | |||
| + | In this thesis, I address these questions using observational and modeling-based analyses | ||
| + | of data collected during three U.S. field campaigns in summer 2014 and the winters of 2015 and | ||
| + | 2017. I first present observations from summer 2014 and results from an observationally- | ||
| + | constrained, photochemical box model. This study was the first to quantify the contribution of oil | ||
| + | and natural gas emissions to local O<sub>3</sub> pollution in the Colorado Front Range, a region that has | ||
| + | been out of compliance with national air quality standards for O<sub>3</sub> since 2008. I next present the | ||
| + | first wintertime aircraft determinations of aerosol uptake coefficients of dinitrogen pentoxide | ||
| + | (N<sub>2</sub>O<sub>5</sub>) and production yields of nitryl chloride (ClNO<sub>2</sub>). These parameters were derived from a | ||
| + | custom, iterative, inorganic nocturnal nitrogen chemistry box model, fit to aircraft observations | ||
| + | collected over the U.S. east coast in 2015. Field-determinations of these parameters are further | ||
| + | compared to laboratory-based parameterizations to evaluate the current representation of these | ||
| + | processes in global models. Lastly, I present results from the first aircraft observations in Salt | ||
| + | Lake Valley, Utah. Observations and box model simulations are combined to assess the | ||
| + | contribution of heterogeneous reactive nitrogen chemistry to wintertime PM formation in this | ||
| + | region, which frequently violates PM air quality standards during wintertime pollution events." | ||
| + | |||
| + | ==Spring 2018== | ||
| + | <font size="4">Monday, 30 April 2018</font> | ||
| + | |||
| + | <b>Inhalation and Sublingual Delivery of Medical Cannabinoids and Vaccines</b> | ||
| + | |||
| + | Robert Sievers,<br> | ||
| + | University of Colorado, Environmental Program | ||
| + | |||
| + | "The development of cannabis products that conform to pharmaceutical level quality standards would be of great benefit to those attempting to use cannabis for medicinal purposes. Currently, the cannabis industry generates products that vary substantially in consistency of dosing, time of onset, and safety of administration route. The bioavailability of these products is not optimal due to a combination of inefficient absorption, first pass metabolism effects, and cannabinoid degradation. To combat these issues, we have developed a cannabinoid-containing dry powder that utilizes isolated, highly purified cannabinoids and excipients that are generally regarded as safe (GRAS) or have recently been determined in clinical trials of formulations to be safe for inhalation. The powder is suitable for respiratory delivery from a simple dry powder inhaler (DPI). Delivery to the lungs in this manner provides a consistent dose with a rapid onset of effects and avoids the bioavailability issues and first-pass metabolism encountered with other methods of administration. Various cannabinoids can also be combined in customized ratios targeted for the treatment of specific pathologies. A new US Patent #9,895,321 to the five authors (Sievers; Robert E., Cape; Stephen P., McAdams; David, Manion; J'aime, Pathak; Pankaj) was issued on February 20, 2018. | ||
| + | Authors: Robert Sievers, Lia Rebits, Xuno Gildelamadrid" | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Observations of particle organic nitrates from airborne/ground platforms: | ||
| + | Insights into method improvement, vertical/geographical distribution, gas/particle partitioning, losses, and contribution to total particle nitrate</b> | ||
| + | |||
| + | Doug Day, <br> | ||
| + | University of Colorado, ANYL Research Scientist | ||
| + | |||
| + | "Organic nitrate formation in the atmosphere represents a sink of NO<sub>x</sub> and termination of the | ||
| + | HO<sub>x</sub> /NO<sub>x</sub> ozone formation cycles, can act as a NO<sub>x</sub> reservoir transporting reactive nitrogen, and | ||
| + | contributes to secondary organic aerosol formation. However, particle-phase organic nitrates (pRONO<sub>2</sub>) | ||
| + | are rarely measured and thus poorly understood. Due to the increasing prevalence of aerosol mass | ||
| + | spectrometer (AMS) field measurements and promise of its use in determining quantitative bulk organic | ||
| + | nitrate functional group contribution to aerosols, a detailed evaluation of quantification methods is | ||
| + | timely. A simple method that relies on the relative intensities of NO + and NO<sub>2</sub> + intensities in the AMS | ||
| + | spectrum, calibrated NO<sub>x</sub> + ratio for NH<sub>4</sub>NO<sub>3</sub> , and inferred ratio for pRONO<sub>2</sub> has been previously proposed | ||
| + | as a way to apportion the total nitrate signal to NH<sub>4</sub>NO<sub>3</sub> and pRONO<sub>2</sub>, and been used by several groups | ||
| + | using a variety of different methods and assumptions. An extensive survey of NO<sub>x</sub> + ratios measured for | ||
| + | various pRONO<sub>2</sub> compounds and mixtures from multiple instruments, groups, and laboratory and field | ||
| + | measurements shows that, in the absence of a pRONO<sub>2</sub> standard, the pRONO<sub>2</sub> NO<sub>x</sub> + ratio can be | ||
| + | estimated using a ratio referenced to the calibrated NH<sub>4</sub>NO<sub>3</sub> ratio, a so-called Ratio-of- Ratios (RoR). We | ||
| + | systematically explore the viability, accuracy, and errors associated with quantifying pRONO<sub>2</sub> with the | ||
| + | AMS RoR NO<sub>x</sub> + ratio method using ground and aircraft field measurements conducted over a large range | ||
| + | of conditions. Positive Matrix Factorization (PMF) of thermal denuder measurements was conducted to | ||
| + | further explore the efficacy of the RoR NO<sub>x</sub> + ratio method and to construct volatility basis sets (VBS) of | ||
| + | pRONO<sub>2</sub> for several campaigns. A broad survey of ground and aircraft AMS measurements, applying the | ||
| + | RoR NO<sub>x</sub> + ratio method, shows a pervasive trend of higher contribution of pRONO<sub>2</sub> to total nitrate with | ||
| + | lower total nitrate concentrations. | ||
| + | |||
| + | <p>Simultaneous measurements of pRONO<sub>2</sub> (applying the AMS RoR NO<sub>x</sub> ratio method) and of total (gas+particle) | ||
| + | organic nitrate (totRONO<sub>2</sub>), organic aerosols (OA), and ammonium nitrate from aircraft and several | ||
| + | ground campaigns were used to investigate vertical/geographical distributions, gas/particle partitioning, | ||
| + | losses, and contributions to total particle nitrate (pTotNO<sub>3</sub>) over North America. pRONO<sub>2</sub> and totRONO<sub>2</sub> | ||
| + | concentrations show strong vertical gradients, with a steep decrease from the top of the boundary layer | ||
| + | (BL) up through the residual layer. However, pRONO<sub>2</sub> was 10-30% of totRONO<sub>2</sub> with little vertical | ||
| + | gradient in gas/particle partitioning from the BL to upper troposphere (UT). pRONO<sub>2</sub> contribution to OA | ||
| + | shows a moderate increase with decreasing OA in the BL and free troposphere (~2-3% by mass of nitrate | ||
| + | group) with higher contributions at the lowest OA (5-8%), mostly observed in the UT. In the BL, RONO<sub>2</sub> | ||
| + | gas/particle partitioning shows a trend with temperature, with higher particle-phase fraction at lower | ||
| + | temperatures, as expected from partitioning theory. However, the temperature trend is much weaker | ||
| + | than for single compound partitioning, which may be due to a broad mixture of species. Little to no | ||
| + | dependence of pRONO<sub>2</sub> /OA on RH or estimated particle water was observed in the BL, suggesting that | ||
| + | losses of pRONO<sub>2</sub> due to hydrolysis are too rapid to observe in this dataset and there may be a | ||
| + | substantial fraction of pRONO<sub>2</sub> species that are not prone to rapid hydrolysis." | ||
| + | <br><br> | ||
| + | <font size="4">Wednesday, 25 April 2018</font> | ||
| + | <br> | ||
| + | <b>Chemistry on Mars: The Search for Habitable Environments with Curiosity</b> | ||
| + | <br> | ||
| + | Melissa Trainer, <br> | ||
| + | Planetary Environments Laboratory, NASA Goddard Space Flight Center<br> | ||
| + | |||
| + | "Following on decades of exploration of Mars, our knowledge of our neighboring planet has | ||
| + | advanced well beyond observations of canals to the comprehensive characterization of surface | ||
| + | topology and regional mineralogy. There are clear lines of evidence for past liquid water and a | ||
| + | complex climate history. Yet some of the fundamental questions remain: Was there ever life on | ||
| + | Mars? Could there have been life on Mars? The Curiosity rover carries the most advanced | ||
| + | analytical laboratory sent to another planet, and over the past four and half years the mission | ||
| + | has performed a detailed in situ investigation of Gale Crater. The Sample Analysis at Mars | ||
| + | (SAM) instrument suite in particular has quantified geochemical indicators that demonstrate | ||
| + | the environment could have supported life, and has achieved detection of the first organic | ||
| + | molecules on Mars. Atmospheric measurements by SAM have identified signatures of planetary | ||
| + | change over billions of years and monitored modern activity. This presentation will recount the | ||
| + | most important findings on the chemistry of Mars to date, and will discuss the implications for | ||
| + | our understanding of whether the red planet was ever habitable." | ||
| + | <br><br> | ||
| + | <font size="4">Tuesday, 24 April 2018</font> | ||
| + | <br> | ||
| + | <b>Laboratory Studies of Planetary Atmospheres and Organic Hazes</b><br> | ||
| + | |||
| + | Melissa Ugelow, <br> | ||
| + | ANYL Student CU Boulder<br><br> | ||
| + | |||
| + | "Atmospheric organic hazes are present in many planetary and satellite atmospheres, possibly including the ancient Earth. Haze composition and how a haze influences surface and atmospheric processes will greatly depend on the atmospheric composition of the planetary body. Therefore, laboratory studies are necessary to determine these atmosphere specific haze properties. This thesis focuses on the chemical, optical and physical properties of Titan and Archean Earth organic haze analogs, along with gas phase neutral and ion measurements during haze analog formation. | ||
| + | |||
| + | <p>Titan haze analogs were formed by ultraviolet (UV) excitation and spark discharge excitation of various concentrations of methane in nitrogen in a flow through reactor. The optical properties of these hazes were measured as a function of methane concentration and were found to have increasing light absorption with increasing aromatic and nitrogen content. To monitor the gas phase during haze analog formation, a new recirculating reactor was used. The concentration of smaller chained hydrocarbons and nitriles, and the isotopic fractionation of carbon in the methane and evolved ethane, was measured as a function of reaction time. Both methane and ethane become enriched in 13C relative to the starting gas mixture. </p> | ||
| + | |||
| + | <p>Archean Earth haze analogs were formed by UV excitation of methane, carbon dioxide, nitrogen and increasing amounts of molecular oxygen in a flow through reactor. As precursor molecular oxygen increases, the particles become more oxidized and non-absorbing. Therefore, haze produced in an oxygen containing atmosphere could form a non-absorbing haze.</p> | ||
| + | |||
| + | <p>Moreover, since Titan’s haze is influenced by ion-neutral chemistry, it is possible similar chemistry occurred in the Archean Earth’s atmosphere. Archean Earth haze analog production and negative ion concentrations were found to be inversely related, with aerosol mass loading decreasing with increasing precursor molecular oxygen. Additionally, the nitrogen in the ions switches from mainly organic nitrogen to inorganic nitrogen with increasing precursor molecular oxygen, possibly indicative of the chemistry that occurred during the rise of oxygen in Earth’s atmosphere. Due to the differences in haze formation and haze properties based on precursor gases, the results of this thesis demonstrate the importance of considering the atmospheric species present during haze formation." | ||
| + | <br><br> | ||
| + | <font size="4">Monday, 23 April 2018</font> | ||
| + | <br> | ||
| + | <b>Mechanistic modeling of reactive soil nitrogen emissions on a continental scale</b><br /> | ||
| + | |||
| + | Quazi Ziaur Rasool<br> | ||
| + | Rice University | ||
| + | <br><br> | ||
| + | "Nitrogen is an essential building block of all proteins and thus an essential nutrient for all life, including crops. Biological Nitrogen Fixation is the natural source of soil nitrogen available for biogeochemical transformations. However, anthropogenic perturbation to nitrogen cycle through the combustion of fossil fuels and consistently increasing fertilization is now larger than natural sources in the United States and globally. Recent global nitrogen budgets estimate that soil reactive nitrogen (N<sub>r</sub>) emissions (predominantly from biochemical transformations in soil) have increased by a factor of 2-3 from pre-industrial levels. These increases are especially pronounced in agricultural regions. These emissions from biogeochemical transformations can be in reduced (NH<sub>3</sub>) or oxidized (NO, HONO, N<sub>2</sub>O) form, depending on complex biogeochemical transformations of soil nitrogen reservoirs.</p> | ||
| + | <p>Reactive nitrogen in the atmosphere is a precursor for ozone and particulate matter formation and contributes to nutrient loading by being washed out by precipitation and the deposition of atmospheric nitrogen gases and aerosols. Until recently, little progress has been made in modeling of the cascade of nitrogen from soil to the atmosphere due to the complexity of and uncertainty in its transport and transformation. The lack of understanding of these multimedia transport processes is due to the typical focus of research on specific media and the difficulty in parameterizing the anthropogenically fixed nitrogen and their input into the atmosphere, primarily through mineral fertilizer application to crops, the largest source of environmental reactive nitrogen.</p> | ||
| + | <p>This talk will focus on modeling of the exchange of gaseous nitrogen species between the soil and the | ||
| + | atmosphere, with an emphasis on Nitrogen oxides (NO, HONO). Contemporary air quality models like U.S. | ||
| + | EPA’s Community Mulitscale Air Quality (CMAQ) model, typically neglect soil emissions of HONO and N<sub>2</sub>O. | ||
| + | Previous soil nitrogen parametrizations in CMAQ have focused on NO emissions only and in a manner | ||
| + | inconsistent with how soil NH<sub>3</sub> emissions (i.e. accounting for anthropogenically fixed nitrogen from fertilizer | ||
| + | application and atmospheric deposition). Thus, there is a need to more mechanistically and consistently | ||
| + | represent the soil N processes that lead to emissions to the atmosphere. The new mechanistic scheme | ||
| + | addresses the spatial-temporal variability of different reactive nitrogen emissions from soil through complex | ||
| + | transport and transformation of soil nitrogen pools in both agricultural and non-agricultural soils. The CMAQ | ||
| + | model with a new mechanistic scheme for modeling reactive nitrogen emissions from soil will be described | ||
| + | and evaluated against observations of atmospheric particulate matter and NO<sub>x</sub> emissions. The use of | ||
| + | multimedia and biome-specific measurements to constrain model parameters, and how this can improve | ||
| + | continental scale (Continental US) models will be presented. These findings will be presented with an | ||
| + | emphasis on the sensitivity of the modeling system to different air-soil exchange parameterizations and how | ||
| + | the representation of these emissions can be improved." | ||
| + | |||
| + | <br><br><font size="4">Monday, 16 April 2018</font><br> | ||
| + | <b>Optical Properties of Absorbing Organic Aerosol</b><br> | ||
| + | |||
| + | Kevin Jansen,<br> | ||
| + | CU Boulder, ANYL 3rd year<br> | ||
| + | |||
| + | "Until relatively recently, it has been assumed that organic aerosol only scatters light, thereby having a negative radiative forcing and global cooling effect. However, aerosol formed from the aqueous-phase reaction of small di-carbonyl compounds, such as glyoxal and methylglyoxal, with ammonium salts have the potential to form light-absorbing brown carbon (BrC) aerosol. Studies of BrC formation mechanisms and optical properties have been primarily preformed using bulk-aqueous solutions, although bulk-phase studies are not perfect simulations of reactions occurring within aerosol particles. In order to characterize BrC aerosol formed in the aerosol phase, we utilize Cavity ring-down (CRD) and Photoacoustic spectroscopies (PAS) to monitor the absorption and extinction of BrC aerosol formed from reactions of glyoxal and ammonium sulfate aerosol within reaction chamber. The PAS allows for the detection of BrC absorption even under conditions in which a few μg/m<sup>3</sup> of weakly absorbing material is made. In addition, depending on the RH of the aerosol and if the aerosol was exposed to light, we observed differing losses in absorption by the BrC aerosol, which may indicate that BrC persists longer in the atmosphere than predicted from bulk phase experiments." | ||
| + | |||
| + | <br><br><font size="4">Tuesday, 10 April 2018</font><br> | ||
| + | <b>Chemistry of Volatile Organic Compounds in a Changing Atmosphere</b><br> | ||
| + | |||
| + | [http://cires.colorado.edu/council-fellows/joost-de-gouw Joost de Gouw],<br> | ||
| + | CU Boulder | ||
| + | |||
| + | "Volatile organic compounds (VOCs) in the atmosphere can react to form important pollutants such as | ||
| + | ozone and secondary organic aerosol and can also have direct effects on human health. In this seminar, I | ||
| + | will present several new insights into the sources and chemistry of VOCs in urban air, from oil and | ||
| + | natural gas production and in biomass burning emissions. | ||
| + | Emissions of VOCs from motor vehicles have strongly declined for decades and as a result, other | ||
| + | emission sources such as from the use of volatile chemical products (e.g. cleaners, glues, coatings, | ||
| + | solvents and personal care products) have become more important in urban air. I will show how | ||
| + | measurements in urban air can be used to determine emissions of reactive VOCs, despite the fact that | ||
| + | they can be removed and/or formed in between the time of emission and sampling. As many volatile | ||
| + | chemical products are used inside buildings, I will show how measurements of indoor air can be used to | ||
| + | determine emissions. | ||
| + | Electric power generation by wind and solar is expanding rapidly, but the use of natural gas | ||
| + | power plants to make up demand will likely remain in the foreseeable future. The production of natural | ||
| + | gas in the U.S. is currently at an all-time high. Methane emissions associated with this activity have | ||
| + | received much attention, because they offset the climate benefits of this lower-carbon fuel. Less | ||
| + | attention has been paid to the emissions of air pollutants such as VOCs and nitrogen oxides (NOx). Using | ||
| + | results from airborne measurements during the NOAA SONGNEX campaign, I will show that a significant | ||
| + | fraction of VOC emissions over the lifecycle of oil and natural gas takes place during production. Using | ||
| + | remote sensing measurements made from the Ozone Monitoring Instrument onboard the NASA Aura | ||
| + | satellite, I will show that both the drilling of new wells as well as the extraction of fossil fuels that follows | ||
| + | contribute to emissions of NOx. | ||
| + | Wildfires in the U.S. have become more frequent and extensive during a longer wildfire season. | ||
| + | Due to the complexity of fuel composition and burning conditions, biomass burning emissions are | ||
| + | among the most challenging to analyze chemically, which makes it difficult to describe the atmospheric | ||
| + | fate and health effects. Measurements of VOCs from biomass burning emissions were made at the Fire | ||
| + | Sciences Laboratory in Missoula, MT during the NOAA FIREX study. I will show how different processes | ||
| + | such as distillation and pyrolysis can explain the composition of VOC emissions for different fuels and | ||
| + | phases of a burn. I will also show the results from laboratory experiments aimed at elucidating the | ||
| + | chemistry of functionalized aromatic compounds that are common from biomass burning. | ||
| + | Finally, I will briefly discuss the development and characterization of new instrumentation for | ||
| + | VOC measurements that will likely lead to new discoveries in our science." | ||
| + | |||
| + | <br><br><font size="4">Monday, 9 April 2018<br></font> | ||
| + | <b>Ecosystem-atmosphere fluxes of volatile organic compounds: Which ones matter? </b><br> | ||
| + | |||
| + | [https://atmoschem.umn.edu/ Dylan Millet],<br> | ||
| + | University of Minnesota<br> | ||
| + | |||
| + | "Volatile organic compounds (VOCs) play several key roles in the atmosphere: their oxidation leads to the formation of health- and climate-relevant pollutants; they affect the nitrogen cycle by interacting with atmospheric NOx; and they modulate the atmosphere’s oxidizing capacity and therefore the lifetimes of greenhouse gases and other pollutants. Terrestrial ecosystems are simultaneously the largest source and a major sink of atmospheric VOCs; however, our understanding of these fluxes and their atmospheric impacts is challenged by a number of outstanding uncertainties, including complex emission sources, bidirectional exchange with the land surface, and chemical interactions with anthropogenic pollutants. In this talk I will present results from my group’s research applying observations to better characterize these ecosystem-atmosphere interactions and their effects on atmospheric chemistry. Discussion will focus on two specific themes. I will first present results focusing specifically on formic acid, which is a major source of atmospheric acidity and an integrated marker of hydrocarbon oxidation, but which has large missing sources. I will then discuss new measurements from my group using high-resolution time-of-flight mass spectrometry to directly measure forest-atmosphere fluxes of VOCs simultaneously across the entire mass spectrum, and explore these results with the aim of better understanding i) how well our models capture this 2-way land-atmosphere carbon exchange, and ii) to what degree the fluxes for the large number of ions outside of the traditionally-measured subset matter for tropospheric composition." | ||
| + | |||
| + | <br><br><font size="4">Monday, 2 April 2018</font><br> | ||
| + | |||
| + | <b>The peroxy radical chemistry that drives atmospheric nano-particle growth</b> | ||
| + | <br> | ||
| + | [https://www.atmos.washington.edu/~thornton/ Joel Thornton]<br> | ||
| + | University of Washington | ||
| + | <br><br> | ||
| + | "Organic carbonaceous material is a ubiquitous and often significant fraction of atmospheric particulate mass, and can be responsible for driving the growth of atmospheric nanoparticles formed from nucleation up to cloud condensation nuclei sizes. Development of a molecular-level understanding of the processes governing nano-particle growth by organic condensation has been a long running challenge. I will present new insights into to the chemistry of biogenic hydrocarbons that contribute to this process, using both in situ observations as well as controlled simulation chamber studies. An underlying theme is the role novel instrumentation techniques have had recently in allowing direct observation and quantification of a wide suite of organic molecules and chemical processes contributing to particle growth." | ||
| + | |||
| + | <br><br><font size="4">Monday, 19 March 2018</font><br><br> | ||
| + | |||
| + | <b>Separation of NO<sub>x</sub> emissions from drilling, and oil and gas extraction in the U.S. using monthly data from the Ozone Monitoring Instrument</b> | ||
| + | <br> | ||
| + | Joep de Bruin<br> | ||
| + | Visiting graduate student, de Gouw lab, CU Boulder | ||
| + | <br><BR> | ||
| + | "Hydraulic fracturing and horizontal drilling have increased unconventional oil and gas extraction from shale reserves in the US in the last decade, making up half of total US oil and gas production at present. This activity results in NO<sub>x</sub> emissions in the extraction regions that are measurable from space using the Ozone Monitoring Instrument (OMI) on the NASA Aura satellite. The NO<sub>x</sub> emissions are a result of two different processes: (1) the drilling and hydraulic fracturing of new wells, and (2) the extraction of oil and gas after the well is completed. To distinguish the effects of drilling and extraction on the NO<sub>x</sub> emissions, a multiple linear regression to the NO<sub>2</sub> columns as a function of time is calculated for 9 extraction regions using the number of drilling rigs and the oil and gas production data from 2007 until 2018. In 3 regions (Permian, Bakken, Eagle Ford) a significant correlation between measured and calculated NO<sub>2</sub> columns is found, of which the Permian region shows the highest correlation. The analysis shows that half of the total NO<sub>x</sub> concentration in this region can be attributed to emissions from oil and gas processes, and that both the drilling and extraction processes have an equal share in these emissions. In other extraction regions, NO<sub>2</sub> columns show poor correlation with the oil and gas activity due to the proximity of urban areas (Barnett, Denver-Julesburg regions), power plants (San Juan) or variations in the drilling and extraction activity over time that are too small (Uintah, Upper Green River)." | ||
| + | |||
| + | <br><br><font size="4">Monday, 12 March 2018</font><BR><BR> | ||
| + | |||
| + | <b>Diffusion of organics in secondary organic aerosol</b> | ||
| + | <BR> | ||
| + | Allan Bertram<br> | ||
| + | University of British Columbia | ||
| + | <BR><BR> | ||
| + | "Secondary organic aerosol (SOA) can modify Earth’s climate by scattering and absorbing solar | ||
| + | radiation and by modifying the properties of clouds. SOA can also negatively affect air quality | ||
| + | and reduce visibility in urban environments. To predict the role of SOA in climate, air quality | ||
| + | and visibility, a good understanding of the diffusion of organics within SOA particles is required. | ||
| + | Through a series of laboratory studies, we have shown that the diffusion rate of organics within | ||
| + | SOA particles depends strongly on several factors including the relative humidity and the | ||
| + | precursors used to generate SOA. More recently, we have used these results to estimate the | ||
| + | mixing times of organics within SOA particles in the planetary boundary layer and free | ||
| + | troposphere." | ||
| + | |||
| + | <br><br><font size="4">Monday, 5 March 2018</font><BR> | ||
| + | |||
| + | <b>International air quality, health, and climate impacts of cookstoves, diesel NOx, and other anthropogenic sectors via PM2.5 and O3</b><BR> | ||
| + | |||
| + | Daven Henze<br> | ||
| + | CU-Boulder Mech. Eng.<BR><BR> | ||
| + | |||
| + | "Diesel cars, trucks, and buses produce ~70% of global land transportation emissions of nitrogen oxides (NO<sub>x</sub>), a key PM<sub>2.5</sub> and ozone precursor. Globally over 3 billion people presently use solid fuel for meal preparation. What are the impacts of these activities on the environmental through atmospheric chemistry and transport? Which species dominates the local and long-range health impacts of air pollution? I will first discuss the use of models and remote sensing measurements to evaluate the domestic and international contributions to PM<sub>2.5</sub> and O<sub>3</sub>, and their impacts on human health and climate. Source-receptor relationships are developed using adjoint sensitivity analysis, constraints from remote sensing observations, and parameterized climate model sensitivities. This talk will then delve into application of these relationships to estimate impacts of diesel NO<sub>x</sub> emissions standards and solid fuel use in major markets and source regions worldwide. We find that the per-cookstove impacts on ambient air quality and global temperature changes are pronounced in several countries not typically targeted in cookstove mitigation efforts (e.g., Ukraine and Romania). We also show that real-world diesel NO<sub>x</sub> emissions in 11 markets representing ~80% of global diesel vehicle sales are significantly higher than certification limits indicate. This excess NO<sub>x</sub> contributed an estimated ~39,000 additional ozone- and PM<sub>2.5</sub>-related premature deaths globally in 2015, with a larger portion of this owing to excessive emissions from heavy duty vehicles than from defeat devices on light duty vehicles. Lastly, we present recent evaluation of the premature deaths and preterm births associated with global O<sub>3</sub> exposure, showing that the former is possibly several times larger than previously expected, rivaling the health impacts of PM<sub>2.5</sub> in severity." | ||
| + | |||
| + | <br><br><font size="4">Monday, 26 February 2018</font><BR><BR> | ||
| + | |||
| + | <b>Chemical Composition of Positive Ions During Laboratory Simulations of Titan’s Haze Formation</b><BR> | ||
| + | |||
| + | Jennifer Berry<br> | ||
| + | CU Boulder<BR><BR> | ||
| + | |||
| + | "Titan, one of Saturn’s moons, is the only other planetary object in our solar system with a thick nitrogen atmosphere. Titan’s haze formation is initiated by energetic electrons and UV photons leading to a complex series of ion-neutral and radical reactions that result in high molecular weight hydrocarbons and nitrogen containing species. A long history of laboratory and modelling studies of ion-neutral reactions and haze particle composition exist, but there has been little experimental work on the role of ions on haze production. Here, we use an Aerodyne/Tofwerk Atmospheric Pressure interface Time-of-Flight Mass Spectrometer (APi-ToF-MS) to measure the ion chemical composition and relative abundance during the formation of haze analogs initiated by irradiating CH<sub>4</sub> in N<sub>2</sub> by a deuterium lamp. The instrument’s high resolution (R>7000) allows us to detect multiple ions at the same unit mass, leading to clear identification of hydrocarbons and nitrogen containing compounds with the same nominal mass. Families of C<sub>x</sub>H<sub>y</sub>+, C<sub>x</sub>H<sub>y</sub>N<sub>w</sub>+, C<sub>x</sub>N<sub>w</sub>+, and H<sub>y</sub>N<sub>w</sub>+ are detected with increasing saturation at higher masses during the irradiation of CH<sub>4</sub>/N<sub>2</sub> mixtures. Nitrogen incorporation into compounds could hold significance in determining the prebiotic chemistry occurring on Titan." | ||
| + | |||
| + | <br><br><font size="4">Monday, 12 February 2018</font><BR> | ||
| + | |||
| + | <b>Assessing the Sources of Elevated Front Range Ozone Based on | ||
| + | Observations from the Boulder Atmospheric Observatory</b><BR> | ||
| + | |||
| + | Emily Fischer<br> | ||
| + | Colorado State University<BR> | ||
| + | |||
| + | "The Denver Metro/North Front Range area currently violates the National | ||
| + | Ambient Air Quality Standard (NAAQS) for ozone. I will discuss | ||
| + | observations of ozone, its precursors, and other secondary species | ||
| + | collected at the Boulder Atmospheric Observatory during summer 2014 | ||
| + | and 2015. My group has used our observations to 1) untangle the | ||
| + | contribution of different classes of volatile organic compounds to ozone | ||
| + | production in our region, and 2) identify how the addition of aged wildfire | ||
| + | smoke can change the abundance of ozone precursors." | ||
| + | |||
| + | <br><br><font size="4">Monday, 21 January 2018</font><br> | ||
| + | |||
| + | <b>Atmospheric Chemistry of Volatile Organic Compounds</b><br> | ||
| + | |||
| + | Joost de Gouw<br> | ||
| + | CIRES & Department of Chemistry and Biochemistry<br> | ||
| + | |||
| + | "Volatile organic compounds (VOCs) are released to the atmosphere from many different sources, both natural and man-made. In the atmosphere, VOCs are removed on time scales of minutes to months and this chemistry leads to formation of ozone and organic aerosol, two air pollutants that also affect climate. We have studied the emissions and chemistry of VOCs using a combination of field and laboratory measurements with mass spectrometry and gas chromatography. I will present some examples of the projects that I have worked on with graduate students over the years and of future research directions." | ||
| + | <br><br> | ||
| + | and | ||
| + | <br> | ||
| + | |||
| + | |||
| + | <b>Every Drop Counts…Looking for Water on Mars</b> | ||
| + | |||
| + | Maggie Tolbert<br> | ||
| + | CIRES & Department of Chemistry and Biochemistry<br> | ||
| + | |||
| + | "Mars is a cold, dry planet where pure liquid water is not stable. However, recent | ||
| + | observations of “recurring slope lineae” (RSL) on Mars may be evidence of current liquid | ||
| + | water flows. Several different hygroscopic salts are known to exist in the Martian soil, | ||
| + | and deliquescence of those salts could provide small amounts of liquid water temporarily. | ||
| + | Our research group uses Raman microscopy and an environmental cell to probe the | ||
| + | conditions under which such liquid brines can form and persist under low Martian | ||
| + | temperatures. In addition, an optical trap is used to probe phase changes of individual | ||
| + | levitated droplets. In this talk, I will discuss laboratory results for the brine-forming | ||
| + | ability of several different Mars-relevant salts and salt mixtures and implications for | ||
| + | water on Mars." | ||
| + | |||
| + | ==Fall 2017== | ||
| + | |||
| + | <font size="4">Monday, 4 December 2017</font> | ||
| + | |||
| + | <b>Measurements of Positive Ambient Ions as Part of HISCALE II Field Campaign</b> | ||
| + | |||
| + | Aroob Abdelhamid<br /> | ||
| + | CU Boulder, ANYL 4th year | ||
| + | |||
| + | "Atmospheric ions control the electrical properties of the atmosphere, influence chemical | ||
| + | composition via ion-molecule and/or ion-catalyzed reactions, and affect new particle formation. | ||
| + | Understanding the role of ions in these processes requires knowledge of ionic chemical | ||
| + | composition. Due to the low concentration of ions, chemical composition measurements have | ||
| + | historically been challenging. Recent advances in mass spectrometry, such as the atmospheric | ||
| + | pressure interface time-of- flight mass spectrometer (APi-TOF), are now making these | ||
| + | measurements more feasible. Here, we present measurements of ambient cations during the | ||
| + | HISCALE II field campaign (August- September 2016) in Lamont, OK. We discuss how the | ||
| + | chemical composition of cations varies over the course of the campaign including before, during, | ||
| + | and after new particle formation events. We specifically focus on the composition of organic | ||
| + | nitrogen ions due to the potential importance of these compounds in atmospheric nucleation. We | ||
| + | compare our results to measurements of neutral organic nitrogen compounds in order to gain | ||
| + | insight into how organic nitrogen is chemically transformed in the atmosphere and how this | ||
| + | influences new particle formation" | ||
| + | |||
| + | <font size="4">Monday, 13 November 2017</font> | ||
| + | |||
| + | <b>Optimization of Surface-Initiated Atom Transfer Radical Polymerization for Application to Small Analyte Detection</b> | ||
| + | |||
| + | Nathan Reed<br /> | ||
| + | CU Boulder, ANYL 1st year | ||
| + | |||
| + | "The research project I contributed to as an undergraduate student sought to investigate molecular transport as a means of signal enhancement in small analyte detection. Selective transport of analyte molecules to a nanosensor location will lower the limit of detection compared to analyte collection from free diffusion alone. Polymer brushes can be grown off a detector surface directly and can then be used to bring small molecules closer in proximity to the detector. The small molecules will selectively partition into the polymer-brush surface based on attractions caused by hydrophobic effects, hydrogen bonding, or ionic interactions. To foster such controlled and specific interactions on a device surface, uniform polymer thin films needed to be synthesized and the appropriate materials used for transport of target analytes needed to be understood. Additionally, there was a need for the method to be standardized so that non-polymer scientists and engineers could replicate it with ease. Atom transfer radical polymerization (ATRP) presented a polymerization technique able meet these needs. ATRP is a controlled polymerization technique whose parameters may be tuned to meet specific needs such as functionality and length. In addition, ATRP is an approachable technique to scientists unfamiliar with polymerization techniques once synthetic methods are unified and optimized. My undergraduate research has investigated ATRP reaction conditions applicable to a broad range of polymer types, both in solution and surface-tethered, to develop a unified and optimized synthetic approach." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Global simulation of brown carbon and single scattering albedo calculation using a chemical transport model</b> | ||
| + | |||
| + | Duseong Jo<br /> | ||
| + | CU Boulder, Postdoc | ||
| + | |||
| + | |||
| + | "Recent observations suggest that a certain fraction of organic carbon (OC) aerosol effectively absorbs solar radiation, which is also known as brown carbon (BrC) aerosol. Despite much observational evidence of its presence, very few global modeling studies have been conducted because of poor understanding of global BrC emissions. I will present an explicit global simulation of BrC in a global 3-D chemical transport model (GEOS-Chem), including global BrC emission estimates from primary (biomass burning and biofuel) and secondary sources. The BrC absorption leads to a general reduction of NO2 photolysis rates, whose maximum decreases occur in Asia up to 9% (19%) on an annual (spring) mean basis. The inclusion of BrC absorption reduces the overestimation of single scattering albedo (SSA) in the model, but still the model overestimates the observed SSA by AERONET. To further reduce the overestimation, the sensitivity calculations of SSA are conducted by focusing on the physical properties of Black Carbon (BC), the inclusion of Brown Carbon (BrC), and the size distribution of dust. Large variations in the calculated SSA may result from slight changes of the geometric mean radius, geometric standard deviation, real and imaginary refractive indices, and density of BC. The inclusion of BrC and observationally-constrained dust size distributions also significantly affect the SSA, and result in a remarkable improvement for the simulated SSA at 440 compared with the AERONET observations." | ||
| + | |||
| + | <font size="4">Monday, 30 October 2017</font> | ||
| + | |||
| + | <b>Chemistry of peroxy radicals and soot formation at combustion conditions</b> | ||
| + | |||
| + | Prof Sandeep Sharma<br /> | ||
| + | CU Boulder, PChem | ||
| + | |||
| + | "In this presentation, I will focus on the chemistry of peroxy radicals and soot formation at combustion conditions. Both these topics are of immense technological importance and will also allow me to present the latest theoretical techniques used to study them. These include the use of automated mechanism generators to build complex reaction mechanisms, the use of transition state theory to calculate rates of elementary reactions, and the use of the semiclassical method for calculating the partition function of anharmonic vibrational modes. The aim is to physically motivate these techniques and highlight the conditions under which we expect them to be predictive and also discuss their shortcomings. I will end the presentation with a short review of the latest work from my group that aims to address some of the shortcomings." | ||
| + | |||
| + | <font size="4">Monday, 23 October 2017</font> | ||
| + | |||
| + | <b>VOCs off-gassed by laser and inkjet printers, and CH4, CO2, and N2O gas fluxes in created wetlands</b> | ||
| + | |||
| + | Wyatt Brown<br /> | ||
| + | CU Boulder, ANYL first year student | ||
| + | |||
| + | "Indoor air quality has become a more prominently researched subject in recent years, and indoor | ||
| + | office environments are still poorly understood in terms of the volatile organic compounds | ||
| + | (VOCs) associated with them. We have identified and quantified various VOCs off-gassed by | ||
| + | laser and inkjet printers using solid phase micro-extraction in conjunction with GC/MS. | ||
| + | |||
| + | In the outdoor environment, it has been well-established that wetlands are important ecosystems | ||
| + | for the planet and provide a multitude of ecological services. In order to combat the widespread | ||
| + | destruction of wetlands, restoration efforts have recently seen a dramatic increase in prevalence. | ||
| + | However, there is a complex, poorly understood relationship between the functions of created | ||
| + | wetlands and the emission of greenhouse gasses. In this talk, the preliminary data on CH4, CO2, | ||
| + | and N2O gas fluxes in created wetlands is presented. Preliminary dissolved organic matter | ||
| + | (DOM) studies are also presented." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>The reaction of acetyl peroxy radical with hydroperoxy radical</b> | ||
| + | |||
| + | Marla DeVault<br /> | ||
| + | CU Boulder, ANYL first year student | ||
| + | |||
| + | "I spent three summers working in Prof. Keith Kuwata's computational chemistry lab at Macalester College. Primarily, my research focused on improving the steps taken to simulate reactions of organic peroxy radicals (OPR's) in the atmosphere. More specifically, I studied the reaction of acetyl peroxy radical with hydroperoxy radical, which is thought to be a source of hydroxy radical. These steps include determining which simple model chemistry could most accurately model the reaction, investigating the viability of non-reactive conformers, and testing methods for calculating the energy of transition structures." | ||
| + | |||
| + | <font size="4">Friday, 13 October 2017</font> | ||
| + | |||
| + | <b>Indoor air (photo)chemistry: A world-wide concern</b> | ||
| + | |||
| + | Prof Sasho Gligorovski<br /> | ||
| + | State Key Laboratory of Organic Geochemistry, Chinese Academy of Sciences | ||
| + | |||
| + | |||
| + | "The first field campaign performed in a high school in Marseille, France (2011) confirmed the | ||
| + | existence of hydroxyl radicals (OH) that were more in line with typical outdoor concentrations | ||
| + | than indoor concentrations (Gomez Alvarez et al., 2013). It was demonstrated that photolysis of | ||
| + | nitrous acid (HONO) is the most important source of these highly reactive OH radicals in the | ||
| + | indoor air. This set of innovatory first direct measurements of OH radicals indoors was followed | ||
| + | with also avant-garde measurements of the solar actinic fluxes which can penetrate indoors and | ||
| + | photolysis frequencies of key indoor species (Gandolfo et al., 2016). The results obtained are of | ||
| + | enormous repercussions and need to be studied in the next few years much more profoundly due | ||
| + | to their still unexplored implications. The facts just mentioned need to be taken in combination | ||
| + | with the elevated concentrations of HONO present in the indoor air (Gligorovski, 2016). In | ||
| + | addition, HONO is an important indoor air pollutant, which can react with amines leading to | ||
| + | carcinogenic nitrosamines. While our understanding of photochemistry of the indoor surfaces is | ||
| + | still in its infancy, the recent results (Gomez Alvarez et al., 2014, Gandolfo et al., 2015, 2017) | ||
| + | based on the light induced heterogeneous NO2 reactions on domestic surfaces leading to HONO | ||
| + | formation, suggest that it is an area that should be pursued further. Another campaign carried out | ||
| + | in an office in Martigue, France (2016) was dedicated to evaluation of OH radical source | ||
| + | strength. Again, the photochemical reactions play an important role to the oxidation capacity of | ||
| + | indoor atmosphere. If we consider all these facts carefully, the concentration of OH radicals | ||
| + | indoors, could reach levels that would be of serious concern from the standpoint of public health. | ||
| + | For these reasons, it is very important to dedicate more efforts to determine the OH | ||
| + | concentrations that can be attained in various indoor settings, in relation to factors such as light | ||
| + | intensity, HONO concentrations, and humidity, among others." | ||
| + | |||
| + | <font size="4">Monday, 9 October 2017</font> | ||
| + | |||
| + | <b>Better Understanding Climate and Atmospheric Chemistry by Understanding the Formation of Mixed Phase Clouds</b> | ||
| + | |||
| + | Prof Dan Cziczo<br /> | ||
| + | MIT | ||
| + | |||
| + | "Field and laboratory measurements using cloud chambers have been used to understand the atmospheric abundance of droplet and ice nucleating particles and to, in turn, construct parameterizations for mixed-phase and completely glaciated clouds in weather and climate models. This seminar investigates measurements of which particles act as the nuclei for droplets and ice crystals and how we can then mimic those particles in the laboratory to understand how clouds form in our atmosphere. We show here that assumptions about the source of the particles as well as uncertainty in the laboratory and field measurements propagate into uncertainty in our understanding of the Earth’s climate and the chemistry of our atmosphere. | ||
| + | |||
| + | When we consider cloud chambers, uncertainty is likely inherent to varying degrees in all instruments and is caused by a variety of factors including exposure of particles to different humidities and/or temperatures than predicated from theory. This can result in a variable underestimation of reported droplet and ice concentrations. This is a critical issue for models which relay on these data for correct parameterizations of cloud formation. For ice clouds in particular, we find that simulated long wave ice-bearing cloud forcing in a global climate model can vary up to 0.8 W/m2 and can change sign from positive to negative within the experimentally constrained bias range. | ||
| + | |||
| + | We’ll conclude with a discussion of possible instrument improvements and how these can improve our understanding of climate, precipitation and atmospheric chemistry." | ||
| + | |||
| + | <font size="4">Monday, 2 October 2017</font> | ||
| + | |||
| + | <b>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</b> | ||
| + | |||
| + | Prof Paul Ziemann<br /> | ||
| + | CU Boulder | ||
| + | |||
| + | "Laboratory studies provide much of the fundamental data on reaction | ||
| + | kinetics, products, and mechanisms that are needed to understand | ||
| + | atmospheric and indoor air chemistry and to develop models that are | ||
| + | used to establish air quality regulations and predict the effects of | ||
| + | human activities. Research in my laboratory focuses primarily on | ||
| + | environmental chamber studies of the atmospheric chemistry of organic | ||
| + | compounds emitted from natural and anthropogenic sources and the | ||
| + | physical and chemical processes by which oxidized organic reaction | ||
| + | products form aerosol particles. In addition to this we have recently | ||
| + | conducted a number of studies of indoor air chemistry at CU. In this | ||
| + | talk I will describe how we conduct the studies by using a diverse | ||
| + | array of measurement techniques." | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Recent Results and Upcoming Projects to Investigate Aerosol Sources, Properties, Processes, and Fate</b> | ||
| + | |||
| + | Prof Jose Jimenez<br /> | ||
| + | CU Boulder | ||
| + | |||
| + | "Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, properties, and evolution are poorly understood. In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students. Ongoing projects include global aerosol measurements and analysis as part of the NASA ATOM project, which is sampling (almost) pole-to-pole across the vertical profile. Initial model comparisons suggest the importance of fast OA removal channels. We have used an Oxidation Flow Reactor (OFR) at multiple field studies and find consistent patterns, where SOA formation by O3 and NO3 are consistent with models, while formation by OH is substantially underpredicted unless semivolatile and intermediate volatility species are accounted for. We are using the GECKO-A fully explicit model to investigate this underprediction, as well as to characterize the similarities and differences of the chemical regimes across the OFR, large chambers, and the atmosphere. We are investigating gas/particle partitioning in the laboratory for different types of particles. Finally, we are continuing to explore the chemistry of indoor air with ToF-CIMS and other instruments. Future outdoor campaigns may include the study of emissions and chemical evolution of smoke from real fires in the western US (NASA FireChem) with AMS and soft-ionization EESI-TOF. Future indoor campaigns will involve sampling at locations such as dining halls, gyms, and art museums." | ||
| + | |||
| + | <font size="4">Monday, 25 Sept 2017</font> | ||
| + | <b>Ambient Ions in Planetary Atmospheres</b> | ||
| + | |||
| + | Prof Eleanor Browne<br /> | ||
| + | CU Boulder | ||
| + | |||
| + | "Atmospheric ions influence the chemical composition of planetary atmospheres through ion-molecule reactions, ion-catalyzed reactions, and ion-particle interactions. Characterizing the chemical composition of ambient ions is necessary for a comprehensive understanding of these processes. In this talk, I will discuss our recent work using atmospheric pressure interface mass spectrometry to measure the chemical composition of ions in field and laboratory settings. I will discuss our measurements of ions in Earth’s atmosphere during the 2015 Holistic Interaction of Shallow Clouds, Aerosols, and Land-Ecosystems (HIS-CALE) II campaign in Lamont, OK as well as our laboratory investigation of ions resulting from the irradiation of CH4-N2 mixtures. " | ||
| + | |||
| + | and | ||
| + | |||
| + | <b>Air Quality and Climate in the Anthropocene: Why we study remote pristine and polluted environments</b> | ||
| + | |||
| + | Prof. Rainer Volkamer<br /> | ||
| + | CU Boulder | ||
| + | |||
| + | "Atmospheric chemistry is at the core of air pollution impacts on public health (ozone, air toxics, and aerosols) and climate (lifetime of greenhouse gases, radiation feedbacks). There is a need for techniques to better quantify emission fluxes of trace gases and aerosols from wildfires, which are deemed responsible for up to 40% of the global CO emissions. The Volkamer group develops instruments, conducts field and laboratory experiments to better probe, test and predict emissions, and study fundamental physical atmospheric chemistry principles of relevance in remote pristine and polluted air. | ||
| + | We offer graduate research opportunities for one student in either of the following three areas: [1] Solar Occultation Flux spectroscopy (mobile SOF) is a new tool under development to better quantify emission fluxes of ozone, radical- and aerosol precursor gases (NH3, NO, NO2, HONO, VOCs, OVOCs, acids, etc) from wildfires. The CU airborne SOF instrument is currently being tested aboard the Wyoming King Air aircraft, in preparation for a field experiment to study emissions and plume chemistry from wildfires in the Pacific Northwest during summer 2018. [2] As part of the CLOUD consortium at CERN, Geneva we study nucleation and aerosol-cloud interactions in the pristine marine, polluted urban and remote free troposphere. For example, measurements of iodine monoxide (IO), iodine dioxide (OIO) radicals, molecular iodine (I2) and other species can constrain the rate of iodine atom release, and iodic acid formation that is deemed responsible for new particle formation in coastal environments, but this chemistry is currently poorly understood. [3] Field measurements of IO, bromine oxide (BrO) radicals and short live oxygenated hydrocarbons at Big Island, Hawaii (Mauna Loa Observatory, at 19.5°N, 155.6°W, 3.4 km AMSL) and La Reunion island, France (Maïdo Observatory, at 21.1°S, 55.4°E, 2.2 km AMSL) are part of an ongoing and larger effort to understand new particle formation, halogen chemistry and OVOC sources in the remote troposphere. An intensive operating period at Maïdo will bring together groups from Europe, Japan, and the US during spring/summer 2018. " | ||
| + | |||
| + | <font size="4">Monday, 18 Sept 2017</font> | ||
| + | |||
| + | <b>Comprehensive Analysis of the Gas- and Particle-Phase Products of VOC Oxidation</b> | ||
| + | |||
| + | Julia Bakker-Arkema<br> | ||
| + | ANYL 3rd-year student, Ziemann lab | ||
| + | |||
| + | "Controlled environmental chamber studies are important for determining atmospheric reaction mechanisms and gas and aerosol products formed in the oxidation of volatile organic compounds (VOCs). Such information is necessary for developing detailed chemical models for use in predicting the atmospheric fate of VOCs and also secondary organic aerosol (SOA) formation. However, complete characterization of atmospheric oxidation reactions, including gas- and particle-phase product yields, and reaction branching ratios, are difficult to achieve. In this work, we investigated the reactions of terminal alkenes with OH radicals in the presence of NOx in an attempt to fully characterize the chemistry of these systems while minimizing and accounting for the inherent uncertainties associated with environmental chamber experiments. Gas-phase products (aldehydes formed by alkoxy radical decomposition) and particle-phase products (alkyl nitrates, β-hydroxynitrates, dihydroxynitrates, 1,4-hydroxynitrates, 1,4-hydroxycarbonyls, and dihydroxycarbonyls) formed through pathways involving addition of OH to the C=C double bond as well as H-atom abstraction were identified and quantified using a suite of analytical techniques. The full product identification and quantitation, with careful minimization of uncertainties for the various components of the experiment and analyses, demonstrates our capability to comprehensively and accurately analyze the complex chemical composition of products formed in the oxidation of organic compounds in laboratory chamber studies." | ||
| + | |||
| + | <font size="4">Monday, 11 Sept 2017</font> | ||
| + | |||
| + | <b>Investigating emissions of ultrafine aerosols from consumer products: A study on 3D printers</b> | ||
| + | |||
| + | Prof. Nina Vance <br> | ||
| + | CU Boulder | ||
| + | |||
| + | "It is well established that inhalation of ultrafine aerosols (particulate matter < 100 nm) is associated with adverse health effects, however the extent of people’s everyday exposure to these pollutants is still poorly known. The introduction of novel consumer product applications and processes present the potential to introduce new ultrafine aerosols and nanoparticles to indoor environments such as homes, office buildings, schools, hotels, and hospitals. In this presentation, I will discuss the use of methods at the intersection of air resources engineering, nanotechnology, and exposure science to quantify and characterize people’s exposure to ultrafine aerosols and nanomaterials in everyday activities. This will be specifically demonstrated by a case study on aerosol emissions from 3D printers. The aerosol emission rates and size distributions that can be characterized by this type of work can serve as input to risk assessment models, to guide the selection of relevant particle doses in toxicity testing, and to engineer product improvements or develop regulations to ensure consumer safety." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Wednesday, 12 July 2017 </font> | ||
| + | |||
| + | <b>Regional Influence of Wildfires on Atmospheric Aerosol in the Western US and Insights into Emission and Aging of Biomass Burning Organic Aerosol</b> | ||
| + | |||
| + | Prof. Qi Zhang<br> | ||
| + | UC Davis | ||
| + | |||
| + | "Biomass burning (BB) is one of the most important contributors to atmospheric aerosols on a global scale and a large source of emissions that impact regional air quality and global climate. In this study, aerosols in wildfire emissions in the Pacific Northwest region of the United States were studied from the Mt. Bachelor Observatory (MBO, ~ 2700 m a.s.l.) in Central Oregon and from the G-1 aircraft in summer 2013 during the DOE Biomass Burning Observation Project (BBOP) field campaign. Episodes of high aerosol concentrations were observed at MBO due to the impacts of plumes transported from large wildfire clusters upwind of the site. Organic aerosols (OA) were a dominant component of particles in these plumes and the regional enhancements of BBOA concentration normalized by amount of fuel burned were found to be mainly driven by the modified combustion efficiency (MCE), an index of the combustion processes of a fire. The ratio of the enhancement of OA mass to the enhancement of CO above their respective backgrounds (ΔOA/ΔCO) in fire plumes appeared to be constant independent of plume aging although BBOA composition was significantly influenced by atmospheric aging. Three types of BBOA were identified during this study, including a semivolatile BBOA-1 (~ 20% of OA mass) which appeared to represent BB POA and two more oxidized BBOAs (BBOA-2 and BBOA-3) that appeared to represent BB SOA. BBOA-3 was highly oxidized (O/C = 1.06; 31% of OA mass), contained no levoglucosan, showed very low volatility with only ~ 40% mass loss at 200°C, and had a similar mass spectrum as low-volatility oxygenated OA (LV-OOA) commonly observed in regional airmass. The chemical evolution of BBOA was examined for an episode when fire plumes originated from a single fire source were sampled continuously for 36 hours. Longer solar radiation evidently led to a higher mass fraction of the chemically aged BBOA-2 and BBOA-3 and substantially more oxidized OA although negligible net amount of OA production was observed in plumes photochemically aged compared to plumes transported primarily during night. These results suggest that SOA formation was balanced by BBOA volatilization, leading to almost no net amount of OA mass added during aging in wildfire plumes." | ||
| + | |||
| + | ==Spring 2017== | ||
| + | <br /> | ||
| + | <font size="4"> Thursday, 27 April 2017 </font> | ||
| + | |||
| + | <b>New Insights into Fossil Fuel Volatile Organic Compound Emissions and Chemistry Using H3O<sup>+</sup> and NO<sup>+</sup> Chemical Ionization Mass Spectrometry</b> | ||
| + | |||
| + | Abigail Koss<br> | ||
| + | ANYL PhD Candidate | ||
| + | |||
| + | "Volatile organic compounds (VOCs) are central to tropospheric chemistry. These species harm human health and contribute to formation of ozone and secondary organic aerosol. VOCs are challenging to measure because they are frequently present in small concentrations, they can have high spatial and temporal variability, and many different functional groups can be present. One of the most powerful techniques used to measure VOCs in the atmosphere is proton transfer reaction mass spectrometry (PTR-MS). In this talk, I discuss my contributions to the improvement and application of this technique. | ||
| + | |||
| + | We developed a new PTR-time-of-flight-MS (PTR-ToF-MS) instrument based on the Aerodyne ToF-CIMS. This instrument has higher sensitivity than its PTR-MS predecessors, and has enabled faster measurement and the measurement of many more VOCs. We extensively characterized this instrument, including the effect of ion guide components, and the response to changing humidity. | ||
| + | The PTR-ToF-MS instrument was deployed on the NOAA P3 aircraft to measure VOCs associated with oil and natural gas producing regions. In the US, these regions have experienced a recent increase in fossil fuel production, sparking concerns about local and regional air quality. Few PTR-ToF measurements in these regions exist, and the interpretation of mass spectra can be quite different from that in urban or forested areas. I developed a guide to interpret these measurements. We detected several previously unreported species, including cyclic nitrogen-containing compounds, which may provide clues about emissions and could be important for overall nitrate reactivity. Comparison between basins answers major campaign science questions about mixing ratios of air toxics. | ||
| + | |||
| + | I expanded the capability of the ToF-CIMS instrument by adapting it to use NO+ reagent ion chemistry. Through a set of experiments using modular GC-CIMS and parallel NO+-CIMS and GC-EI-MS, I determined the selectivity and sensitivity of NO+ CIMS for several classes of compounds that PTR-MS cannot easily measure, including cyclic alkanes, long-chain alkanes, isomerically specific measurements of carbonyls, and alcohols. The NO+ CIMS instrument was deployed on a mobile laboratory to measure real-world highway vehicle emissions. Application of positive matrix factorization clearly identified the important emission sources, their VOC composition, and contribution to total VOC emissions." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 24 April 2017 </font> | ||
| + | |||
| + | <b>Turning brown in the sun: Aldehydes, aqueous aerosol, and evaporating cloud droplets</b> | ||
| + | |||
| + | Prof. David De Haan<br> | ||
| + | University of San Diego | ||
| + | |||
| + | "Much of what we think we know about aqueous aerosol chemistry – reaction rates, products, mechanisms, and photolytic pathways – comes from extrapolating bulk aqueous-phase lab simulations to atmospheric conditions. Based on this approach, it is now commonly assumed that small, water-soluble aldehydes can react at night with ammonium salts to slowly form light-absorbing brown carbon (BrC). These BrC products are thought to be quickly destroyed by sunlight. When aqueous aerosol processes are studied in aqueous aerosol particles, however, this common narrative turns out to be only partially true. In this talk, results will be presented from recent chamber studies on ammonium and amine-containing aerosol particles as they interact with aldehyde species, solar simulator lamps, and clouds. In some cases, sunlight actually accelerates BrC formation during cloud processing. Chemical analysis of the aerosol produced in these experiments suggests that mechanisms initiated by photolytically-produced radical species are the dominant source of oligomers, and by extension, of BrC." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4">Monday, 17 April 2017</font> | ||
| + | |||
| + | <b>Products and yields from the gas-phase oxidation of benzenediols</b> | ||
| + | |||
| + | Zachary Finewax <br> | ||
| + | University of Colorado, Boulder | ||
| + | |||
| + | "Biomass burning (i.e. wildfires, prescribed burns) emits significant amounts of organic carbon to the atmosphere. However, the secondary processing of these emissions is poorly understood. In this work, catechol and resorcinol (1,2-benzenediol and 1,3-benzenediol, respectively) were reacted with hydroxyl radical (OH) in the presence of NOx, or with nitrate radical (NO3) in an environmental chamber. These compounds were previously identified and quantified as significant emissions from biomass burning. Both benzenediol isomers produced a significant quantity of secondary organic aerosol (SOA) after reaction with OH or NO3. 4-nitrocatechol was identified as the dominant product in the SOA from catechol oxidation by both OH and NO3, whereas the product distribution from resorcinol oxidation included benzenetriols, nitroaromatics, and hydroxybenzoquinones. Formation mechanisms of these products are discussed." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 10 April 2017 </font> | ||
| + | |||
| + | <b>North American pollution measurements from geostationary orbit with Tropospheric Emissions: Monitoring of Pollution (TEMPO)</b> | ||
| + | |||
| + | Dr. Kelly Chance<br> | ||
| + | Harvard-Smithsonian Center for Astrophysics | ||
| + | |||
| + | "TEMPO is the first NASA Earth Venture Instrument. It launches between 2019 and 2021 to measure atmospheric pollution from Mexico City and Cuba to the Canadian oil sands, and from the Atlantic to the Pacific. It does this hourly at high spatial resolution, ~10 km 2 , to measure the key elements of air pollution chemistry. Geostationary daytime measurements capture the variability in the diurnal cycle of emissions and chemistry at sub-urban scale to improve emission inventories, monitor population exposure, and enable emission-control strategies. TEMPO measures UV/visible Earth reflectance spectra to retrieve O 3 , NO 2 , SO 2 , H 2 CO, C 2 H 2 O 2 , H 2 O, BrO, OClO, IO, aerosols, cloud parameters, and UVB radiation. It tracks aerosol loading. It provides near-real- time air quality products. TEMPO is the North American component of upcoming the global geostationary constellation for pollution monitoring, together with the European Sentinel-4 and the Korean Geostationary Environmental Monitoring Spectrometer (GEMS). | ||
| + | |||
| + | TEMPO science studies may include: Solar-induced fluorescence from chlorophyll over land and in the ocean to study tropical dynamics, primary productivity and carbon uptake, to detect red tides, and to study phytoplankton; measurements of stratospheric intrusions that cause air quality exceedances; measurements at peaks in vehicle travel to capture the variability in emissions from mobile sources; measurements of thunderstorm activity, including outflow regions to better quantify lightning NO x and O 3 production; cropland measurements to follow the temporal evolution of emissions after fertilizer application and from rain-induced emissions from semi-arid soils; investigating the chemical processing of primary fire emissions and the secondary formation of VOCs and ozone; examining ocean halogen emissions and their impact on the oxidizing capacity of coastal environments; measuring spectra of nighttime lights as markers for human activity, energy conservation, and compliance with outdoor lighting standards intended to reduce light pollution." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Friday, 7 April 2017 </font> | ||
| + | |||
| + | <b>Dissertation Defense: Development and Application of an Oxidation Flow Reactor to Study Secondary Organic Aerosol Formation from Ambient Air</b> | ||
| + | |||
| + | Brett Palm<br> | ||
| + | University of Colorado, Boulder | ||
| + | |||
| + | "Secondary organic aerosols (SOA) in the atmosphere play an important role in air quality, human health, and climate. However, the sources, formation pathways, and fate of SOA are poorly constrained. In this dissertation, I will present development and application of the oxidation flow reactor (OFR) technique for studying SOA formation from OH, O3, and NO3 oxidation of ambient air. With a several-minute residence time and a portable design with no inlet, OFRs are particularly well suited for this purpose. | ||
| + | |||
| + | I will first introduce the OFR concept, and discuss several advances I have made in performing and interpreting OFR experiments. This includes estimating oxidant exposures, modeling the fate of low-volatility gases in the OFR (wall loss, condensation, and oxidation), and comparing SOA yields of single precursors in the OFR with yields measured in environmental chambers. When these experimental details are carefully considered, SOA formation in an OFR can be more reliably compared with ambient SOA formation processes. | ||
| + | |||
| + | I will then present an overview of what OFR measurements have taught us about SOA formation in the atmosphere. I will present a comparison of SOA formation from OH, O3, and NO3 oxidation of ambient air in a wide variety of environments, from rural forests to urban air. In a rural forest, the SOA formation correlated with biogenic precursors (e.g., monoterpenes). In urban air, it correlated instead with reactive anthropogenic tracers (e.g., trimethylbenzene). | ||
| + | |||
| + | In mixed-source regions, the SOA formation did not correlate well with any single precursor, but could be predicted by multilinear regression from several precursors. Despite these correlations, the concentrations of speciated ambient VOCs could only explain approximately 10-50% of the total SOA formed from OH oxidation. In contrast, ambient VOCs could explain all of the SOA formation observed from O3 and NO3 oxidation. Evidence suggests that lower-volatility gases (semivolatile and intermediate-volatility organic compounds; S/IVOCs) were present in ambient air and were the likely source of SOA formation that could not be explained by VOCs. These measurements show that S/IVOCs likely play an important intermediary role in ambient SOA formation in all of the sampled locations, from rural forests to urban air." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 3 April 2017 </font> | ||
| + | |||
| + | <b>Measurements of emissions from agricultural fires and wildfires in the U.S</b> | ||
| + | |||
| + | Dr. Xiaoxi Liu <br> | ||
| + | University of Colorado, Boulder | ||
| + | |||
| + | "Biomass burning (BB) produces significant amounts of trace gases and aerosol, which play important roles in atmospheric chemistry and climate. This study presents detailed airborne measurements of emissions from 15 agricultural fires and 3 wildfires in the U.S. during the 2013 Studies of Emissions and Atmospheric Composition, Clouds and Climate Coupling by Regional Surveys (SEAC4RS) and the Biomass Burning Observation Project (BBOP). A detailed set of emission factors (EFs) for 25 trace gases and 6 components of submicron particulate matter (PM1) was reported for the agricultural fires located in the southeastern U.S. Observed EFs are generally consistent with previous measurements of crop residue burning, but the fires studied here emitted high amounts of oxygenated volatile organic compounds, sulfur dioxide, and fine particles. Filter-based measurements of aerosol light absorption implied that brown carbon was ubiquitous in the plumes. The rapid chemical evolution of the primary emissions in 7 out of 15 agricultural plumes was examined in detail for ~1.2 hr. A Lagrangian plume cross-section model was used to simulate the evolution of ozone, reactive nitrogen species, and organic aerosol (OA). For the western wildfires, we measured an extensive set of EFs for over 80 gases and 5 PM1 species. The wildfires emitted high amounts of PM1 (in which OA comprised most of the mass) with an average EF that is over two times of prescribed fire EFs. The EFs were used to estimate the annual regional emissions from agricultural fires and wildfires for CO, NOx, total non-methane organic compounds, and PM1. Our wildfire PM1 emission estimate from 11 western states is over three times that of the 2011 National Emissions Inventory (NEI) PM2.5 estimate, mainly due to our high EF(PM1), and also higher than the PM2.5 emitted from all other sources in these states according to NEI. This supports the practice of prescribed burning that could reduce fine particle emissions." | ||
| + | |||
| + | ''And'' | ||
| + | |||
| + | <b>Atmospheric chemistry of aliphatic amines: reaction mechanisms and temperature effects</b> | ||
| + | |||
| + | Dr. Derek Price<br> | ||
| + | University of Colorado, Boulder | ||
| + | |||
| + | "Aliphatic amines come from both anthropogenic and biogenic sources, including agricultural/livestock emissions and biomass burning. Radical oxidation of aliphatic amines can produce organic aerosol as well as aminium salt aerosol. The extent to which organic/aminium-salt aerosol is formed is dependent on the type of radical and precursor amine. Temperature has an important impact on SOA formation from aliphatic amines. Temperature variations can occur both seasonally and through vertical mixing in the atmosphere. In this presentation, I will discuss (1) the reaction pathways identified in smog chamber studies of hydroxyl and nitrate radical oxidation of aliphatic amines through comparison of mass spectra from both aerosol (HR-ToF-AMS and PILS-ToF-MS) and gas phase (SIFT-MS) instrumentation, and (2) the effects of temperature changes on the system." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 20 March 2017 </font> | ||
| + | |||
| + | <b>Atmospheric Organics: The Cubism of Atmospheric Chemistry</b> | ||
| + | |||
| + | Colette Heald<br> | ||
| + | MIT | ||
| + | |||
| + | "Reactive organic carbon (ROC) is the fuel of atmospheric chemistry: the oxidation of these species leads to the formation of ozone, aerosols, and CO2, with both air quality and climate impacts. Organic aerosol is an important, often dominant, contributor to atmospheric aerosol, and yet the complexity of its formation and evolution in the atmosphere preclude a good understanding of its impacts. In this talk I will highlight some recent work from my group on tropospheric organics, including: (1) the global budget of reactive organic carbon (2) the deposition of organic carbon and potential constraints on the lifecycle of ROC, and (3) the decadal trend in organic aerosol over the United States." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 13 Mar 2017 </font> | ||
| + | |||
| + | <b>Science Diplomacy: Lessons from recent updates to the Montreal Protocol</b> | ||
| + | |||
| + | Dr. Federico San Martini, <br> | ||
| + | Secretariat for the Implementation of the Montreal Protocol | ||
| + | |||
| + | "On 15 October 2016, after 7 years of consultations, the Parties to the Montreal Protocol adopted an amendment in Kigali, Rwanda that added hydrofluorocarbons (HFCs) to the list of substances controlled under the Protocol. Under the Amendment, countries committed to phase down the production and consumption of HFCs by more than 80 percent, and agreed to provisions to control HFC-23 by-product emissions. Implementation of the Amendment will avoid more than 80 billion metric tons of carbon dioxide equivalent emissions by 2050. The Montreal Protocol has been called the most successful environmental treaty to date, and the Kigali Amendment the single largest contribution to date towards keeping global temperature rise below 2 oC. Why is the Montreal Protocol considered so successful, how will the amendment contribute to climate protection efforts, and how did science inform the policy process? This talk will explore how science informed policy in the Montreal Protocol, and what lessons could be applied to other fora. Time permitting, we will also | ||
| + | discuss international air quality monitoring efforts at diplomatic facilities. | ||
| + | |||
| + | Dr. San Martini has worked for many years on science-policy issues related to air quality, short-lived climate forcers and stratospheric ozone protection. He obtained his PhD with Prof. McRae at MIT, and postdoced with Prof Mario Molina working in Air Pollution in Mexico City, before taking a position with the Board on Chemical Sciences and Technology at the National Academies. He moved to the State Department as a AAAS Science and Technology Policy Fellow, first working on water resources, and then on air quality, short-lived climate forcers and stratospheric ozone protection. While at the State Department Ico was instrumental in establishing the air quality monitoring network at U.S. diplomatic facilities and ensuring that air quality data is publicly available to researchers. More recently he joined the Montreal Protocol Multilateral Fund Secretariat where he works on projects related to ozone- and climate-protection." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 6 March 2017 </font> | ||
| + | |||
| + | <b>Secondary Organic Aerosol Formation from Atmospheric Oxidation of Isoprene: Implications for Air Quality, Climate and Public Health</b> | ||
| + | |||
| + | Prof. Jason Surratt<br> | ||
| + | University of North Carolina | ||
| + | |||
| + | "Atmospheric fine particulate matter (PM 2.5 ) plays a key role in climate and is associated with adverse effects on air quality and human health. The largest mass fraction of PM 2.5 is organic, which is mostly derived from secondary organic aerosol (SOA) formed by the atmospheric oxidation of hydrocarbons. Atmospheric oxidation of isoprene (2-methyl- 1,3-butadiene), the most abundant non-methane hydrocarbon emitted into the atmosphere, is now recognized as one of the largest contributors to PM 2.5 . Despite its abundance, the exact manner in which isoprene- derived SOA is formed has been recently examined through the combination of synthetic organic and analytical chemistry with flow reactor, smog chamber, and field studies. Through these recent studies, we have identified reactive epoxides and hydroperoxides produced from the atmospheric oxidation of isoprene under low-nitric oxide (NO) conditions that are crucial to the formation of ambient SOA. Notably, certain isoprene-derived SOA constituents have been recently observed in cloud water samples and shown to contribute to light-absorbing aerosol, indicating that isoprene-derived SOA may be important for aerosol climate effects. We have found that anthropogenic pollutants, such as acidic sulfate aerosol, significantly enhance isoprene SOA. This is of great public health importance since isoprene is primarily emitted from terrestrial vegetation, and thus, is not controllable, whereas anthropogenic emissions are controllable. Whether SOA derived from this source contributes to the adverse health effects induced by exposure to ambient PM 2.5 reported in epidemiological studies is largely unknown. Using an in vitro model of human airway epithelial cells (BEAS-2B), we have also evaluated the potential early biological effects induced by exposure to isoprene-derived epoxides and hydroperoxides and their resultant SOA constituents. Exposure induced cytotoxicity and expression of oxidative stress and inflammation-associated genes have been assessed. Our initial findings suggest that isoprene-derived epoxides, hydroperoxides and the resultant SOA constituents induce altered oxidative stress and inflammation-associated gene expression in human lung cells under non-cytotoxic conditions. These recent findings highlight the importance of future work aimed at linking PM 2.5 source, composition, exposure biomarkers and health outcomes." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 27 February 2017 </font> | ||
| + | |||
| + | <b>Cannabidiol-dependent modulation of cognitive learning and synaptic function</b> | ||
| + | |||
| + | Prof. Jeff Smith <br> | ||
| + | CSU Pueblo | ||
| + | |||
| + | "The National Institutes of Health, National Institute on Drug Abuse, currently lists Cannabidiol as having potential therapeutic value for treating neurological disorders that include a strong learning and memory component including; anxiety, psychosis, pain, and substance use disorders. It is also being studied for its potential to modulate various neurodegenerative disorders that profoundly affect learning and memory including Alzheimer’s disease. Despite this potential therapeutic importance, the current scientific understanding of exactly how Cannabidiol affects various forms of learning and memory, and the underlying cellular mechanisms that it targets, is inadequate to guide its most efficacious and least harmful use for treating such disorders. Our research advances knowledge in this area by showing that Cannabidiol modulates trace fear conditioning in mice. These experiments model cognitive learning and memory processes and involve multiple brain regions, including the hippocampus, which has a critical role in the learning and memory disorders listed above, and it is essential for achieving normal trace fear conditioning in rodents. Our work further shows that Cannabidiol modulates basal synaptic transmission in mouse hippocampal slices by affecting conduction velocity in the Shaffer collateral and Mossy Fiber pathways, and by modulating synaptic plasticity in these regions. Impulse propagation and synaptic plasticity are essential fundamental mechanisms that support learning and memory, therefore our results present a clearer picture of how Cannabidiol might be most useful, and least harmful for treating neurological disorders that have a strong cognitive learning and memory component." | ||
| + | |||
| + | Download the video [http://cires1.colorado.edu/jimenez-group/Seminars/smith_seminar_2-27-2017.mp4 here] | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 20 February 2017 </font> | ||
| + | |||
| + | <b>Atmospheric Chemistry of Nitrogen Oxides at Soil-Air Interfaces</b> | ||
| + | |||
| + | Prof. Jonathan Raff, <br> | ||
| + | Indiana University | ||
| + | |||
| + | "There remain large uncertainties in the terrestrial sources and sinks of reactive oxides of nitrogen (NOy = NO, NO2, and HONO)—gases that play an important role in regulating the oxidizing capacity of the atmosphere. Most terrestrial surfaces are covered in soil particles, either as fine coatings of air-blown dust or as topsoil in which countless organisms live. In addition, soil particles possess large intrinsic surface areas and myriad reactive surface sites on which chemical reactions occur. These properties make soil a potentially important consideration in understanding the heterogeneous reactions that control the atmospheric NOy budget. In this presentation I will discuss results of laboratory experiments conducted on soil and individual soil constituents aimed at testing the hypothesis that redox couples involving soil organic matter and minerals mediate HONO conversion from NO 2 at night and from nitrate during the daytime. In addition, we carried out kinetics studies using a coated wall flow reactor and surface composition studies using nano-DESI and nanoSIMS [at the Environmental Molecular Sciences Laboratory (EMSL), Pacific Northwest National Laboratory] to explore the role of minerals and organic matter as sinks for HONO in soil." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 13 February 2017 </font> | ||
| + | |||
| + | <b>Understanding the Molecular Signature of Atmospheric Organic Aerosols using Ion Mobility Mass Spectrometry</b> | ||
| + | |||
| + | Dr. Xuan Zhang <br> | ||
| + | NCAR | ||
| + | |||
| + | "I will present some recent developments on the Ion Mobility Mass Spectrometer (IMS) that measures the collision cross section () and mass-to- charge ratio (m/z) of charged molecules. A two-dimensional space based on these two quantities is developed to facilitate the comprehensive investigation of complex organic mixtures in the atmosphere. Species of the same chemical class, despite variations in the molecular structures, tend to develop a unique distribution pattern by following a trend line on the space. The characteristics of trend lines for a variety of functionalities that are commonly present in the atmosphere can be predicted by the core model simulations, which provide a useful tool to identify the chemical class to which an unknown species belongs on the space. Furthermore, molecular characterization of labile species such as multi-functional organic nitrates and highly oxidized organic molecules in the condensed phase using the IMS technique is discussed." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 6 February 2017</font> | ||
| + | |||
| + | <b>Aerosol Liquid Water: A Valentine to the Clean Air Act</b> | ||
| + | |||
| + | Prof. Ann Marie Carlton<br> | ||
| + | University of California, Irvine | ||
| + | |||
| + | "Water is a ubiquitous and abundant component of atmospheric particles. It influences light scattering, the hydrological cycle, atmospheric chemistry, and secondary formation of particulate matter (PM) in the atmosphere. Despite the critical importance of aerosol liquid water, actual mass concentrations are not well documented in the literature. Routine air quality networks that measure particle mass [e.g., the U.S. Environmental Protection Agency’ s Interagency Monitoring of Protected Visual Environments(IMPROVE)] and most particle measurement techniques [e.g., the aerosol mass spectrometer (AMS)] remove water and other semivolatile compounds during sampling and/or during filter equilibration. Using speciated ion and meteorological data we estimate water mass concentrations to better understand the geospatial patterns and historical trends of aerosol liquid water in the context of improved air quality and recently noted reductions in particulate organic carbon (OC) mass. We find a decrease in aerosol water mass concentrations that is correlated with decreasing organic particle mass, for which we can provide a plausible mechanistic explanation. These findings are consistent with the hypotheses that aerosol liquid water facilitates formation of biogenic secondary organic aerosol (SOA) and that biogenically derived SOA is modulated in the presence of anthropogenic perturbations. Decreasing aerosol liquid water mass can be explained, in part, by environmental regulations aimed at reducing sulfur emissions to alleviate environmental problems associated with acid rain and inorganic particle mass, suggesting the Clean Air Act is more successful than accounted for." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 30 January 2017 </font> | ||
| + | |||
| + | <b>Closing the loop on phase equilibrium: connecting fundamental volatility measurements of complex fluids with applications of headspace detection</b> | ||
| + | |||
| + | Megan Harries<br> | ||
| + | ANYL 3rd Year Student, CU Boulder<br> | ||
| + | |||
| + | "Almost all fluids that are useful to us are complex mixtures consisting of many components with differing properties. An understanding of the phase equilibrium of these fluids is essential to many scientific disciplines as well as industry. For example, knowledge gaps in this area affect applications ranging from the development of alternative fuels to the deployment of forensic methods based on vapor characterization. These applications depend on our ability to bridge the gap – governed by thermodynamics – between vapor and liquid composition. This is difficult for even a binary, but beyond a ternary mixture, it becomes a real hair-puller. To unite these two pieces of the complete picture, I am adapting two relatively new techniques: the advanced distillation curve (ADC) method for volatility measurement and PLOT-cryoadsorption for vapor sampling. A modification of the ADC method, in development, provides simultaneous determination of temperature, pressure, and the composition of both vapor and condensed phases of a fluid mixture, while PLOT-cryoadsorption provides the field-ready vapor analysis. I will present a preliminary study that demonstrates the method and our ability to reconcile the measurements with models. Finally, I will discuss two related projects demonstrating applications, one using ADC to evaluate two alternative fuels and the second demonstrating environmental applications of a field portable PLOT-cryoadsorption device." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 23 January 2017</font> | ||
| + | |||
| + | <b>UV Photochemistry of Carboxylic Acids: A source of unsaturated VOC and OVOC in marine air</b> | ||
| + | |||
| + | Randall Chiu<br> | ||
| + | ANYL 3rd Year Student, CU Boulder<br> | ||
| + | |||
| + | "Photochemistry plays an important role in marine dissolved organic carbon (DOC) degradation, but the mechanisms that convert DOC into volatile organic compounds (VOCs) remain poorly understood. We irradiated carboxylic acids (C7-C9) on a simulated ocean surface with UV light (<320 nm) in a photochemical flow reactor, and transferred the VOC products into a dark ozone reactor. Glyoxal was detected as a secondary product from heptanoic, octanoic, and NA films, but not from octanol. Primary glyoxal emissions were not observed, nor was glyoxal formed in the absence of ozone. Addition of a photosensitizer had no noticeable effect. The concurrent detection of heptanal in the NA system suggests that the ozonolysis of 2-nonenal is the primary chemical mechanism that produces glyoxal. This source can potentially sustain few 10 pptv glyoxal over oceans, and helps to explain why glyoxal fluxes in marine air are directed from the atmosphere into the ocean." | ||
| + | |||
| + | <br /> | ||
| + | <font size="4">Thursday, 12 January 2017</font> | ||
| + | |||
| + | <b>Secondary Organic Aerosols: Development and Application of New Techniques in Chamber and Field Experiments</b> | ||
| + | Jordan Krechmer (Advisor: Jose-Luis Jimenez)<br> | ||
| + | ANYL student PhD Dissertation Defense, CU Boulder<br> | ||
| + | |||
| + | Secondary organic aerosols (SOA) have detrimental effects on human health and can influence the Earth’s climate by altering radiative forcing. Their sources, fates, and chemical composition across the globe, however, remain poorly constrained. In this talk I will present new developments in the analysis of SOA field and laboratory experiments. First, I will describe the development of a method to accurately quantify the loss of gaseous compounds to the Teflon walls of environmental chambers using real-time measurements. The method used short bursts of light to produce oxidants in situ, which in turn produced several gas-phase products with differing volatilities. Gas-phase products were observed in real time with a chemical ionization mass spectrometer (CIMS). The time scale of this process was short (< 700 s) enough to be on the order of other processes in SOA chamber experiments and is thus important enough to necessitate accounting for. | ||
| + | |||
| + | Second, I describe how the same method was then applied to chamber experiments with liquid organic seed aerosol present to quantify the effect of gas-wall partitioning on aerosol mass yield experiments. A well-characterized simple chemical system is used to produce low volatility organic compounds at a rapid rate, which are taken up by liquid organic seed particles and/or the Teflon chamber walls. Both gas-phase products and aerosol concentrations are continuously monitored. A simple but comprehensive box model is used to quantify gas-particle partitioning and α. We discuss the implications for quantifying gas-particle partitioning under more complex conditions. | ||
| + | |||
| + | ==Fall 2016== | ||
| + | <br /> | ||
| + | <font size="4"> Monday, 14 November 2016 </font> | ||
| + | |||
| + | <B>Industrial Hemp: Opportunities in R & D in Colorado’s Fastest Growing Industry</B> | ||
| + | |||
| + | Bob Sievers<br> | ||
| + | Department of Chemistry and Biochemistry, Environmental Program and CIRES <br> | ||
| + | University of Colorado Boulder | ||
| + | |||
| + | “Industrial Hemp” is legally defined as Cannabis Sativa containing less than 0.3% THC, and “Marijuana” is defined as containing more than 0.3% THC. In 2016 in Colorado, the industrial hemp and marijuana industries created revenues totaling $1 billion dollars. To put this in context, this exceeds revenues from grain growing, sports and performing arts venues, or residential construction. It is now possible at CU (and other universities) to perform R & D on the growth, separation, purification, and applications of industrial hemp or marijuana under strict regulations, which will be discussed. Examples of possible uses range from precursors to novel materials and various medical applications such as those outlined by the National Institute for Drug Abuse. Examples are treatment of stress and pain, epileptic seizures, and substance abuse. Development of new separation, analysis methods, and pharmaceutical delivery are important to the success of this industry. Methods for improving bioavailability of cannabidiol have been invented. | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 7 November 2016 </font> | ||
| + | |||
| + | <B>Exploring the Evolution of Biomass-Burning Aerosol in Chambers and the Atmosphere</B> | ||
| + | |||
| + | Jeff Pierce | ||
| + | <br>Associate Professor, Department of Atmospheric Science | ||
| + | <br>Colorado State University | ||
| + | |||
| + | I will discuss theoretical work on the evolution of biomass-burning organic aerosol in smog chambers and in ambient plumes that shows: (1) vapor wall losses appear to matter greatly in smog-chamber oxidation experiments of biomass-burning aerosol, and (2) differences in fire size and meteorology might impact the organic aerosol (and aerosol size distribution) evolution in ambient plumes as much as differences in emission factors or chemical mechanisms. Laboratory and field experiments should carefully consider these complicating factors when comparing across different experiments and plumes. | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, 31 October 2016 </font> | ||
| + | |||
| + | <B>Cell Membrane Conditions on C-Reactive Protein Binding</B> | ||
| + | |||
| + | Mitchell Alton, First Year Graduate Student | ||
| + | <br>Department of Chemistry and Biochemistry | ||
| + | <br>University of Colorado Boulder | ||
| + | |||
| + | C-reactive protein (CRP) is an important human protein involved in identification of apoptotic (dying) cells and acts as a general marker of inflammation. CRP functions by binding to the lipid, phosphatidylcholine (PC), which is present in all cells. However CRP does not bind to normal, healthy cells. It is theorized that CRP has different binding affinities for healthy versus dying cells due to differences in membrane curvatures and the presence of oxidized lipids in dying cells. Additionally, CRP has two different conformations that affect binding affinities. I will discuss how these conditions affect the binding of CRP to membranes. | ||
| + | |||
| + | <B>Measurements of Peroxy Radical Loss Rates on Laboratory Surfaces</B> | ||
| + | |||
| + | Benjamin Deming, First Year Graduate Student | ||
| + | <br>Department of Chemistry and Biochemistry | ||
| + | <br>University of Colorado Boulder | ||
| + | |||
| + | Peroxy radicals are important intermediates in the oxidative processing of volatile organic compounds in the atmosphere. Numerous instruments to measure atmospheric concentrations of these short-lived species are in active development. Wall losses of these compounds should be considered when designing such an instrument due to losses within the inlet. In this work, an ECHAMP peroxy radical detector was used to determine the wall-loss rates of HO 2 , CH 3 O 2 , C 2 H 5 O 2 , and isoprene peroxy radicals on PFA Teflon, quartz, halocarbon wax, and other materials. In addition to concentration and relative humidity dependencies, the use of FEP vs PFA Teflon was investigated. | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, October 17, 2016 </font> | ||
| + | |||
| + | <B>Liquid water on Mars? Stable or metastable salt solutions formed via deliquescence</B> | ||
| + | |||
| + | Raina Gough | ||
| + | <br>Department of Chemistry and Biochemistry and CIRES | ||
| + | <br>University of Colorado Boulder | ||
| + | |||
| + | Several different hygroscopic salts are known to exist in the Martian soil. Although Mars is a cold, dry planet where pure liquid water is not stable, deliquescence of perchlorate and other species can likely provide small amounts of liquid water temporarily. We use Raman microscopy and an environmental cell to probe the conditions under which such liquid brines can form and persist under low Martian temperatures. We will discuss the brine-forming ability of several different Mars-relevant salts and salt mixtures, as well as the potential habitability or toxicity of these aqueous solutions to bacterial spores. We discuss the relevance of these studies to the recently publicized “recurring slope lineae” (RSL) on Mars that may be evidence of liquid water flows. We also discuss the results of experiments we have done on salty sediment samples from the Dry Valleys of Antarctica, the only terrestrial analog site for the Martian RSL. | ||
| + | |||
| + | |||
| + | <B>Electronic Properties and Composition of GaAs1-xPx Grown by Close-Spaced Vapor Transport</B> | ||
| + | |||
| + | Allison Davis, First Year Graduate Student | ||
| + | <br>Department of Chemistry and Biochemistry and CIRES | ||
| + | <br>University of Colorado Boulder | ||
| + | |||
| + | Abstract | ||
| + | |||
| + | Semiconductor thin films are widely utilized in the production of photovoltaic devices. GaAs is a III-V binary semiconductor with exceptional optoelectronic properties such as a direct band gap of 1.42eV and a lattice parameter of 5.6Å, making it an attractive material for the construction of high efficiency solar cells. GaAs can be combined with GaP, another III-V semiconductor with a 2.25 eV band gap, which allows the band gap and the lattice parameter to be tuned. Combining these binary materials to make the ternary alloy GaAs1-xPx is useful for multi-junction cells. This study aims to produce and characterize GaAs1-xPx thin films grown via CSVT. | ||
| + | |||
| + | <br /> | ||
| + | <font size="4"> Monday, October 3, 2016 </font> | ||
| + | |||
| + | <B>Characterization of Organic Nitrogen in the Atmosphere</B> | ||
| + | |||
| + | Ellie Browne | ||
| + | <br>Department of Chemistry and Biochemistry and CIRES | ||
| + | <br>University of Colorado Boulder | ||
| + | |||
| + | Abstract | ||
Organic nitrogen is a ubiquitous atmospheric component typically accounting for between one-quarter and one-third of reactive nitrogen deposition. The chemical complexity and reactivity of organic nitrogen, however, has made it challenging to study. Consequently, little is known about the atmospheric processing of organic nitrogen and the resulting implications for biogeochemistry, air quality, and climate. Research in my group uses mass spectrometry to identify the organic nitrogen compounds present in the atmosphere and to investigate the chemical processing of these compounds. This talk will describe the techniques that we use in the lab will discuss results from our recent field measurements. | Organic nitrogen is a ubiquitous atmospheric component typically accounting for between one-quarter and one-third of reactive nitrogen deposition. The chemical complexity and reactivity of organic nitrogen, however, has made it challenging to study. Consequently, little is known about the atmospheric processing of organic nitrogen and the resulting implications for biogeochemistry, air quality, and climate. Research in my group uses mass spectrometry to identify the organic nitrogen compounds present in the atmosphere and to investigate the chemical processing of these compounds. This talk will describe the techniques that we use in the lab will discuss results from our recent field measurements. | ||
| Line 65: | Line 3,835: | ||
Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, properties, and evolution are poorly understood. In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students. Ongoing projects include global aerosol measurements and analysis as part of the NASA ATOM project, which is sampling (almost) pole-to-pole across the vertical profile. Initial model comparisons suggest the importance of fast OA removal channels. We have used an Oxidation Flow Reactor (OFR) at multiple field studies and find consistent patterns, where SOA formation by O3 and NO3 are consistent with models, while formation by OH is substantially underpredicted unless semivolatile and intermediate volatility species are accounted for. We are starting to use the GECKO-A fully explicit model to investigate this underprediction, as well as to characterize the similarities and differences of the chemical regimes across the OFR, large chambers, and the atmosphere. We are investigating wall losses of vapors on large chambers, and using the results to enable the investigation of gas/particle partitioning for different types of particles. Finally, we are continuing to explore the chemistry of indoor air with ToF-CIMS and other instruments, where we measured 60+ organic acids in a classroom, some of which are related to humans. Future campaigns will involve sampling at locations such as dining halls, gyms, and art museums. | Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, properties, and evolution are poorly understood. In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students. Ongoing projects include global aerosol measurements and analysis as part of the NASA ATOM project, which is sampling (almost) pole-to-pole across the vertical profile. Initial model comparisons suggest the importance of fast OA removal channels. We have used an Oxidation Flow Reactor (OFR) at multiple field studies and find consistent patterns, where SOA formation by O3 and NO3 are consistent with models, while formation by OH is substantially underpredicted unless semivolatile and intermediate volatility species are accounted for. We are starting to use the GECKO-A fully explicit model to investigate this underprediction, as well as to characterize the similarities and differences of the chemical regimes across the OFR, large chambers, and the atmosphere. We are investigating wall losses of vapors on large chambers, and using the results to enable the investigation of gas/particle partitioning for different types of particles. Finally, we are continuing to explore the chemistry of indoor air with ToF-CIMS and other instruments, where we measured 60+ organic acids in a classroom, some of which are related to humans. Future campaigns will involve sampling at locations such as dining halls, gyms, and art museums. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 19, 2016 </font> | ||
<B>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</B> | <B>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</B> | ||
| Line 88: | Line 3,859: | ||
Volatile organic compounds (VOCs) in the atmosphere are emitted from many different natural and man-made sources. In the presence of nitrogen oxides, VOCs are precursors for the formation of secondary pollutants like ozone and fine particles, which are both important for climate and air quality. We measure VOCs in the atmosphere using mass spectrometry from research aircraft, mobile laboratories and ground sites. I will focus on measurements made with a newly developed instrument with a focus on compounds that have not been widely reported. | Volatile organic compounds (VOCs) in the atmosphere are emitted from many different natural and man-made sources. In the presence of nitrogen oxides, VOCs are precursors for the formation of secondary pollutants like ozone and fine particles, which are both important for climate and air quality. We measure VOCs in the atmosphere using mass spectrometry from research aircraft, mobile laboratories and ground sites. I will focus on measurements made with a newly developed instrument with a focus on compounds that have not been widely reported. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 12, 2016 </font> | ||
<B>Going Through a Phase: Loss of aerosol water upon contact</B> | <B>Going Through a Phase: Loss of aerosol water upon contact</B> | ||
| Line 112: | Line 3,884: | ||
The growing world population in the Anthropocene is impacting atmospheric composition on global scales. The last decade has marked a turning point in human history, as for the first time more people are now living in urban rather than rural environments on our planet. Atmospheric chemistry is at the core of the impacts of Air Pollution on human health (ozone and aerosols) and Climate (lifetime of greenhouse gases, aerosol radiation feedbacks). The Volkamer group studies remote pristine atmospheric environments to better understand how natural sources of tropospheric halogens and marine organic carbon destroy ozone, oxidize atmospheric mercury, modify oxidative capacity and aerosols. Why was ozone so low in pre-industrial times? We currently do not have the answer to this question, but it determines the baseline under which anthropogenic impacts have modified the radiative forcing associated with ozone as an important greenhouse gas. We also develop mobile Solar Occultation Flux spectroscopy (mobile SOF) to better quantify total emission fluxes of ozone and aerosol precursor gases (NH3, NO2, C2H6, etc) from agriculture, oil & natural gas production, urban areas and wildfires. The CU mobile SOF instrument is a unique prototype in the US, and limited by the need to develop retrievals to measure a large variety of gases that absorb at mid-infrared wavelengths. Finally, we are conducting laboratory experiments to understand the reaction mechanisms that determine the sources and sinks of our field observations, test and improve atmospheric models, and satellites. | The growing world population in the Anthropocene is impacting atmospheric composition on global scales. The last decade has marked a turning point in human history, as for the first time more people are now living in urban rather than rural environments on our planet. Atmospheric chemistry is at the core of the impacts of Air Pollution on human health (ozone and aerosols) and Climate (lifetime of greenhouse gases, aerosol radiation feedbacks). The Volkamer group studies remote pristine atmospheric environments to better understand how natural sources of tropospheric halogens and marine organic carbon destroy ozone, oxidize atmospheric mercury, modify oxidative capacity and aerosols. Why was ozone so low in pre-industrial times? We currently do not have the answer to this question, but it determines the baseline under which anthropogenic impacts have modified the radiative forcing associated with ozone as an important greenhouse gas. We also develop mobile Solar Occultation Flux spectroscopy (mobile SOF) to better quantify total emission fluxes of ozone and aerosol precursor gases (NH3, NO2, C2H6, etc) from agriculture, oil & natural gas production, urban areas and wildfires. The CU mobile SOF instrument is a unique prototype in the US, and limited by the need to develop retrievals to measure a large variety of gases that absorb at mid-infrared wavelengths. Finally, we are conducting laboratory experiments to understand the reaction mechanisms that determine the sources and sinks of our field observations, test and improve atmospheric models, and satellites. | ||
| − | == Monday, April 18, 2016 | + | ==Spring 2016== |
| + | <br /> | ||
| + | <font size="4"> Monday, April 18, 2016 </font> | ||
<b>Effects of Atmospheric Conditions on the Composition of Secondary Organic Aerosol Formed from the Oxidation of Isoprene and Monoterpenes</b> | <b>Effects of Atmospheric Conditions on the Composition of Secondary Organic Aerosol Formed from the Oxidation of Isoprene and Monoterpenes</b> | ||
| Line 124: | Line 3,898: | ||
Motivated by the Southern Oxidant and Aerosol Study (SOAS) field campaign of 2013, a series of environmental chamber experiments have been conducted to study the effect of environmental conditions on aerosol composition formed from biogenic VOC precursors. The experiments, performed under conditions that mimic those observed during SOAS, study the secondary organic aerosol (SOA) formed from the oxidation of isoprene and select monoterpenes. The composition of the SOA formed was characterized using spectrophotometric functional group analysis methods to quantify the amount of carbonyl [C(O)], carboxylic acid [C(O)OH], hydroxyl [CHOH], ester [C(O)OR], peroxide [CHOOR] and nitrate [CHONO2] groups in the aerosol. Further molecular analysis was done on select systems using various mass spectrometry methods. By comparing the composition of the chamber aerosol with field samples collected during the SOAS campaign, we will attempt to identify the chemistry that leads to aerosol formation in the southeast US. | Motivated by the Southern Oxidant and Aerosol Study (SOAS) field campaign of 2013, a series of environmental chamber experiments have been conducted to study the effect of environmental conditions on aerosol composition formed from biogenic VOC precursors. The experiments, performed under conditions that mimic those observed during SOAS, study the secondary organic aerosol (SOA) formed from the oxidation of isoprene and select monoterpenes. The composition of the SOA formed was characterized using spectrophotometric functional group analysis methods to quantify the amount of carbonyl [C(O)], carboxylic acid [C(O)OH], hydroxyl [CHOH], ester [C(O)OR], peroxide [CHOOR] and nitrate [CHONO2] groups in the aerosol. Further molecular analysis was done on select systems using various mass spectrometry methods. By comparing the composition of the chamber aerosol with field samples collected during the SOAS campaign, we will attempt to identify the chemistry that leads to aerosol formation in the southeast US. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 11, 2016 </font> | ||
<b>On the Lifetime of Nitrogen Oxides in the Continental Boundary Layer</b> | <b>On the Lifetime of Nitrogen Oxides in the Continental Boundary Layer</b> | ||
| Line 138: | Line 3,913: | ||
Nitrogen oxides drive the chemistry of the troposphere, catalyzing production of oxidants, ozone and aerosol. Textbooks describe this chemistry as starting with emission of NO and terminating with the gas phase reaction of OH with NO2 to produce HNO3. Here, I will describe approaches to observing and characterizing the lifetime of nitrogen oxides using in situ and space based observations. In contrast to the textbook story we find the lifetime of nitrogen oxides on the continents are primarily controlled by organic nitrate formation. | Nitrogen oxides drive the chemistry of the troposphere, catalyzing production of oxidants, ozone and aerosol. Textbooks describe this chemistry as starting with emission of NO and terminating with the gas phase reaction of OH with NO2 to produce HNO3. Here, I will describe approaches to observing and characterizing the lifetime of nitrogen oxides using in situ and space based observations. In contrast to the textbook story we find the lifetime of nitrogen oxides on the continents are primarily controlled by organic nitrate formation. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 4, 2016 </font> | ||
<b>Heterogeneous Efflorescence by Mineral Dust Particles</b> | <b>Heterogeneous Efflorescence by Mineral Dust Particles</b> | ||
| Line 150: | Line 3,926: | ||
One of the key factors that determines particle growth and heterogeneous reaction efficiency of aerosols is whether the particle is liquid or solid. Salt aerosols transition between these phases through efflorescence and deliquescence. Although homogeneous efflorescence has been studied in detail, heterogeneous efflorescence is not well understood. Here we examined heterogeneous efflorescence by optically levitating single droplets of salt solutions and exposed the droplet to a flow of mineral dust particles. The impact of mineral dust on efflorescence and the difference between immersion and contact efflorescence will be discussed. | One of the key factors that determines particle growth and heterogeneous reaction efficiency of aerosols is whether the particle is liquid or solid. Salt aerosols transition between these phases through efflorescence and deliquescence. Although homogeneous efflorescence has been studied in detail, heterogeneous efflorescence is not well understood. Here we examined heterogeneous efflorescence by optically levitating single droplets of salt solutions and exposed the droplet to a flow of mineral dust particles. The impact of mineral dust on efflorescence and the difference between immersion and contact efflorescence will be discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 28, 2016 </font> | ||
<b>Chemistry of Multifunctional Hydroperoxides in Secondary Organic Aerosol</b> | <b>Chemistry of Multifunctional Hydroperoxides in Secondary Organic Aerosol</b> | ||
| Line 162: | Line 3,939: | ||
Multifunctional hydroperoxides have been identified as a product of autoxidation reactions and have been proposed as structures for extremely low volatility compounds (ELVOCs) observed in secondary organic aerosol. The chemistry of these hydroperoxides is complex and challenging to study, as many conventional analytical techniques disturb the peroxide functionality and cause additional chemistry to occur. In this work we are able to produce multifunctional hydroperoxides in environmental chamber reactions as well as in solution, allowing us to investigate their chemistry under a variety of conditions. Observed intramolecular reactions, reactions with water, acid-catalyzed acetal formation and acid-catalyzed peroxyacetal formation are all discussed. | Multifunctional hydroperoxides have been identified as a product of autoxidation reactions and have been proposed as structures for extremely low volatility compounds (ELVOCs) observed in secondary organic aerosol. The chemistry of these hydroperoxides is complex and challenging to study, as many conventional analytical techniques disturb the peroxide functionality and cause additional chemistry to occur. In this work we are able to produce multifunctional hydroperoxides in environmental chamber reactions as well as in solution, allowing us to investigate their chemistry under a variety of conditions. Observed intramolecular reactions, reactions with water, acid-catalyzed acetal formation and acid-catalyzed peroxyacetal formation are all discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 14, 2016 </font> | ||
<b>The Seasonality of Tropospheric Ozone and Reactive Nitrogen: From Measurements to Models</b> | <b>The Seasonality of Tropospheric Ozone and Reactive Nitrogen: From Measurements to Models</b> | ||
| Line 174: | Line 3,952: | ||
Tropospheric ozone is a potent greenhouse gas that forms through the oxidation of volatile organic compounds (VOCs) in the presence of nitrogen oxides (NOx). Previous field studies have largely focused on summertime ozone photochemistry that varies non-linearly with NOx and VOCs. First I will present results from OH reactivity, ozone production efficiency, and box-model analyses that quantify the impacts of regional oil and natural gas (O&NG) emissions on photochemical ozone in the Northern Front Range (NFR) of Colorado. The NFR is unique in that it has been non-compliant with ozone air quality standards since 2007 and has ozone precursor emissions from urban-Denver in close proximity to a rapidly developing O&NG basin. Second I will present an initial analysis of winter field data and discuss planned work aimed at addressing scientific uncertainties in wintertime O3 proces | Tropospheric ozone is a potent greenhouse gas that forms through the oxidation of volatile organic compounds (VOCs) in the presence of nitrogen oxides (NOx). Previous field studies have largely focused on summertime ozone photochemistry that varies non-linearly with NOx and VOCs. First I will present results from OH reactivity, ozone production efficiency, and box-model analyses that quantify the impacts of regional oil and natural gas (O&NG) emissions on photochemical ozone in the Northern Front Range (NFR) of Colorado. The NFR is unique in that it has been non-compliant with ozone air quality standards since 2007 and has ozone precursor emissions from urban-Denver in close proximity to a rapidly developing O&NG basin. Second I will present an initial analysis of winter field data and discuss planned work aimed at addressing scientific uncertainties in wintertime O3 proces | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 7, 2016 </font> | ||
<B>Effect of a Functional Group on SOA Yields and Particle Composition from OH Radical Initiated Reactions of Alkanes in the Presence of NOx</B> | <B>Effect of a Functional Group on SOA Yields and Particle Composition from OH Radical Initiated Reactions of Alkanes in the Presence of NOx</B> | ||
| Line 186: | Line 3,965: | ||
In a series of laboratory experiments we have identified major products and determined SOA yields from the reactions of C10 alcohol and C12 ketone position-isomers with OH radicals in the presence of NOx. Reactions were studied with a particle beam mass spectrometer (PBMS), gas chromatograph with flame ionization detection (GC-FID), and a scanning mobility particle sizer (SMPS), among other instruments. Functional group position had a considerable effect on SOA yields, which is fully explainable by the fate of alkoxy radical intermediates and vapor pressures of resulting products. Important observed differences from alkane chemistry were enhanced decomposition via α-cleavage, ability of the preexisting ketone to play a role in cyclic hemiacetal formation, and significantly hindered isomerization when the ketone was part of the transition-state ring. | In a series of laboratory experiments we have identified major products and determined SOA yields from the reactions of C10 alcohol and C12 ketone position-isomers with OH radicals in the presence of NOx. Reactions were studied with a particle beam mass spectrometer (PBMS), gas chromatograph with flame ionization detection (GC-FID), and a scanning mobility particle sizer (SMPS), among other instruments. Functional group position had a considerable effect on SOA yields, which is fully explainable by the fate of alkoxy radical intermediates and vapor pressures of resulting products. Important observed differences from alkane chemistry were enhanced decomposition via α-cleavage, ability of the preexisting ketone to play a role in cyclic hemiacetal formation, and significantly hindered isomerization when the ketone was part of the transition-state ring. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 29, 2016 </font> | ||
<B>Supercooling and ice formation of perchlorate and | <B>Supercooling and ice formation of perchlorate and | ||
| Line 199: | Line 3,979: | ||
Perchlorate and chloride salts, discovered in the Martian regolith at multiple landing sites, may provide pathways for liquid water stability on current Mars. It has previously been assumed that perchlorate and chloride brines form in the Martian regolith via melting or deliquescence, they would be present only briefly because efflorescence into a crystal or freezing to ice would soon occur. Here, we used a Raman microscope to study the temperature and relative humidity (RH) conditions at which magnesium perchlorate and magnesium chloride brines will deliquesce into aqueous droplets, form ice, and effloresce back into crystalline particles. | Perchlorate and chloride salts, discovered in the Martian regolith at multiple landing sites, may provide pathways for liquid water stability on current Mars. It has previously been assumed that perchlorate and chloride brines form in the Martian regolith via melting or deliquescence, they would be present only briefly because efflorescence into a crystal or freezing to ice would soon occur. Here, we used a Raman microscope to study the temperature and relative humidity (RH) conditions at which magnesium perchlorate and magnesium chloride brines will deliquesce into aqueous droplets, form ice, and effloresce back into crystalline particles. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 22, 2016 </font> | ||
<B> Investigating hydrocarbon emissions in oil and gas basins using mobile platforms</B> | <B> Investigating hydrocarbon emissions in oil and gas basins using mobile platforms</B> | ||
| Line 206: | Line 3,987: | ||
<br>Deparmtment of Chemistry and Biochemistry | <br>Deparmtment of Chemistry and Biochemistry | ||
<br>University of Colorado | <br>University of Colorado | ||
| + | |||
| + | <br>Cires Auditorium | ||
Abstract | Abstract | ||
| Line 211: | Line 3,994: | ||
Methane emissions are of concern due to methane’s contribution to global climate change and tropospheric ozone formation. Sources of methane include landfills, agriculture, and oil and natural gas operations. Recent studies have focused on determining the extent to which oil and gas operations contribute to methane emissions. The National Oceanic and Atmospheric Administration’s (NOAA) Global Monitoring Division has conducted fieldwork in oil and gas basins in order to characterize and quantify emissions of methane and non-methane hydrocarbons. Field campaigns from the past two years will be presented. Measurement techniques and methods, including the use of a mobile laboratory and aircraft, will be discussed. | Methane emissions are of concern due to methane’s contribution to global climate change and tropospheric ozone formation. Sources of methane include landfills, agriculture, and oil and natural gas operations. Recent studies have focused on determining the extent to which oil and gas operations contribute to methane emissions. The National Oceanic and Atmospheric Administration’s (NOAA) Global Monitoring Division has conducted fieldwork in oil and gas basins in order to characterize and quantify emissions of methane and non-methane hydrocarbons. Field campaigns from the past two years will be presented. Measurement techniques and methods, including the use of a mobile laboratory and aircraft, will be discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 15, 2016 </font> | ||
<b>Chemistry in of the coldest places in the atmosphere: | <b>Chemistry in of the coldest places in the atmosphere: | ||
| Line 238: | Line 4,022: | ||
The WINTER aircraft campaign was a recent field experiment to probe the sources and evolution of gas and aerosol pollutants in Northeast US urban and industrial plumes during the winter. A highly customized Aerodyne aerosol mass spectrometer was flown on the NCAR C-130 to characterize submicron aerosol composition and evolution. Work towards constraining wintertime secondary organic aerosol formation and evolution will be presented from a case study of urban outflow from NYC. Observations and results of wintertime aerosol nitrate, power plant plume acidity, as well as measurements made from an oxidation flow reactor, flown for the first time, will be discussed. | The WINTER aircraft campaign was a recent field experiment to probe the sources and evolution of gas and aerosol pollutants in Northeast US urban and industrial plumes during the winter. A highly customized Aerodyne aerosol mass spectrometer was flown on the NCAR C-130 to characterize submicron aerosol composition and evolution. Work towards constraining wintertime secondary organic aerosol formation and evolution will be presented from a case study of urban outflow from NYC. Observations and results of wintertime aerosol nitrate, power plant plume acidity, as well as measurements made from an oxidation flow reactor, flown for the first time, will be discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 1, 2016 </font> | ||
<b> Measurement of volatile organic compounds in the atmosphere using NO+ chemical ionization mass spectrometry</b> | <b> Measurement of volatile organic compounds in the atmosphere using NO+ chemical ionization mass spectrometry</b> | ||
| Line 250: | Line 4,035: | ||
The underutilized technique of NO+ chemical ionization mass spectrometry (NO+ CIMS) may improve volatile organic compound (VOC) measurement in the troposphere. In this talk I describe the development of an NO+ CIMS instrument and evaluate the usefulness of the NO+ technique. The evaluation is established through labwork using a gas-chromatography (GC) interface, in-situ measurement of urban air using a GC interface, and direct measurement of urban air. NO+ is useful for fast (1Hz) measurement of carbonyl isomers, for small aliphatics, and large (C13-C15) n-alkanes. The NO+ CIMS technique may be an extremely useful approach for studies of SOA formation, photochemistry, and emissions from fossil fuels and biomass burning. | The underutilized technique of NO+ chemical ionization mass spectrometry (NO+ CIMS) may improve volatile organic compound (VOC) measurement in the troposphere. In this talk I describe the development of an NO+ CIMS instrument and evaluate the usefulness of the NO+ technique. The evaluation is established through labwork using a gas-chromatography (GC) interface, in-situ measurement of urban air using a GC interface, and direct measurement of urban air. NO+ is useful for fast (1Hz) measurement of carbonyl isomers, for small aliphatics, and large (C13-C15) n-alkanes. The NO+ CIMS technique may be an extremely useful approach for studies of SOA formation, photochemistry, and emissions from fossil fuels and biomass burning. | ||
| − | == Monday, December 7, 2015 | + | ==Fall 2015== |
| + | <br /> | ||
| + | <font size="4"> Monday, December 7, 2015 </font> | ||
<b>Parameterization and evaluation of airborne halogen oxide | <b>Parameterization and evaluation of airborne halogen oxide | ||
| Line 264: | Line 4,051: | ||
Tropospheric halogen oxides catalytically destroy ozone, modify oxidative capacity and oxidize atmospheric mercury. Ozone is an important precursor for OH, which determines the lifetime of methane, an important greenhouse gas. About 75% of the global tropospheric ozone and methane loss occurs at tropical latitudes, where further the ozone radiative forcing is most sensitive to changes in the ozone budget. We have measured bromine and iodine monoxide (BrO and IO) by Airborne Multi-Axis Differential Optical Absorption Spectroscopy (AMAX-DOAS) over the Western, Central and Eastern tropical Pacific Ocean. AMAX-DOAS measures solar scattered light along changing lines of sight to detect trace gases at different altitudes. Light path lengths at instrument altitude can reach up to a few hundred km in thin air, which enables detection limits of about 0.3 pptv for BrO and 0.05 pptv for IO for 60s and 30s integration time respectively. The initial result of a DOAS analysis is the integrated concentration along the line of sight, i.e. a column measurement. To derive volume mixing ratios from MAX-DOAS data typically involves the time consuming process of simulating the light path contributions to each measured spectrum by radiative transfer modeling. Here we present a method to parameterize radiative transfer, which allows for a fast conversion of column measurements into volume mixing ratios along the flight track. We will compare parameterized results with vertical profiles we retrieved by inversion techniques and discuss advantages and limitations of our new method. | Tropospheric halogen oxides catalytically destroy ozone, modify oxidative capacity and oxidize atmospheric mercury. Ozone is an important precursor for OH, which determines the lifetime of methane, an important greenhouse gas. About 75% of the global tropospheric ozone and methane loss occurs at tropical latitudes, where further the ozone radiative forcing is most sensitive to changes in the ozone budget. We have measured bromine and iodine monoxide (BrO and IO) by Airborne Multi-Axis Differential Optical Absorption Spectroscopy (AMAX-DOAS) over the Western, Central and Eastern tropical Pacific Ocean. AMAX-DOAS measures solar scattered light along changing lines of sight to detect trace gases at different altitudes. Light path lengths at instrument altitude can reach up to a few hundred km in thin air, which enables detection limits of about 0.3 pptv for BrO and 0.05 pptv for IO for 60s and 30s integration time respectively. The initial result of a DOAS analysis is the integrated concentration along the line of sight, i.e. a column measurement. To derive volume mixing ratios from MAX-DOAS data typically involves the time consuming process of simulating the light path contributions to each measured spectrum by radiative transfer modeling. Here we present a method to parameterize radiative transfer, which allows for a fast conversion of column measurements into volume mixing ratios along the flight track. We will compare parameterized results with vertical profiles we retrieved by inversion techniques and discuss advantages and limitations of our new method. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, November 30, 2015 </font> | ||
<b>Immediate fates of the world’s oldest pesticide | <b>Immediate fates of the world’s oldest pesticide | ||
| Line 277: | Line 4,065: | ||
Elemental sulfur (S0) has been used as an effective pesticide in agricultural systems since ancient Egyptian civilization. Today, in California’s winegrowing regions, applications of S0 dust are used as a preventative against powdery mildew infestation. Throughout the growing season, average applications are 150 kg S ha-1 yr1, and over Napa Valley vineyards alone, the applications total 810 Mg S0. Based on decades of research in northeastern U.S. forests that documented devastating ecosystem consequences of inadvertent reactive S and nitrogen deposition, I was inspired to understand how intensive, widespread, purposeful additions of S0 affect local-to-regional scale soil and water quality in agricultural systems. In Napa Valley vineyards, I have explored (1) What are the immediate fates of S0 locally in soils? and (2) What are the unintended consequences of its intensive, widespread use for downgradient ecosystems? In this talk, I will show the connection between hydrologic controls and the fate of S0 in California vineyards, and discuss how the pattern and consequences of continued applications might change under current drought conditions in the State. Ultimately, decisions about S pesticide management and water use in California’s winegrowing regions have implications for the sustainability of this industry, as well as the function of surrounding terrestrial and aquatic ecosystems. | Elemental sulfur (S0) has been used as an effective pesticide in agricultural systems since ancient Egyptian civilization. Today, in California’s winegrowing regions, applications of S0 dust are used as a preventative against powdery mildew infestation. Throughout the growing season, average applications are 150 kg S ha-1 yr1, and over Napa Valley vineyards alone, the applications total 810 Mg S0. Based on decades of research in northeastern U.S. forests that documented devastating ecosystem consequences of inadvertent reactive S and nitrogen deposition, I was inspired to understand how intensive, widespread, purposeful additions of S0 affect local-to-regional scale soil and water quality in agricultural systems. In Napa Valley vineyards, I have explored (1) What are the immediate fates of S0 locally in soils? and (2) What are the unintended consequences of its intensive, widespread use for downgradient ecosystems? In this talk, I will show the connection between hydrologic controls and the fate of S0 in California vineyards, and discuss how the pattern and consequences of continued applications might change under current drought conditions in the State. Ultimately, decisions about S pesticide management and water use in California’s winegrowing regions have implications for the sustainability of this industry, as well as the function of surrounding terrestrial and aquatic ecosystems. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, November 16, 2015 </font> | ||
<b>Light-absorbing impurities and spectral albedo of the Juneau Icefield</b> | <b>Light-absorbing impurities and spectral albedo of the Juneau Icefield</b> | ||
| Line 300: | Line 4,089: | ||
Mineral dust aerosol is a major contributor to global ice nucleation, although atmospheric processing of mineral dust by sulfuric and nitric acids has been found to alter its ice nucleation ability. Samples of kaolinite and montmorillonite, two aluminosilicate clay minerals, were treated with aqueous sulfuric and nitric acid to simulate atmospheric processing. The samples were studied X-ray diffraction, transmission electron microscopy, and inductively coupled plasma–atomic emission spectroscopy were used to study the structural changes due to acid treatment, which were correlated to observations found in ice nucleation experiments. On the basis of lattice spacing arguments, the observed reduction in ice nucleation activity of acid-treated minerals were correlated to physical and chemical alterations to the mineral structure and the formation aqueous salts on the mineral surface. | Mineral dust aerosol is a major contributor to global ice nucleation, although atmospheric processing of mineral dust by sulfuric and nitric acids has been found to alter its ice nucleation ability. Samples of kaolinite and montmorillonite, two aluminosilicate clay minerals, were treated with aqueous sulfuric and nitric acid to simulate atmospheric processing. The samples were studied X-ray diffraction, transmission electron microscopy, and inductively coupled plasma–atomic emission spectroscopy were used to study the structural changes due to acid treatment, which were correlated to observations found in ice nucleation experiments. On the basis of lattice spacing arguments, the observed reduction in ice nucleation activity of acid-treated minerals were correlated to physical and chemical alterations to the mineral structure and the formation aqueous salts on the mineral surface. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, November 9, 2015 </font> | ||
<b>The Genetics of Terpenoid Production in Cannabis</b> | <b>The Genetics of Terpenoid Production in Cannabis</b> | ||
| Line 313: | Line 4,103: | ||
The Cannabaceae family produces an astonishing diversity of cannabinoids and related terpenes, which are thought to collectively provide the complex flavor and aroma of hops, as well as the psychoactive and medical effects of Cannabis. New research provides important insights into the genetic variation underlying this phytochemical diversity, and sheds light on approaches that can be used to improve breeding efforts to up- or down-regulate the production of these compounds. | The Cannabaceae family produces an astonishing diversity of cannabinoids and related terpenes, which are thought to collectively provide the complex flavor and aroma of hops, as well as the psychoactive and medical effects of Cannabis. New research provides important insights into the genetic variation underlying this phytochemical diversity, and sheds light on approaches that can be used to improve breeding efforts to up- or down-regulate the production of these compounds. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, November 2, 2015 </font> | ||
<b>Diversity of bioaerosols in indoor and outdoor air across the U.S.</b> | <b>Diversity of bioaerosols in indoor and outdoor air across the U.S.</b> | ||
| Line 338: | Line 4,129: | ||
The samples were analysed with a Nanospray Desorption Electrospray Ionization (nano-DESI) high-resolution mass spectrometer in both the positive and negative modes to reveal that the faster growing, and thus larger diameter, branch had a higher oxygen-to-carbon (O:C) ratio when compared with the lower branch, suggesting that the faster growing branch is more oxidized. Furthermore, the most intense peaks from the two branches were compositionally different, but a significant proportion of the formulas identified had N:C ratios of ~0.1, which suggests that organic nitrates are a large component of the aerosol products. | The samples were analysed with a Nanospray Desorption Electrospray Ionization (nano-DESI) high-resolution mass spectrometer in both the positive and negative modes to reveal that the faster growing, and thus larger diameter, branch had a higher oxygen-to-carbon (O:C) ratio when compared with the lower branch, suggesting that the faster growing branch is more oxidized. Furthermore, the most intense peaks from the two branches were compositionally different, but a significant proportion of the formulas identified had N:C ratios of ~0.1, which suggests that organic nitrates are a large component of the aerosol products. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 26, 2015 </font> | ||
<b>High-resolution mass spectrometry: peak fitting and aerosol partitioning</b> | <b>High-resolution mass spectrometry: peak fitting and aerosol partitioning</b> | ||
| Line 362: | Line 4,154: | ||
Real-time determination of chemically-resolved particles size distributions and their evolution in laboratory and ambient studies can be used to investigate changing source contributions, growth rates, secondary aerosol yields, cloud condensation nuclei potential and other physicochemical processes. The Aerodyne Aerosol Mass Spectrometers (AMSs) provides fast size and chemical composition measurements of particles from ~50 nanometers to ~1 micrometer. This work addresses the resolution of the size measurement via particle time-of-flight (PToF) in this instrument. An algebraic model is used to estimate size resolution for different configurations of the instrument as well as particle diameters and vaporization rates. Under typical AMS configurations, for a given particle size, resolution is primarily controlled by the uncertainties in particle time-of-flight due to the finite opening time of the particle gating chopper and particle vaporization. The model was tested with a range of chopper gate widths, particle sizes, and particle compositions, and agreements and disagreements are investigated. The methodology to estimate the size resolution can be applied to all instruments that use particle time-of-flight to infer particle size. Instrument and data acquisition configurations can be optimized for a range of different particle sampling conditions including ambient sampling under low or high loadings, for aircraft platforms, for laboratory applications where loadings may be controllable or narrow particle size ranges are present, and when single-particle chemical or light-scattering detection is possible. | Real-time determination of chemically-resolved particles size distributions and their evolution in laboratory and ambient studies can be used to investigate changing source contributions, growth rates, secondary aerosol yields, cloud condensation nuclei potential and other physicochemical processes. The Aerodyne Aerosol Mass Spectrometers (AMSs) provides fast size and chemical composition measurements of particles from ~50 nanometers to ~1 micrometer. This work addresses the resolution of the size measurement via particle time-of-flight (PToF) in this instrument. An algebraic model is used to estimate size resolution for different configurations of the instrument as well as particle diameters and vaporization rates. Under typical AMS configurations, for a given particle size, resolution is primarily controlled by the uncertainties in particle time-of-flight due to the finite opening time of the particle gating chopper and particle vaporization. The model was tested with a range of chopper gate widths, particle sizes, and particle compositions, and agreements and disagreements are investigated. The methodology to estimate the size resolution can be applied to all instruments that use particle time-of-flight to infer particle size. Instrument and data acquisition configurations can be optimized for a range of different particle sampling conditions including ambient sampling under low or high loadings, for aircraft platforms, for laboratory applications where loadings may be controllable or narrow particle size ranges are present, and when single-particle chemical or light-scattering detection is possible. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 19, 2015 </font> | ||
<b>Portable Air Pollution Monitors and the Global Ozone (GO3) Project</b> | <b>Portable Air Pollution Monitors and the Global Ozone (GO3) Project</b> | ||
| Line 376: | Line 4,169: | ||
The Global Ozone or “GO3” Project was founded as a non-profit organization for educational outreach in 2009. In the GO3 Project, middle and high school students at more than 100 schools around the world measure air pollutants and meteorological parameters outside their schools and upload their data to a public database every 15 minutes. The data may be graphed online and displayed on Google Earth. Students interpret their results and discuss their findings in blogs and forums in a social network similar to Facebook. The GO3 Project includes an online, interactive curriculum where students learn about atmospheric environmental problems and how they are interrelated, including: ground level ozone, stratospheric ozone depletion ("ozone hole"), acid rain and global climate change. The GO3 Project also includes a hands-on Black Carbon Experiment where students measure this important air pollutant by collecting particles on a filter and measuring the optical transmission through the filter using a simple but accurate photometer. In our newest project, GO3 Treks, students hypothesize how air pollutants vary along treks of their own design, and then carry out the treks using pocket-sized ozone and black carbon monitors. Treks are displayed on Google Earth within blogs where students discuss | The Global Ozone or “GO3” Project was founded as a non-profit organization for educational outreach in 2009. In the GO3 Project, middle and high school students at more than 100 schools around the world measure air pollutants and meteorological parameters outside their schools and upload their data to a public database every 15 minutes. The data may be graphed online and displayed on Google Earth. Students interpret their results and discuss their findings in blogs and forums in a social network similar to Facebook. The GO3 Project includes an online, interactive curriculum where students learn about atmospheric environmental problems and how they are interrelated, including: ground level ozone, stratospheric ozone depletion ("ozone hole"), acid rain and global climate change. The GO3 Project also includes a hands-on Black Carbon Experiment where students measure this important air pollutant by collecting particles on a filter and measuring the optical transmission through the filter using a simple but accurate photometer. In our newest project, GO3 Treks, students hypothesize how air pollutants vary along treks of their own design, and then carry out the treks using pocket-sized ozone and black carbon monitors. Treks are displayed on Google Earth within blogs where students discuss | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 12, 2015 </font> | ||
<b>CE-DOAS at its Detection Limit</b> | <b>CE-DOAS at its Detection Limit</b> | ||
| Line 407: | Line 4,201: | ||
Volkamer et al. (2015): Aircraft measurements of BrO, IO, glyoxal, NO2, H2O, O2-O2 and aerosol extinction profiles in the tropics: Comparison with aircraft-/ship-based in situ and lidar measurements, Atmos. Meas. Tech. 8, 2121-2148, 2015. doi:10.5194/amt-8-2121-2015. | Volkamer et al. (2015): Aircraft measurements of BrO, IO, glyoxal, NO2, H2O, O2-O2 and aerosol extinction profiles in the tropics: Comparison with aircraft-/ship-based in situ and lidar measurements, Atmos. Meas. Tech. 8, 2121-2148, 2015. doi:10.5194/amt-8-2121-2015. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 5, 2015 </font> | ||
<b>Building molecular complexity with sunlight at aqueous interfaces</b> | <b>Building molecular complexity with sunlight at aqueous interfaces</b> | ||
| Line 430: | Line 4,225: | ||
Organic nitrogen is a ubiquitous atmospheric component typically accounting for between one-quarter and one-third of reactive nitrogen deposition, however, its chemical complexity and its reactivity has made it challenging to study. Consequently, little is known about the atmospheric processing of organic nitrogen and the resulting implications for biogeochemistry, air quality, and climate. Organic nitrogen can be broadly separated into two groups: oxidized organic nitrogen compounds such as acyl peroxy nitrates and reduced organic nitrogen compounds such as amines. Historically, our knowledge of the chemistry of reduced organic nitrogen has lagged behind that of oxidized organic nitrogen due to the dominance of oxidized nitrogen sources. Recent enactment of air quality regulations in the United States and parts of Europe, however, has resulted in decreased emissions of oxidized nitrogen while emissions of reduced nitrogen have remained constant or have increased due to expansion of agriculture and increased use of fertilizer. Thus, it is timely to study the chemistry of reduced organic nitrogen. I will discuss trends in emissions of reduced nitrogen, its implications for reduced organic nitrogen, and future studies on the chemistry of reduced organic nitrogen. | Organic nitrogen is a ubiquitous atmospheric component typically accounting for between one-quarter and one-third of reactive nitrogen deposition, however, its chemical complexity and its reactivity has made it challenging to study. Consequently, little is known about the atmospheric processing of organic nitrogen and the resulting implications for biogeochemistry, air quality, and climate. Organic nitrogen can be broadly separated into two groups: oxidized organic nitrogen compounds such as acyl peroxy nitrates and reduced organic nitrogen compounds such as amines. Historically, our knowledge of the chemistry of reduced organic nitrogen has lagged behind that of oxidized organic nitrogen due to the dominance of oxidized nitrogen sources. Recent enactment of air quality regulations in the United States and parts of Europe, however, has resulted in decreased emissions of oxidized nitrogen while emissions of reduced nitrogen have remained constant or have increased due to expansion of agriculture and increased use of fertilizer. Thus, it is timely to study the chemistry of reduced organic nitrogen. I will discuss trends in emissions of reduced nitrogen, its implications for reduced organic nitrogen, and future studies on the chemistry of reduced organic nitrogen. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 21, 2015 </font> | ||
<b>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</b> | <b>Chemistry of Organic Compounds in the Atmosphere and Indoor Air</b> | ||
| Line 446: | Line 4,242: | ||
<br>CIRES Fellow, University of Colorado, Boulder | <br>CIRES Fellow, University of Colorado, Boulder | ||
| − | < | + | ==Spring 2015== |
| + | <br /> | ||
| + | <font size="4"> Monday, April 13, 2015 </font> | ||
| − | + | Optical properties of brown carbon aerosol in the near-ultraviolet spectral region</b> | |
| − | < | + | <br>Rebecca Washenfelder |
| − | |||
| − | Rebecca Washenfelder | ||
<br>NOAA Chemical Sciences Division | <br>NOAA Chemical Sciences Division | ||
| Line 463: | Line 4,259: | ||
During the Southern Oxidant and Aerosol Study in summer 2013, we acquired field measurements of aerosol optical extinction at 360-420 nm. We combined these data with direct absorption measurements of water-soluble organic carbon obtained from a UV/VIS-WSOC instrument, and with aerosol composition measurements. I will present the magnitude of brown and black carbon absorption and the relative contributions of biomass burning, anthropogenic, and secondary organic aerosol contributions to brown carbon absorption in the Southeast U.S. during the summer. | During the Southern Oxidant and Aerosol Study in summer 2013, we acquired field measurements of aerosol optical extinction at 360-420 nm. We combined these data with direct absorption measurements of water-soluble organic carbon obtained from a UV/VIS-WSOC instrument, and with aerosol composition measurements. I will present the magnitude of brown and black carbon absorption and the relative contributions of biomass burning, anthropogenic, and secondary organic aerosol contributions to brown carbon absorption in the Southeast U.S. during the summer. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 6, 2015 </font> | ||
| − | <b>Forensics, Metabolomics and Molecular Imaging by Mass Spectrometry</b> | + | <b> Forensics, Metabolomics and Molecular Imaging by Mass Spectrometry</b> |
Facundo M. Fernández, Ph. D. | Facundo M. Fernández, Ph. D. | ||
| Line 476: | Line 4,273: | ||
Mass Spectrometry (MS) is one of the key analytical methods used to identify and characterize small quantities of biological molecules embedded in complex matrices. Although MS has found widespread use, technical improvements in its instrumentation are needed to extend its application to the grand challenges that face the environmental, chemical and biomedical sciences. In this talk I will present insights into new approaches for generating ions under atmospheric pressure in an “open air” format followed by mass spectrometric detection. That research has enabled our group to perform a series of experiments in the fields of forensics, imaging and metabolomics. I will describe how “open air” MS has helped us detect the components in and track the sources of counterfeit drugs in developing countries, perform high throughput metabolic fingerprinting of patients with cancer, and image a variety of surfaces. I will also describe more fundamental work involving finite element simulations and Schlieren imaging of ion transport processes at the atmospheric pressure interface of the mass spectrometer. Finally, I will describe new results where plasma ion sources are used for better coupling of LC to MS. | Mass Spectrometry (MS) is one of the key analytical methods used to identify and characterize small quantities of biological molecules embedded in complex matrices. Although MS has found widespread use, technical improvements in its instrumentation are needed to extend its application to the grand challenges that face the environmental, chemical and biomedical sciences. In this talk I will present insights into new approaches for generating ions under atmospheric pressure in an “open air” format followed by mass spectrometric detection. That research has enabled our group to perform a series of experiments in the fields of forensics, imaging and metabolomics. I will describe how “open air” MS has helped us detect the components in and track the sources of counterfeit drugs in developing countries, perform high throughput metabolic fingerprinting of patients with cancer, and image a variety of surfaces. I will also describe more fundamental work involving finite element simulations and Schlieren imaging of ion transport processes at the atmospheric pressure interface of the mass spectrometer. Finally, I will describe new results where plasma ion sources are used for better coupling of LC to MS. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 30, 2015 </font> | ||
<b>Advances in the Quantitation of Atmospheric Organosulfates</b> | <b>Advances in the Quantitation of Atmospheric Organosulfates</b> | ||
| Line 490: | Line 4,288: | ||
Organosulfates are significant components of secondary organic aerosols (SOA) formed under acidic conditions. This class of compounds contains a characteristic sulfate ester functional group (R-O-SO3-) and is suggested to be a significant contributor to organic carbon in fine particles in the atmosphere. However, the quantification of individual organosulfates molecules has proven challenging due to the lack of authentic quantification standards and suitable methods of analysis. To overcome these challenges, a series of organosulfates standards was synthesized and used to develop and validate of a new analytical method for their quantification. Recent results from the application of this method to ambient aerosol collected in Centreville, Alabama during the Southeast Atmosphere Study (SAS) in 2013 will be discussed. | Organosulfates are significant components of secondary organic aerosols (SOA) formed under acidic conditions. This class of compounds contains a characteristic sulfate ester functional group (R-O-SO3-) and is suggested to be a significant contributor to organic carbon in fine particles in the atmosphere. However, the quantification of individual organosulfates molecules has proven challenging due to the lack of authentic quantification standards and suitable methods of analysis. To overcome these challenges, a series of organosulfates standards was synthesized and used to develop and validate of a new analytical method for their quantification. Recent results from the application of this method to ambient aerosol collected in Centreville, Alabama during the Southeast Atmosphere Study (SAS) in 2013 will be discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 16, 2015 </font> | ||
<b>Tropospheric Reactive Chlorine: Observations, Sources and Effects</b> | <b>Tropospheric Reactive Chlorine: Observations, Sources and Effects</b> | ||
| Line 505: | Line 4,304: | ||
Ambient measurements have detected tropospheric reactive chlorine concentrations much higher than predicted by state-of-the-art air quality models, challenging current understanding of the emissions and atmospheric chemistry of chlorinated compounds. For example, measurements in the Dallas Fort Worth (DFW) area routinely observed HCl concentrations of 1 ppb or more, peaking in the late afternoon. Modeling work has investigated several hypotheses for the source of HCl in DFW, and results suggest emissions of an organic chloride, potentially from hydraulic fracturing activity, as the most likely explanation. In the presence of particulate chloride reactive chlorine can also be formed from heterogeneous reactions on the particles’ surface. Laboratory chamber experiments were conducted to quantify the rate of heterogeneous production of chlorine, and results suggest that this path could explain observed sources of Cl2. Higher concentrations of reactive chlorine can lead to increased production secondary organic aerosol (SOA). Mass yields of SOA formed from chlorine-radical-initiated oxidation of hydrocarbons were measured in laboratory chamber experiments. Volatility basis set parameters from these experiments can be incorporated in air quality models to more accurately represent reactive chlorine concentrations and its effects on atmospheric composition. | Ambient measurements have detected tropospheric reactive chlorine concentrations much higher than predicted by state-of-the-art air quality models, challenging current understanding of the emissions and atmospheric chemistry of chlorinated compounds. For example, measurements in the Dallas Fort Worth (DFW) area routinely observed HCl concentrations of 1 ppb or more, peaking in the late afternoon. Modeling work has investigated several hypotheses for the source of HCl in DFW, and results suggest emissions of an organic chloride, potentially from hydraulic fracturing activity, as the most likely explanation. In the presence of particulate chloride reactive chlorine can also be formed from heterogeneous reactions on the particles’ surface. Laboratory chamber experiments were conducted to quantify the rate of heterogeneous production of chlorine, and results suggest that this path could explain observed sources of Cl2. Higher concentrations of reactive chlorine can lead to increased production secondary organic aerosol (SOA). Mass yields of SOA formed from chlorine-radical-initiated oxidation of hydrocarbons were measured in laboratory chamber experiments. Volatility basis set parameters from these experiments can be incorporated in air quality models to more accurately represent reactive chlorine concentrations and its effects on atmospheric composition. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 9, 2015 </font> | ||
<b>Secondary Organic Aerosol Modeling using the Statistical Oxidation Model</b> | <b>Secondary Organic Aerosol Modeling using the Statistical Oxidation Model</b> | ||
| Line 516: | Line 4,316: | ||
Multi-generational gas-phase oxidation of organic vapors can influence the abundance, composition and properties of organic particulate matter or organic aerosol. Most air quality and climate models lack or include an ad hoc treatment of multi-generational oxidation. In this seminar, I will highlight our recent work where we coupled a semi-explicit multi-generational oxidation model for organics (fully constrained by experimental smog chamber data that includes both functionalization and fragmentation reactions) with a gas-phase chemical mechanism in a regional 3-D air quality model. The seminar will discuss results where we (a) investigated the role of multi-generational gas-phase chemistry on the mass, composition, volatility and oxidation state of SOA and (b) explored the influence of vapor wall-losses on ambient concentrations and properties of SOA. Based on those results, I will argue that 3-D models need to include (this or similar) multi-generational oxidation schemes to accurately describe the atmospheric evolution of OA in air quality and climate models. | Multi-generational gas-phase oxidation of organic vapors can influence the abundance, composition and properties of organic particulate matter or organic aerosol. Most air quality and climate models lack or include an ad hoc treatment of multi-generational oxidation. In this seminar, I will highlight our recent work where we coupled a semi-explicit multi-generational oxidation model for organics (fully constrained by experimental smog chamber data that includes both functionalization and fragmentation reactions) with a gas-phase chemical mechanism in a regional 3-D air quality model. The seminar will discuss results where we (a) investigated the role of multi-generational gas-phase chemistry on the mass, composition, volatility and oxidation state of SOA and (b) explored the influence of vapor wall-losses on ambient concentrations and properties of SOA. Based on those results, I will argue that 3-D models need to include (this or similar) multi-generational oxidation schemes to accurately describe the atmospheric evolution of OA in air quality and climate models. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 2, 2015 </font> | ||
<b>Optical Properties of Titan Haze Analogs Using Photoacoustic and Cavity Ring-Down Spectroscopy</b> | <b>Optical Properties of Titan Haze Analogs Using Photoacoustic and Cavity Ring-Down Spectroscopy</b> | ||
| Line 527: | Line 4,328: | ||
The organic haze that surrounds Saturn's moon Titan is formed through the photolysis and electron initiated dissociation of methane and nitrogen. Both the chemical pathways leading to the haze formation and the resulting haze optical properties are still highly uncertain. Here we examine the optical properties of simulated haze aerosol to better understand its scattering and absorption properties, and the impact of haze on Titan's radiative balance. To determine the complex refractive index of haze particles, we combine two spectroscopic techniques, one that measures absorption and one that measures extinction: photoacoustic spectroscopy coupled with cavity ring-down spectroscopy (PASCaRD). This technique provides the benefit of a high precision determination of the imaginary component of the refractive index (k), along with the highly sensitive determination of the real component of the refractive index (n) in a flow system set up. The Titan aerosol analogs studied are produced by two energy sources, UV excitation and spark discharge excitation. The refractive indices are determined at two wavelengths, 405 and 532 nm, using the PASCaRD system. I will present preliminary data on the complex refractive indices of laboratory generated Titan aerosol analogs at both wavelengths using both energy sources. The high precision values determined from this method should be useful for modelers and for data retrieval from spacecraft and remote sensing instruments. | The organic haze that surrounds Saturn's moon Titan is formed through the photolysis and electron initiated dissociation of methane and nitrogen. Both the chemical pathways leading to the haze formation and the resulting haze optical properties are still highly uncertain. Here we examine the optical properties of simulated haze aerosol to better understand its scattering and absorption properties, and the impact of haze on Titan's radiative balance. To determine the complex refractive index of haze particles, we combine two spectroscopic techniques, one that measures absorption and one that measures extinction: photoacoustic spectroscopy coupled with cavity ring-down spectroscopy (PASCaRD). This technique provides the benefit of a high precision determination of the imaginary component of the refractive index (k), along with the highly sensitive determination of the real component of the refractive index (n) in a flow system set up. The Titan aerosol analogs studied are produced by two energy sources, UV excitation and spark discharge excitation. The refractive indices are determined at two wavelengths, 405 and 532 nm, using the PASCaRD system. I will present preliminary data on the complex refractive indices of laboratory generated Titan aerosol analogs at both wavelengths using both energy sources. The high precision values determined from this method should be useful for modelers and for data retrieval from spacecraft and remote sensing instruments. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 23, 2015 </font> | ||
<b>Chemical Nucleation in the Atmosphere: | <b>Chemical Nucleation in the Atmosphere: | ||
| Line 544: | Line 4,346: | ||
<br>The seminar will describe the bases for these conclusions. | <br>The seminar will describe the bases for these conclusions. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 16, 2015 </font> | ||
<b>Rethinking Secondary Organic Aerosol formation and removal in 3D models based on explicit chemistry</b> | <b>Rethinking Secondary Organic Aerosol formation and removal in 3D models based on explicit chemistry</b> | ||
| Line 552: | Line 4,355: | ||
Current secondary organic aerosol (SOA) parameterizations fail to explain the observed amounts and properties of tropospheric SOA, which results in large uncertainties in their effects on radiation and climate. We use the Generator of Explicit Chemistry and Kinetics of Organics in the Atmosphere (GECKO-A) to simulate SOA gas-phase chemistry in various environments (urban, forest, low/high NOx). Our results show that GECKO-A predicts significant SOA mass growth in urban plumes, and formation of less volatile and more soluble organic products, than predicted by parameterizations typically used in 3D models. The results also indicate that atmospheric processing of gas-phase oxidation intermediates, which are poorly represented in 3D models, can significantly modulate SOA formation and lifetime. We discuss the possible effect of dry deposition and organic photo-fragmentation reactions on condensable organic vapors and SOA. We derive new parameterizations that reproduce GECKO-A behavior in terms of volatility, yields and solubility for use in 3D models. These parameterizations are applied at regional and global scales to re-evaluate SOA concentrations, burden and removal. | Current secondary organic aerosol (SOA) parameterizations fail to explain the observed amounts and properties of tropospheric SOA, which results in large uncertainties in their effects on radiation and climate. We use the Generator of Explicit Chemistry and Kinetics of Organics in the Atmosphere (GECKO-A) to simulate SOA gas-phase chemistry in various environments (urban, forest, low/high NOx). Our results show that GECKO-A predicts significant SOA mass growth in urban plumes, and formation of less volatile and more soluble organic products, than predicted by parameterizations typically used in 3D models. The results also indicate that atmospheric processing of gas-phase oxidation intermediates, which are poorly represented in 3D models, can significantly modulate SOA formation and lifetime. We discuss the possible effect of dry deposition and organic photo-fragmentation reactions on condensable organic vapors and SOA. We derive new parameterizations that reproduce GECKO-A behavior in terms of volatility, yields and solubility for use in 3D models. These parameterizations are applied at regional and global scales to re-evaluate SOA concentrations, burden and removal. | ||
| − | = | + | <br /> |
| + | <font size="4"> Wednesday, February 11, 2015 </font> | ||
<b> Isoprene aerosol yields quantified with space-based relationships between aerosol optical depth and formaldehyde: contrasting regimes in Africa and the southeast US</b> | <b> Isoprene aerosol yields quantified with space-based relationships between aerosol optical depth and formaldehyde: contrasting regimes in Africa and the southeast US</b> | ||
| Line 560: | Line 4,364: | ||
<b>Abstract</b>: Isoprene is an important precursor of organic aerosol (OA), but yields of OA are uncertain. We obtain satellite-derived yields of isoprene OA for Africa and the southeast US by exploiting spatial and temporal consistency between aerosol optical depth (AOD) from MODIS and formaldehyde, ΩHCHO, from OMI. GEOS-Chem chemical transport model yields of isoprene OA using reversible partitioning of isoprene OA are low (<1%) and the resultant AOD is lower than MODIS AOD. GEOS-Chem shows the observed slope from regression of AOD against ΩHCHO is a sensitive indicator of yields of isoprene OA and reproduces observed slopes when partitioning to the aerosol phase is irreversible and regional mean isoprene OA yields are 5.5±1.8% for Africa and 8.6±2.4% for the southeast US. Gas-phase isoprene oxidation is predominantly by NO in the southeast US, and mixed NO and HO2 in Africa. Higher satellite-derived yields under NO dominant conditions is consistent with higher yields observed with chambers operating close to ambient NO2 to NO ratios. We infer lower yields of isoprene OA (3.0%) with boundary-layer southeast US SEAC4RS aircraft observations, as GEOS-Chem calculation of AOD is sensitive to vertical distribution of isoprene OA. Simulated yields of isoprene OA are uniform in GEOS-Chem, but SEAC4RS-derived yields increase to 4.2% at 2-4 km. The AOD we simulate in Africa and the southeast US with our satellite-derived yields implies larger direct radiative forcing from isoprene OA than previous model estimates. | <b>Abstract</b>: Isoprene is an important precursor of organic aerosol (OA), but yields of OA are uncertain. We obtain satellite-derived yields of isoprene OA for Africa and the southeast US by exploiting spatial and temporal consistency between aerosol optical depth (AOD) from MODIS and formaldehyde, ΩHCHO, from OMI. GEOS-Chem chemical transport model yields of isoprene OA using reversible partitioning of isoprene OA are low (<1%) and the resultant AOD is lower than MODIS AOD. GEOS-Chem shows the observed slope from regression of AOD against ΩHCHO is a sensitive indicator of yields of isoprene OA and reproduces observed slopes when partitioning to the aerosol phase is irreversible and regional mean isoprene OA yields are 5.5±1.8% for Africa and 8.6±2.4% for the southeast US. Gas-phase isoprene oxidation is predominantly by NO in the southeast US, and mixed NO and HO2 in Africa. Higher satellite-derived yields under NO dominant conditions is consistent with higher yields observed with chambers operating close to ambient NO2 to NO ratios. We infer lower yields of isoprene OA (3.0%) with boundary-layer southeast US SEAC4RS aircraft observations, as GEOS-Chem calculation of AOD is sensitive to vertical distribution of isoprene OA. Simulated yields of isoprene OA are uniform in GEOS-Chem, but SEAC4RS-derived yields increase to 4.2% at 2-4 km. The AOD we simulate in Africa and the southeast US with our satellite-derived yields implies larger direct radiative forcing from isoprene OA than previous model estimates. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 9, 2015 </font> | ||
<b>Formation of Low Volatility Compounds and SOA from Isoprene | <b>Formation of Low Volatility Compounds and SOA from Isoprene | ||
| Line 574: | Line 4,379: | ||
Many of the sources and formation mechanisms of secondary organic aerosol (SOA) are still poorly understood. In this talk, I will present evidence of gas-phase low volatility organic compounds (LVOC), produced from the OH oxidation of isoprene hydroxy hydroperoxide (ISOPOOH), condensing and forming secondary organic aerosol (SOA) during the Caltech FIXCIT chamber study. Decreases in LVOC concentrations directly corresponded to the appearance and growth in organic aerosol, indicating that LVOC were condensing and forming SOA (at OA levels below 1 μg m-3) with a wall-loss corrected mass yield of roughly 1.5% (from isoprene). This previously uncharacterized formation pathway from isoprene could account for up to 8.0 Tg yr-1 of SOA, or 5% of the estimated global SOA source. The experimental conditions and AMS SOA spectrum indicate that SOA formation in this study is separate and not explained by previously described IEPOX uptake. Condensing species have 4-5 carbons and volatilities consistent with multiple hydroxyl groups, as well as carbonyl and possibly hydroperoxide groups. These species are not extremely low volatility VOCs (ELVOC) as previously observed from monoterpene oxidation by O3, but most likely LVOC with saturation concentrations ranging from 10-2 to 10¬ μg m-3. Their ability to condense at low OA levels at room temperature and to grow nanoparticles indicate that their importance may be largest in environments with lower OA concentrations. The same LVOC compounds were observed in the atmosphere during the SOAS campaign in the SE US. The results of efforts to extract mechanistic information from an ion mobility spectrometer-mass spectrometer (IMS-MS) during the SOAS field study and subsequent laboratory work will also be presented. | Many of the sources and formation mechanisms of secondary organic aerosol (SOA) are still poorly understood. In this talk, I will present evidence of gas-phase low volatility organic compounds (LVOC), produced from the OH oxidation of isoprene hydroxy hydroperoxide (ISOPOOH), condensing and forming secondary organic aerosol (SOA) during the Caltech FIXCIT chamber study. Decreases in LVOC concentrations directly corresponded to the appearance and growth in organic aerosol, indicating that LVOC were condensing and forming SOA (at OA levels below 1 μg m-3) with a wall-loss corrected mass yield of roughly 1.5% (from isoprene). This previously uncharacterized formation pathway from isoprene could account for up to 8.0 Tg yr-1 of SOA, or 5% of the estimated global SOA source. The experimental conditions and AMS SOA spectrum indicate that SOA formation in this study is separate and not explained by previously described IEPOX uptake. Condensing species have 4-5 carbons and volatilities consistent with multiple hydroxyl groups, as well as carbonyl and possibly hydroperoxide groups. These species are not extremely low volatility VOCs (ELVOC) as previously observed from monoterpene oxidation by O3, but most likely LVOC with saturation concentrations ranging from 10-2 to 10¬ μg m-3. Their ability to condense at low OA levels at room temperature and to grow nanoparticles indicate that their importance may be largest in environments with lower OA concentrations. The same LVOC compounds were observed in the atmosphere during the SOAS campaign in the SE US. The results of efforts to extract mechanistic information from an ion mobility spectrometer-mass spectrometer (IMS-MS) during the SOAS field study and subsequent laboratory work will also be presented. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 2, 2015 </font> | ||
<b>Photon and Water Mediated Sulfur Chemistry in Planetary Atmospheres</b> | <b>Photon and Water Mediated Sulfur Chemistry in Planetary Atmospheres</b> | ||
| Line 586: | Line 4,392: | ||
Sulfur compounds have been observed in the atmospheres of a number of planetary bodies in our solar system including Earth and Venus. The global cloud cover on Venus located at an altitude between 50 and 80 kilometers is composed primarily of sulfuric acid (H2SO4) and water. Planetary photochemical models have attempted to explain observations of sulfuric acid and sulfur oxides with significant discrepancies remaining between models and observation. I will report on recent laboratory studies attempting to measure the kinetics of reactions of SO2 and water to form sulfurous acid and progress made in instrument development. | Sulfur compounds have been observed in the atmospheres of a number of planetary bodies in our solar system including Earth and Venus. The global cloud cover on Venus located at an altitude between 50 and 80 kilometers is composed primarily of sulfuric acid (H2SO4) and water. Planetary photochemical models have attempted to explain observations of sulfuric acid and sulfur oxides with significant discrepancies remaining between models and observation. I will report on recent laboratory studies attempting to measure the kinetics of reactions of SO2 and water to form sulfurous acid and progress made in instrument development. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, January 26, 2015 </font> | ||
<b>Weeding Out the Myths: Introduction to Medical Marijuana</b> | <b>Weeding Out the Myths: Introduction to Medical Marijuana</b> | ||
| Line 599: | Line 4,406: | ||
Medical marijuana laws and funded studies in Colorado make it increasingly important to understand the characteristics and appropriate use of medical marijuana. This presentation will use an evidence-based approach to introduce the endocannabinoid system and the pharmacology of marijuana as a potential treatment for various conditions. The proposed mechanisms of action for marijuana provide valuable information about its potential benefits and risks. Additionally, the pharmacokinetics of marijuana, including its absorption, distribution, metabolism, and elimination, will be discussed to help participants determine how various dosage forms may be used. The overall goal is to help participants learn more about how marijuana works and how that relates to the risks and benefits of using medical marijuana. | Medical marijuana laws and funded studies in Colorado make it increasingly important to understand the characteristics and appropriate use of medical marijuana. This presentation will use an evidence-based approach to introduce the endocannabinoid system and the pharmacology of marijuana as a potential treatment for various conditions. The proposed mechanisms of action for marijuana provide valuable information about its potential benefits and risks. Additionally, the pharmacokinetics of marijuana, including its absorption, distribution, metabolism, and elimination, will be discussed to help participants determine how various dosage forms may be used. The overall goal is to help participants learn more about how marijuana works and how that relates to the risks and benefits of using medical marijuana. | ||
| − | == Monday, Nov. 17th, 2014 | + | ==Fall 2014== |
| + | <br /> | ||
| + | <font size="4"> Monday, Nov. 17th, 2014 </font> | ||
<B>Iodine Monoxide observations from CU AMAX-DOAS aboard the | <B>Iodine Monoxide observations from CU AMAX-DOAS aboard the | ||
| Line 612: | Line 4,421: | ||
The CU Airborne Multi-AXis Differential Optical Absorption Spectroscopy (CU AMAX-DOAS) instrument was deployed for the TORERO and CONTRAST field campaigns. These were in the Tropical Eastern Pacific in January-February 2012, and the Tropical Western Pacific in January-February 2014 respectively. We report the first scattered light limb measurements of IO. I will discuss a number of highlights from our observations: 1) instances of enhanced IO mixing ratios in the transition layer 2) a free troposphere background of IO that shows little variation with altitude 3) a hemispheric gradient in total IO column and 4) the first quantification of IO in the lower stratosphere. These measurements provide the best constraints to date on IO outside the planetary boundary layer. They offer the chance to begin assessing our understanding on iodine chemistry in the transition layer, free troposphere, and lower stratosphere and its impacts of climate, particularly tropospheric ozone. | The CU Airborne Multi-AXis Differential Optical Absorption Spectroscopy (CU AMAX-DOAS) instrument was deployed for the TORERO and CONTRAST field campaigns. These were in the Tropical Eastern Pacific in January-February 2012, and the Tropical Western Pacific in January-February 2014 respectively. We report the first scattered light limb measurements of IO. I will discuss a number of highlights from our observations: 1) instances of enhanced IO mixing ratios in the transition layer 2) a free troposphere background of IO that shows little variation with altitude 3) a hemispheric gradient in total IO column and 4) the first quantification of IO in the lower stratosphere. These measurements provide the best constraints to date on IO outside the planetary boundary layer. They offer the chance to begin assessing our understanding on iodine chemistry in the transition layer, free troposphere, and lower stratosphere and its impacts of climate, particularly tropospheric ozone. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Nov. 10th, 2014 </font> | ||
ANALYTICAL & ENVIRONMENTAL CHEMISTRY DIVISION and | ANALYTICAL & ENVIRONMENTAL CHEMISTRY DIVISION and | ||
| Line 646: | Line 4,456: | ||
Oxidation flow reactors (OFRs) using OH produced from low-pressure Hg lamps at 254 nm(OFR254) or both 185 and 254 nm (OFR185) are commonly used in atmospheric chemistry and other fields. OFR254 requires the addition of externally formed O3 since OH is formed from O3 photolysis, while OFR185 does not since O3 is formed in the reactor and OH can also be formed from H2O photolysis. In this study, we use a plug-flow kinetic model to investigate OFR properties under a very wide range of conditions applicable to both field and laboratory studies. We show that the radical chemistry in OFRs can be characterized as a function of UV light intensity, H2O concentration, and total external OH reactivity (e.g., from VOCs, NOx, and SO2). In OFR185, OH exposure is more sensitive to external OH reactivity than in OFR254, because injected O3 in OFR254 promotes the recycling of HO2 to OH, making external perturbations to theradical chemistry less significant. There has been some speculation in the literature about whether “non-tropospheric chemistry” (photolysis at 185 or 254 nm, and/or reactions with O(1D) and O(3P)) may play an important role in these OFRs. For field studies in forested regions or the urban area of Los Angeles, reactants of atmospheric interest are predominantly consumed by OH. Nontropospheric oxidants contribute to the degradation of some species under conditions of low H2O concentration and/or very high external OH reactivity. This appears to have been a problem in some laboratory and source studies, but can be avoided in future studies by experimental planning based on our findings. Some biogenic VOCs can have substantial contributions of reaction with O3 under some operating conditions, especially for OFR254. NO3 may have played an unexpectedly significant role in some past laboratory studies. RO2 fate is similar to that in the atmosphere under low-NOx conditions. A comparison of OFRs with typical environmental chamber studies using UV blacklights and with the atmosphere is presented. OFRs’ key advantages are their short experimental time scales, portability to field sites, enabling a direct connection of field and laboratory studies, and controllable and predictable radical chemistry. This study further establishes the usefulness of these reactors and enables better experiment planning and interpretation. | Oxidation flow reactors (OFRs) using OH produced from low-pressure Hg lamps at 254 nm(OFR254) or both 185 and 254 nm (OFR185) are commonly used in atmospheric chemistry and other fields. OFR254 requires the addition of externally formed O3 since OH is formed from O3 photolysis, while OFR185 does not since O3 is formed in the reactor and OH can also be formed from H2O photolysis. In this study, we use a plug-flow kinetic model to investigate OFR properties under a very wide range of conditions applicable to both field and laboratory studies. We show that the radical chemistry in OFRs can be characterized as a function of UV light intensity, H2O concentration, and total external OH reactivity (e.g., from VOCs, NOx, and SO2). In OFR185, OH exposure is more sensitive to external OH reactivity than in OFR254, because injected O3 in OFR254 promotes the recycling of HO2 to OH, making external perturbations to theradical chemistry less significant. There has been some speculation in the literature about whether “non-tropospheric chemistry” (photolysis at 185 or 254 nm, and/or reactions with O(1D) and O(3P)) may play an important role in these OFRs. For field studies in forested regions or the urban area of Los Angeles, reactants of atmospheric interest are predominantly consumed by OH. Nontropospheric oxidants contribute to the degradation of some species under conditions of low H2O concentration and/or very high external OH reactivity. This appears to have been a problem in some laboratory and source studies, but can be avoided in future studies by experimental planning based on our findings. Some biogenic VOCs can have substantial contributions of reaction with O3 under some operating conditions, especially for OFR254. NO3 may have played an unexpectedly significant role in some past laboratory studies. RO2 fate is similar to that in the atmosphere under low-NOx conditions. A comparison of OFRs with typical environmental chamber studies using UV blacklights and with the atmosphere is presented. OFRs’ key advantages are their short experimental time scales, portability to field sites, enabling a direct connection of field and laboratory studies, and controllable and predictable radical chemistry. This study further establishes the usefulness of these reactors and enables better experiment planning and interpretation. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Nov. 3rd, 2014 </font> | ||
<b>Uncovering the structure and dynamics of a single nanoparticle | <b>Uncovering the structure and dynamics of a single nanoparticle | ||
| Line 657: | Line 4,468: | ||
Modern femtosecond lasers can produce pulses of light that are shorter than the vibrational periods in molecules and generate electric fields stronger than the Coulomb field that binds electrons in atoms. These lasers are ideally suited to studying ultrafast processes in nanomaterials, such as electron transfer in photovoltaic nanostructures. However, all previous laser-nanoparticle experiments have been conducted on nanoparticles suspended in solvent, embedded in a bulk material, or attached to a surface, meaning that powerful gas-phase spectroscopy techniques cannot be used. In 2011 we embarked on a collaboration with the Jimenez group to construct a photoelectron–photoion spectrometer capable of examining isolated nanoparticles in the “gas phase.” To our surprise, the completed spectrometer was capable of recording the complete photoion distribution resulting from the interaction of a single nanoparticle with the femtosecond laser pulse. This breakthrough technique has allowed for the examination of localized light fields in nanostructures, the discovery of shock waves in nanoscale plasmas, and the observation of the evanescent wavefunction in quantum dots. In this talk I describe how we combined the tools of physical chemistry, laser physics, and atmospheric science to construct this new instrument, describe the discoveries to-date, and discuss our plans for future experiments, which include pushing the time-resolution towards the attosecond frontier. | Modern femtosecond lasers can produce pulses of light that are shorter than the vibrational periods in molecules and generate electric fields stronger than the Coulomb field that binds electrons in atoms. These lasers are ideally suited to studying ultrafast processes in nanomaterials, such as electron transfer in photovoltaic nanostructures. However, all previous laser-nanoparticle experiments have been conducted on nanoparticles suspended in solvent, embedded in a bulk material, or attached to a surface, meaning that powerful gas-phase spectroscopy techniques cannot be used. In 2011 we embarked on a collaboration with the Jimenez group to construct a photoelectron–photoion spectrometer capable of examining isolated nanoparticles in the “gas phase.” To our surprise, the completed spectrometer was capable of recording the complete photoion distribution resulting from the interaction of a single nanoparticle with the femtosecond laser pulse. This breakthrough technique has allowed for the examination of localized light fields in nanostructures, the discovery of shock waves in nanoscale plasmas, and the observation of the evanescent wavefunction in quantum dots. In this talk I describe how we combined the tools of physical chemistry, laser physics, and atmospheric science to construct this new instrument, describe the discoveries to-date, and discuss our plans for future experiments, which include pushing the time-resolution towards the attosecond frontier. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 27th, 2014 </font> | ||
<b>The Structure of Atmospheric Particles & Impacts on Atmospheric Chemistry and Climate</b> | <b>The Structure of Atmospheric Particles & Impacts on Atmospheric Chemistry and Climate</b> | ||
| Line 667: | Line 4,479: | ||
The interactions of aerosol particles with light and clouds are determined in part by the structure of atmospheric particles, which is the focus of research in my laboratory. My talk will focus on molecular-level studies of surfaces relevant for cirrus (ice) cloud formation and the phase separation behavior of submicron aerosol particles composed of organic and inorganic components. Through these projects, I will demonstrate the importance of characterizing aerosol structure in determining aerosol physical and chemical properties relevant to atmospheric chemistry and climate. | The interactions of aerosol particles with light and clouds are determined in part by the structure of atmospheric particles, which is the focus of research in my laboratory. My talk will focus on molecular-level studies of surfaces relevant for cirrus (ice) cloud formation and the phase separation behavior of submicron aerosol particles composed of organic and inorganic components. Through these projects, I will demonstrate the importance of characterizing aerosol structure in determining aerosol physical and chemical properties relevant to atmospheric chemistry and climate. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 20th, 2014 </font> | ||
<b>Analysis of arson fire debris by low temperature dynamic headspace adsorption porous layer open tubular columns</b> | <b>Analysis of arson fire debris by low temperature dynamic headspace adsorption porous layer open tubular columns</b> | ||
| Line 686: | Line 4,499: | ||
This talk will explore the research of Dr. Scott Mabury and his work on poly and perfluorinated alkyl substances, or PFAS, in the environment. I will examine the ways that PFAS are transported and transformed in different systems, specifically the atmosphere, soil and water, and biological systems. | This talk will explore the research of Dr. Scott Mabury and his work on poly and perfluorinated alkyl substances, or PFAS, in the environment. I will examine the ways that PFAS are transported and transformed in different systems, specifically the atmosphere, soil and water, and biological systems. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 13th, 2014 </font> | ||
<b>Ozonolysis of a polyunsaturated acid and its primary oxidation products</b> | <b>Ozonolysis of a polyunsaturated acid and its primary oxidation products</b> | ||
| Line 705: | Line 4,519: | ||
Since the mid-1990’s, the Nordstrom lab at the US Geological Survey National Research Program (USGS NRP) has sampled numerous hot springs and rivers in Yellowstone National Park (YNP). The data, collected at least annually, provide a unique record of the inorganic chemistry (including major cations and anions, trace metals, and unusual species such as polythionates) of the geothermal waters at YNP. The data are used by collaborators at various universities to supplement microbiology research and also by USGS researchers investigating the geochemistry of YNP. This talk will summarize some of the current research efforts at YNP, with emphasis on USGS work that has public health and safety implications. | Since the mid-1990’s, the Nordstrom lab at the US Geological Survey National Research Program (USGS NRP) has sampled numerous hot springs and rivers in Yellowstone National Park (YNP). The data, collected at least annually, provide a unique record of the inorganic chemistry (including major cations and anions, trace metals, and unusual species such as polythionates) of the geothermal waters at YNP. The data are used by collaborators at various universities to supplement microbiology research and also by USGS researchers investigating the geochemistry of YNP. This talk will summarize some of the current research efforts at YNP, with emphasis on USGS work that has public health and safety implications. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 6th, 2014 </font> | ||
<b>Nitrogen Oxides and Aerosols (NOaA): Some applications of cavity enhanced | <b>Nitrogen Oxides and Aerosols (NOaA): Some applications of cavity enhanced | ||
| Line 719: | Line 4,534: | ||
Nitrogen oxides (NOx) are emitted from both anthropogenic and natural processes and critically influence the oxidizing capacity of the atmosphere. Aerosols arise from a variety of sources and impact climate through their radiative effects and atmospheric chemistry through heterogeneous and multiphase reactions. Nitrogen oxides in particular undergo distinct heterogeneous reactions that link gas and aerosol phase chemistry. Both nitrogen oxides and aerosols are amenable to measurement by new, high sensitivity optical techniques known as cavity enhanced spectroscopy. I will survey some recent efforts and future projects toward development of this instrumentation, as well as several applications, including nighttime chemistry, wintertime photochemistry and aerosol optical properties. | Nitrogen oxides (NOx) are emitted from both anthropogenic and natural processes and critically influence the oxidizing capacity of the atmosphere. Aerosols arise from a variety of sources and impact climate through their radiative effects and atmospheric chemistry through heterogeneous and multiphase reactions. Nitrogen oxides in particular undergo distinct heterogeneous reactions that link gas and aerosol phase chemistry. Both nitrogen oxides and aerosols are amenable to measurement by new, high sensitivity optical techniques known as cavity enhanced spectroscopy. I will survey some recent efforts and future projects toward development of this instrumentation, as well as several applications, including nighttime chemistry, wintertime photochemistry and aerosol optical properties. | ||
| − | = | + | <br /> |
| − | + | <font size="4"> Monday, September 29, 2014 </font> | |
<b>A new mechanism for solid formation in the atmosphere: contact efflorescence</b> | <b>A new mechanism for solid formation in the atmosphere: contact efflorescence</b> | ||
| Line 742: | Line 4,557: | ||
In this talk I will describe for incoming graduate students the atmospheric chemistry research being conducted in my laboratory. Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to achieve a deep understanding of atmospheric chemistry and to develop detailed and accurate models that are used to establish air quality regulations and to predict the effects of human activities on global climate. Research in my laboratory focuses on the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form microscopic aerosol particles. Studies are conducted in large-volume environmental chambers where experiments are designed to simulate but simplify atmospheric chemistry and conditions in order to obtain information on gas and particle chemical composition; gas and heterogeneous/multiphase reaction rates and equilibria; thermodynamic, hygroscopic, and phase properties of particles; and gas-particle-wall interactions. Obtaining such data is a challenge, but in this talk I will describe how we approach this problem by using a diverse array of measurement techniques including real-time and offline mass spectrometry, temperature-programmed thermal desorption, gas and liquid chromatography, NMR, spectrophotometry, and scanning mobility particle sizing. I will then provide examples of our use of these methods to develop quantitative chemical reaction mechanisms of organic gas and aerosol chemistry and models of SOA formation and particle properties such as hygroscopicity and describe some ongoing research projects. | In this talk I will describe for incoming graduate students the atmospheric chemistry research being conducted in my laboratory. Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to achieve a deep understanding of atmospheric chemistry and to develop detailed and accurate models that are used to establish air quality regulations and to predict the effects of human activities on global climate. Research in my laboratory focuses on the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form microscopic aerosol particles. Studies are conducted in large-volume environmental chambers where experiments are designed to simulate but simplify atmospheric chemistry and conditions in order to obtain information on gas and particle chemical composition; gas and heterogeneous/multiphase reaction rates and equilibria; thermodynamic, hygroscopic, and phase properties of particles; and gas-particle-wall interactions. Obtaining such data is a challenge, but in this talk I will describe how we approach this problem by using a diverse array of measurement techniques including real-time and offline mass spectrometry, temperature-programmed thermal desorption, gas and liquid chromatography, NMR, spectrophotometry, and scanning mobility particle sizing. I will then provide examples of our use of these methods to develop quantitative chemical reaction mechanisms of organic gas and aerosol chemistry and models of SOA formation and particle properties such as hygroscopicity and describe some ongoing research projects. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 22, 2014 </font> | ||
<b>Volatile Organic Compounds in the Atmosphere</b> | <b>Volatile Organic Compounds in the Atmosphere</b> | ||
| Line 764: | Line 4,580: | ||
Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (POA, emitted in the particle phase) and secondary OA (SOA, formed by gas-to-particle conversion). In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students. Ongoing projects include analysis of SOA formation and aging from field studies in urban areas (CalNex-LA), forests (BEACHON-RoMBAS, SOAS, GoAmazon) and over regional scales from aircraft (DC3 and SEAC4RS), including direct measurements of the SOA formation and photochemical evolution from ambient air using an oxidation flow reactor (OFR). We are modeling the oxidation chemistry in the OFR in detail, and under most conditions we can show that non-tropospheric chemical pathways (e.g. reactions with O1D or photolysis at 185 or 254 nm) are negligible. SOA formation in LA is consistent with other urban sites. SOA formation and aging in the OFR is remarkably consistent across sites, showing peak formation at ~2 days of equivalent oxidation and loss of mass at ~2 weeks of oxidation. The detection and aging of SOA formed from isoprene epoxides in the Southeast US with the high-resolution AMS is shown to be robust, resulting in a ~15% contribution to the OA budget in summer and a lifetime of ~2 weeks against gas-phase aging. Gas/particle partitioning is measured directly using a FIGAERO-CIMS, with consistent results with other instruments at the SOAS site. Finally, I will introduce future projects in our group, including an update on the new shared smog chamber facility in the Cristol building (for which testing will start in October), development and application of chemical ionization mass spectrometry to laboratory research on OA gas/particle partitioning and to SOA formation from terpenes and the NO3 radical, and a NASA pole-to-pole aircraft field campaign on aerosol composition. | Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (POA, emitted in the particle phase) and secondary OA (SOA, formed by gas-to-particle conversion). In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students. Ongoing projects include analysis of SOA formation and aging from field studies in urban areas (CalNex-LA), forests (BEACHON-RoMBAS, SOAS, GoAmazon) and over regional scales from aircraft (DC3 and SEAC4RS), including direct measurements of the SOA formation and photochemical evolution from ambient air using an oxidation flow reactor (OFR). We are modeling the oxidation chemistry in the OFR in detail, and under most conditions we can show that non-tropospheric chemical pathways (e.g. reactions with O1D or photolysis at 185 or 254 nm) are negligible. SOA formation in LA is consistent with other urban sites. SOA formation and aging in the OFR is remarkably consistent across sites, showing peak formation at ~2 days of equivalent oxidation and loss of mass at ~2 weeks of oxidation. The detection and aging of SOA formed from isoprene epoxides in the Southeast US with the high-resolution AMS is shown to be robust, resulting in a ~15% contribution to the OA budget in summer and a lifetime of ~2 weeks against gas-phase aging. Gas/particle partitioning is measured directly using a FIGAERO-CIMS, with consistent results with other instruments at the SOAS site. Finally, I will introduce future projects in our group, including an update on the new shared smog chamber facility in the Cristol building (for which testing will start in October), development and application of chemical ionization mass spectrometry to laboratory research on OA gas/particle partitioning and to SOA formation from terpenes and the NO3 radical, and a NASA pole-to-pole aircraft field campaign on aerosol composition. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 15, 2014 </font> | ||
<b>Research Overview of the Joint Center for Artificial Photosynthesis (JCAP): | <b>Research Overview of the Joint Center for Artificial Photosynthesis (JCAP): | ||
| Line 776: | Line 4,593: | ||
JCAP was established in 2010 as one of the DOE’s Energy Innovation Hubs. JCAP’s mission is to build fully integrated solar-fuels generators that utilize earth-abundant semiconductors and catalysts for efficient conversion of water to O2 and H2 and for the reduction of CO2 to liquid fuels. JCAP prototypes are designed to enable separation of products and therefore require membranes and complex interfaces between various material components that will function under realistic operating conditions. JCAP’s long-term goal is to develop a commercially viable, solar-generation technology that simultaneously satisfies the following four criteria: high efficiency, multi-year stability, low module cost, and safe operation. JCAP’s approach to assembling complete artificial photosynthetic systems is to develop robust concepts for complete solar-fuels generators, then to break them down into essential assemblies of components, and finally to adapt or discover the materials needed to fabricate those assemblies. JCAP research bridges basic and applied sciences as well as engineering associated with prototype construction and consideration of scale-up challenges. | JCAP was established in 2010 as one of the DOE’s Energy Innovation Hubs. JCAP’s mission is to build fully integrated solar-fuels generators that utilize earth-abundant semiconductors and catalysts for efficient conversion of water to O2 and H2 and for the reduction of CO2 to liquid fuels. JCAP prototypes are designed to enable separation of products and therefore require membranes and complex interfaces between various material components that will function under realistic operating conditions. JCAP’s long-term goal is to develop a commercially viable, solar-generation technology that simultaneously satisfies the following four criteria: high efficiency, multi-year stability, low module cost, and safe operation. JCAP’s approach to assembling complete artificial photosynthetic systems is to develop robust concepts for complete solar-fuels generators, then to break them down into essential assemblies of components, and finally to adapt or discover the materials needed to fabricate those assemblies. JCAP research bridges basic and applied sciences as well as engineering associated with prototype construction and consideration of scale-up challenges. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 8, 2014 </font> | ||
<b>Reactive trace gases in tropospheric chemistry and climate</b> | <b>Reactive trace gases in tropospheric chemistry and climate</b> | ||
| Line 788: | Line 4,606: | ||
The Volkamer group develops innovative optical spectroscopic instruments (in-situ and remote sensing) to measure trace gases and aerosols, and applies these instruments to conduct field measurements from mobile platforms (vehicles, ships, aircraft) and laboratory measurements in simulation chambers and flowtubes. This talk will present examples of current graduate student projects in the Volkamer group (MAD-CAT, CONTRAST, and FRAPPE projects). Future student opportunities exist in relation with the analysis of data from ongoing field projects, as well as laboratory studies that use in-situ cavity enhanced absorption spectroscopy and mass spectrometry to investigate the role of Setschenow ‘salting-in’ for aerosol formation. Prof. Volkamer is currently on sabbatical in Europe, and is best reached by email. | The Volkamer group develops innovative optical spectroscopic instruments (in-situ and remote sensing) to measure trace gases and aerosols, and applies these instruments to conduct field measurements from mobile platforms (vehicles, ships, aircraft) and laboratory measurements in simulation chambers and flowtubes. This talk will present examples of current graduate student projects in the Volkamer group (MAD-CAT, CONTRAST, and FRAPPE projects). Future student opportunities exist in relation with the analysis of data from ongoing field projects, as well as laboratory studies that use in-situ cavity enhanced absorption spectroscopy and mass spectrometry to investigate the role of Setschenow ‘salting-in’ for aerosol formation. Prof. Volkamer is currently on sabbatical in Europe, and is best reached by email. | ||
| − | = | + | <br /> |
| + | <font size="4"> Tuesday, August 26, 2014 </font> | ||
| − | Explicit Modelling of the Multiphase Oxidation of Organic Compounds | + | '''Explicit Modelling of the Multiphase Oxidation of Organic Compounds''' |
[http://www.lisa.univ-paris12.fr/component/comprofiler/userprofile/aumont Prof. Bernard Aumont], LISA and Univ of Paris XII | [http://www.lisa.univ-paris12.fr/component/comprofiler/userprofile/aumont Prof. Bernard Aumont], LISA and Univ of Paris XII | ||
| Line 799: | Line 4,618: | ||
Recent assessments and applications of the GECKO-A modeling tool will be presented, with a focus given to studies devoted to examine SOA formation and ageing, SOC phase partitioning and multiphase oxidation processes. | Recent assessments and applications of the GECKO-A modeling tool will be presented, with a focus given to studies devoted to examine SOA formation and ageing, SOC phase partitioning and multiphase oxidation processes. | ||
| − | == Monday, May 19, 2014 | + | ==Spring 2014== |
| + | <br /> | ||
| + | <font size="4"> Monday, May 19, 2014 </font> | ||
<b>Surface observations of VOCs over the temperate | <b>Surface observations of VOCs over the temperate | ||
| Line 814: | Line 4,635: | ||
Non-negligible mixing ratios of isoprene, monoterpenes, glyoxal and methyl glyoxal were all observed over the remote ocean indicating an oceanic source of these species. Very high mixing ratios of DMS were observed over phytoplankton blooms during SOAP alongside elevated levels of methanethiol and dimethyl sulfone . The significance of these observations is discussed and observations are compared with those elsewhere in the remote marine boundary layer. | Non-negligible mixing ratios of isoprene, monoterpenes, glyoxal and methyl glyoxal were all observed over the remote ocean indicating an oceanic source of these species. Very high mixing ratios of DMS were observed over phytoplankton blooms during SOAP alongside elevated levels of methanethiol and dimethyl sulfone . The significance of these observations is discussed and observations are compared with those elsewhere in the remote marine boundary layer. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, May 8, 2014 </font> | ||
<b>Development of optical spectroscopic instruments and | <b>Development of optical spectroscopic instruments and | ||
| Line 827: | Line 4,649: | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 28, 2014 </font> | ||
<b>Gas/Particle Partitioning of Organic Acids: instrument development and field deployment</b> | <b>Gas/Particle Partitioning of Organic Acids: instrument development and field deployment</b> | ||
| Line 838: | Line 4,661: | ||
Organic acids are common atmospheric oxidation products and can help our understanding of those mechanisms. In particular their partitioning between the gas and particle phase can lead to a better understanding of their contribution to SOA loadings and lifetime. In this talk I will be presenting the development a new instrument (the FIGAERO-HRToF-CIMS) for real time detection of both gas and particle phase organic acids. Results from lab characterization and a field campaign as part of the Southern Oxidant and Aerosol Study (SOAS) will be shown | Organic acids are common atmospheric oxidation products and can help our understanding of those mechanisms. In particular their partitioning between the gas and particle phase can lead to a better understanding of their contribution to SOA loadings and lifetime. In this talk I will be presenting the development a new instrument (the FIGAERO-HRToF-CIMS) for real time detection of both gas and particle phase organic acids. Results from lab characterization and a field campaign as part of the Southern Oxidant and Aerosol Study (SOAS) will be shown | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 21, 2014 </font> | ||
<b>Can Mass Spectrometry be More Radical?</b> | <b>Can Mass Spectrometry be More Radical?</b> | ||
| Line 850: | Line 4,674: | ||
Radical induced damage to biomolecules is highly relevant to aging, neurodegenerative diseases, and cancers. Fundamental understanding of radical-biomolecule interactions is important for the development of new drugs and therapeutic procedures, but is often missing. We have taken a unique approach to address this issue by studying gas-phase ion/radical reactions. Instrumentation and methods have been developed to enable ion/radical reactions in an electrospray ionization (ESI) plume or inside a linear ion trap mass spectrometer. Accurate mass measurement, stable isotope labeling, tandem mass spectrometry, and theoretical calculations are utilized to provide insight into the reaction mechanisms. | Radical induced damage to biomolecules is highly relevant to aging, neurodegenerative diseases, and cancers. Fundamental understanding of radical-biomolecule interactions is important for the development of new drugs and therapeutic procedures, but is often missing. We have taken a unique approach to address this issue by studying gas-phase ion/radical reactions. Instrumentation and methods have been developed to enable ion/radical reactions in an electrospray ionization (ESI) plume or inside a linear ion trap mass spectrometer. Accurate mass measurement, stable isotope labeling, tandem mass spectrometry, and theoretical calculations are utilized to provide insight into the reaction mechanisms. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 14, 2014 </font> | ||
<b>Chemical Reactions Among Pollutants Indoors – The Human Touch</b> | <b>Chemical Reactions Among Pollutants Indoors – The Human Touch</b> | ||
| Line 861: | Line 4,686: | ||
Regardless of whether a pollutant originates outdoors or indoors, most of our exposure to it occurs indoors. Indoor exposures to ozone and airborne particles of outdoor origin partially explain the mortality ascribed to these pollutants in epidemiological studies. The human body influences indoor concentrations of these and other chemicals in occupied indoor environments. Constituents of human skin oil react with ozone. Lower indoor ozone concentrations lead to less generation of secondary organic aerosols (SOA) from ozone-initiated reactions with terpenes and other unsaturated indoor pollutants. SOA levels influence the levels of co-occurring semivolatile organic compounds (SVOCs). Occupants also inadvertently transfer their skin oils and skin flakes to surfaces, impacting indoor surface chemistry, even when humans are no longer present. In brief, dynamic physical and chemical processes involving occupants and indoor pollutants markedly influence occupant exposures in indoor environments. | Regardless of whether a pollutant originates outdoors or indoors, most of our exposure to it occurs indoors. Indoor exposures to ozone and airborne particles of outdoor origin partially explain the mortality ascribed to these pollutants in epidemiological studies. The human body influences indoor concentrations of these and other chemicals in occupied indoor environments. Constituents of human skin oil react with ozone. Lower indoor ozone concentrations lead to less generation of secondary organic aerosols (SOA) from ozone-initiated reactions with terpenes and other unsaturated indoor pollutants. SOA levels influence the levels of co-occurring semivolatile organic compounds (SVOCs). Occupants also inadvertently transfer their skin oils and skin flakes to surfaces, impacting indoor surface chemistry, even when humans are no longer present. In brief, dynamic physical and chemical processes involving occupants and indoor pollutants markedly influence occupant exposures in indoor environments. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 3, 2014 </font> | ||
<b>Novel phase transitions of optically levitated microdroplets: | <b>Novel phase transitions of optically levitated microdroplets: | ||
| Line 873: | Line 4,699: | ||
The phase state and water content of atmospheric particles influence the particle’s size, optical properties and chemical reactivity with important but poorly constrained effects on air quality and climate. One challenging problem is predicting the ambient conditions required to initiate a phase transformation of a particle from liquid to solid, particularly when consideration is given to multi-particle interactions as well as chemical composition of the liquid droplet. One such example is the phenomenon of contact nucleation. Presented here are the first direct observations of contact nucleation of crystalline ammonium sulfate using a recently developed long-working distance optical trap. Insight is given to the mechanism behind contact nucleation. The effect of amorphous or glassy phase states will also be discussed. | The phase state and water content of atmospheric particles influence the particle’s size, optical properties and chemical reactivity with important but poorly constrained effects on air quality and climate. One challenging problem is predicting the ambient conditions required to initiate a phase transformation of a particle from liquid to solid, particularly when consideration is given to multi-particle interactions as well as chemical composition of the liquid droplet. One such example is the phenomenon of contact nucleation. Presented here are the first direct observations of contact nucleation of crystalline ammonium sulfate using a recently developed long-working distance optical trap. Insight is given to the mechanism behind contact nucleation. The effect of amorphous or glassy phase states will also be discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Feb. 24, 2014 </font> | ||
| − | Chemical and physical processes controlling organic aerosols and tropopause cirrus: An observational perspective | + | '''Chemical and physical processes controlling organic aerosols and tropopause cirrus: An observational perspective''' |
| − | Dr. Andrew Rollins | + | Dr. Andrew Rollins, |
<br>NOAA Chemical Sciences Division | <br>NOAA Chemical Sciences Division | ||
| Line 907: | Line 4,734: | ||
stratosphere. | stratosphere. | ||
| − | = | + | <br /> |
| + | <font size="4"> Thursday, Feb. 20, 2104 </font> | ||
| − | The role of oceanic halogen and sulfur compounds for the middle atmosphere | + | '''The role of oceanic halogen and sulfur compounds for the middle atmosphere''' |
Dr. Susann Tegtmeier | Dr. Susann Tegtmeier | ||
| Line 937: | Line 4,765: | ||
ozone layer for current day conditions and future scenarios. | ozone layer for current day conditions and future scenarios. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 17, 2014 </font> | ||
[http://bertramgroup.ucsd.edu/ Prof. Tim Bertram], UCSD | [http://bertramgroup.ucsd.edu/ Prof. Tim Bertram], UCSD | ||
| Line 959: | Line 4,788: | ||
ocean model. | ocean model. | ||
| − | = | + | <br /> |
| + | <font size="4"> Thursday, Feb. 13, 2014 </font> | ||
<b>Chemical changes to light absorbing carbonaceous aerosol with oxidative aging</b> | <b>Chemical changes to light absorbing carbonaceous aerosol with oxidative aging</b> | ||
| Line 988: | Line 4,818: | ||
aging mechanisms and timescales is needed in order to accurately estimate the climate effects of LAC. | aging mechanisms and timescales is needed in order to accurately estimate the climate effects of LAC. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Feb. 10, 2014 </font> | ||
<b>Mercury cycling in the atmosphere-ocean-land system: Insights from regional and global modeling</b> | <b>Mercury cycling in the atmosphere-ocean-land system: Insights from regional and global modeling</b> | ||
| Line 999: | Line 4,830: | ||
Mercury is a neurotoxin that affects public heath primarily through consumption of marine fish and seafood. Anthropogenic mercury emission sources include coal burning and artisanal gold mining. Mercury undergoes long-range transport in the atmosphere and can cycle multiple times in the atmosphere-land-ocean system before eventually buried in the ocean sediment. To attribute the source of mercury in a certain region thus requires an appreciation of the contribution from legacy and external sources. However, our current understanding of the legacy mercury in the land and ocean subjects to large uncertainties. There are also unresolved questions in the atmospheric chemistry of mercury that affect its long-range transport potential. This talk will highlight recent advances in combining regional/global modeling in the atmosphere/ocean and measurements in different environmental compartments to improve understanding of the mercury cycle. | Mercury is a neurotoxin that affects public heath primarily through consumption of marine fish and seafood. Anthropogenic mercury emission sources include coal burning and artisanal gold mining. Mercury undergoes long-range transport in the atmosphere and can cycle multiple times in the atmosphere-land-ocean system before eventually buried in the ocean sediment. To attribute the source of mercury in a certain region thus requires an appreciation of the contribution from legacy and external sources. However, our current understanding of the legacy mercury in the land and ocean subjects to large uncertainties. There are also unresolved questions in the atmospheric chemistry of mercury that affect its long-range transport potential. This talk will highlight recent advances in combining regional/global modeling in the atmosphere/ocean and measurements in different environmental compartments to improve understanding of the mercury cycle. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 3, 2014 </font> | ||
<b>Tech Transfer in the Life Sciences</b> | <b>Tech Transfer in the Life Sciences</b> | ||
| Line 1,007: | Line 4,839: | ||
Academic research is a creative field in which new ways of thinking often emerge. It is therefore not surprising that many inventions are born out of research. How is it that university inventions can best be transformed into real-world solutions, and what processes have been established to facilitate innovation at CU? Brynmor Rees, a Licensing Manager from CU’s Technology Transfer Office (TTO), will lead a discussion about inventions, intellectual property (including patents), and commercialization at CU. The presentation will include what to do with your invention, information about starting companies, how the patent system works, and real case studies. | Academic research is a creative field in which new ways of thinking often emerge. It is therefore not surprising that many inventions are born out of research. How is it that university inventions can best be transformed into real-world solutions, and what processes have been established to facilitate innovation at CU? Brynmor Rees, a Licensing Manager from CU’s Technology Transfer Office (TTO), will lead a discussion about inventions, intellectual property (including patents), and commercialization at CU. The presentation will include what to do with your invention, information about starting companies, how the patent system works, and real case studies. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, January 13, 2014 </font> | ||
<b>Active Learning: Tips for Success.</b> | <b>Active Learning: Tips for Success.</b> | ||
| Line 1,014: | Line 4,847: | ||
Much evidence has been collected over the past 10 years supporting the efficacy of active learning in college science classrooms. However, many instructors who are interested in change still find it challenging to implement successfully. I will present reasons why it's worth trying active learning, and suggest some approaches and techniques that can be easily tried in any class. | Much evidence has been collected over the past 10 years supporting the efficacy of active learning in college science classrooms. However, many instructors who are interested in change still find it challenging to implement successfully. I will present reasons why it's worth trying active learning, and suggest some approaches and techniques that can be easily tried in any class. | ||
| − | == Monday, December 2, 2013 | + | ==Fall 2013== |
| + | <br /> | ||
| + | <font size="4"> Monday, December 2, 2013 </font> | ||
Fernando Rosario-Ortiz, | Fernando Rosario-Ortiz, | ||
| Line 1,027: | Line 4,862: | ||
The study of the physicochemical properties of organic matter has been of interest to researchers in the areas of chemistry and engineering for many decades. Organic matter influences many different processes in the natural and engineered environment, including fate and transport of organic contaminants and the formation of disinfection byproducts. Over the past decade, emphasis has been placed on the study of the physicochemical properties of effluent organic matter (EfOM), which represents the organic matter found in wastewater effluents. The presence of EfOM in wastewater effluents impacts not only the efficacy of engineered processes towards the removal of organic contaminants, but also the natural fate of these compounds in receiving streams. In this presentation, the photochemical formation of reactive oxygen species (ROS) from EfOM will be discussed. The ROS of interest include singlet oxygen (1O2) and hydroxyl radical (HO). The overall quantum yields for the formation of these species as a function of EfOM composition will be discussed, in addition to additional details with regards the photophysical processes responsible for the formation of these species in the complex mixture that is EfOM. | The study of the physicochemical properties of organic matter has been of interest to researchers in the areas of chemistry and engineering for many decades. Organic matter influences many different processes in the natural and engineered environment, including fate and transport of organic contaminants and the formation of disinfection byproducts. Over the past decade, emphasis has been placed on the study of the physicochemical properties of effluent organic matter (EfOM), which represents the organic matter found in wastewater effluents. The presence of EfOM in wastewater effluents impacts not only the efficacy of engineered processes towards the removal of organic contaminants, but also the natural fate of these compounds in receiving streams. In this presentation, the photochemical formation of reactive oxygen species (ROS) from EfOM will be discussed. The ROS of interest include singlet oxygen (1O2) and hydroxyl radical (HO). The overall quantum yields for the formation of these species as a function of EfOM composition will be discussed, in addition to additional details with regards the photophysical processes responsible for the formation of these species in the complex mixture that is EfOM. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, November 18, 2013 </font> | ||
Roger Atkinson | Roger Atkinson | ||
| Line 1,043: | Line 4,879: | ||
Unsaturated 1,4-dicarbonyls are important products of the atmospheric degradations of aromatic hydrocarbons such as benzene, toluene, xylenes and trimethylbenzenes, which comprise ∼20% of the non-methane volatile organic compounds present in urban air masses in the USA. However, in many cases the measured formation yields of the unsaturated 1,4-dicarbonyls are significantly lower than those of the presumed co-product 1,2-dicarbonyls. These discrepancies could be due to analytical problems and/or rapid photolysis of unsaturated 1,4-keto-aldehydes and unsaturated 1,4-dialdehydes, or to incorrect reaction mechanisms for the OH radical-initiated reactions of aromatic hydrocarbons. Since unsaturated 1,4-dicarbonyls are major products of OH + furans, with apparently simpler product distributions and mechanisms, we have investigated the reactions of OH radicals with furan, 2- and 3-methylfuran, and 2,3- and 2,5-dimethylfuran, in the presence of NO. Using direct air sampling atmospheric pressure ionization tandem mass spectrometry and gas chromatography, the unsaturated 1,4-dicarbonyls were observed and quantified. The measured unsaturated 1,4-dicarbonyl formation yields ranged from 8 ± 2% from OH + 2,3-dimethylfuran to 75 ± 5% from OH + furan. Other products were also formed. These data will be presented and discussed and, time permitting, a brief discussion of in situ nitro-aromatic and nitro-PAH formation from the atmospheric degradations of aromatic hydrocarbons and PAHs will also be presented. | Unsaturated 1,4-dicarbonyls are important products of the atmospheric degradations of aromatic hydrocarbons such as benzene, toluene, xylenes and trimethylbenzenes, which comprise ∼20% of the non-methane volatile organic compounds present in urban air masses in the USA. However, in many cases the measured formation yields of the unsaturated 1,4-dicarbonyls are significantly lower than those of the presumed co-product 1,2-dicarbonyls. These discrepancies could be due to analytical problems and/or rapid photolysis of unsaturated 1,4-keto-aldehydes and unsaturated 1,4-dialdehydes, or to incorrect reaction mechanisms for the OH radical-initiated reactions of aromatic hydrocarbons. Since unsaturated 1,4-dicarbonyls are major products of OH + furans, with apparently simpler product distributions and mechanisms, we have investigated the reactions of OH radicals with furan, 2- and 3-methylfuran, and 2,3- and 2,5-dimethylfuran, in the presence of NO. Using direct air sampling atmospheric pressure ionization tandem mass spectrometry and gas chromatography, the unsaturated 1,4-dicarbonyls were observed and quantified. The measured unsaturated 1,4-dicarbonyl formation yields ranged from 8 ± 2% from OH + 2,3-dimethylfuran to 75 ± 5% from OH + furan. Other products were also formed. These data will be presented and discussed and, time permitting, a brief discussion of in situ nitro-aromatic and nitro-PAH formation from the atmospheric degradations of aromatic hydrocarbons and PAHs will also be presented. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, November 11, 2013 </font> | ||
Shuichi Ushijima, | Shuichi Ushijima, | ||
| Line 1,067: | Line 4,904: | ||
Carbonyls are common intermediates in the overall oxidation of organic volatile compounds (VOCs), a chemical process that can influence tropospheric ozone production, aerosol formation, and climate change. Understanding carbonyl chemistry is imperative to fully understand and model such processes. To further the current knowledge of carbonyl chemistry, which is lacking for larger branched species, the temperature dependence of the gas-phase reaction of three ketones, 3-pentanone (DEK), 3-methyl 2-butanone (MIPK), and 2,4-dimethyl 3-pentanone (DIPK), with Cl atoms were investigated using an atmospheric smog chamber equipped with FT-IR, PTR-MS, and GC-FID. The ketones’ Arrhenius expressions were found to be: kDEK = 4 ±11 x 10-11 cm3 molecules-1 s-1 e(2 ± 7 kJ/mol)/RT, kMIPK = 7 ±23 x 10-11 cm3 molecules-1 s-1 e(0.2± 8 kJ/mol)/RT, kDIPK = 3 ±110 x 10-10 cm3 molecules-1 s-1 e(-3± 8 kJ/mol)/RT. Within the uncertainties the rate coefficients exhibit no temperature dependence. Product yield analysis of DIPK and MIPK consistently displayed a tertiary α-carbon: primary β-carbon (relative to carbonyl) branching ratio of approximately 70% : 30% for H-atom abstraction by Cl atoms over the investigated temperature range. This indicates a temperature-independent mechanism, consistent with the kinetic measurements. | Carbonyls are common intermediates in the overall oxidation of organic volatile compounds (VOCs), a chemical process that can influence tropospheric ozone production, aerosol formation, and climate change. Understanding carbonyl chemistry is imperative to fully understand and model such processes. To further the current knowledge of carbonyl chemistry, which is lacking for larger branched species, the temperature dependence of the gas-phase reaction of three ketones, 3-pentanone (DEK), 3-methyl 2-butanone (MIPK), and 2,4-dimethyl 3-pentanone (DIPK), with Cl atoms were investigated using an atmospheric smog chamber equipped with FT-IR, PTR-MS, and GC-FID. The ketones’ Arrhenius expressions were found to be: kDEK = 4 ±11 x 10-11 cm3 molecules-1 s-1 e(2 ± 7 kJ/mol)/RT, kMIPK = 7 ±23 x 10-11 cm3 molecules-1 s-1 e(0.2± 8 kJ/mol)/RT, kDIPK = 3 ±110 x 10-10 cm3 molecules-1 s-1 e(-3± 8 kJ/mol)/RT. Within the uncertainties the rate coefficients exhibit no temperature dependence. Product yield analysis of DIPK and MIPK consistently displayed a tertiary α-carbon: primary β-carbon (relative to carbonyl) branching ratio of approximately 70% : 30% for H-atom abstraction by Cl atoms over the investigated temperature range. This indicates a temperature-independent mechanism, consistent with the kinetic measurements. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, November 4, 2013 </font> | ||
Amanda McLaughlin, | Amanda McLaughlin, | ||
| Line 1,096: | Line 4,934: | ||
Metal organic frameworks (MOFs) show potential for use in gas sensing and sequestration, with applications in industrial, defense, and biological settings, as well as environmental monitoring. Different gases can be adsorbed into these crystalline networks of organic ligands and metal ions or clusters by changing their sub-nanometer sized pores. An important practical consideration when developing MOFs for gas sensing is the requirement that they operate effectively under humid conditions. Layers of two MOFs, HKUST-1 (Cu3BTC2(H2O)n] and ZIF-8 (2-methylimidazole zinc salt), were formed on silver nanoparticles and the extent to which they adsorbed sample gases and water vapor was determined using localized surface plasmon resonance spectroscopy. | Metal organic frameworks (MOFs) show potential for use in gas sensing and sequestration, with applications in industrial, defense, and biological settings, as well as environmental monitoring. Different gases can be adsorbed into these crystalline networks of organic ligands and metal ions or clusters by changing their sub-nanometer sized pores. An important practical consideration when developing MOFs for gas sensing is the requirement that they operate effectively under humid conditions. Layers of two MOFs, HKUST-1 (Cu3BTC2(H2O)n] and ZIF-8 (2-methylimidazole zinc salt), were formed on silver nanoparticles and the extent to which they adsorbed sample gases and water vapor was determined using localized surface plasmon resonance spectroscopy. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 28, 2013 </font> | ||
Prof. Jose-Luis Jimenez | Prof. Jose-Luis Jimenez | ||
| Line 1,108: | Line 4,947: | ||
In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group since Fall 2008, as well as of upcoming projects of interest to 1st year students. Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (POA, emitted in the particle phase) and secondary OA (SOA, formed by gas-to-particle conversion). Together with others in the community and contrary to the understanding at the time, we demonstrated in the mid-2000s that SOA dominates over POA at most locations. This paradigm shift has led to intense research on the sources, processing, properties, and fate of SOA. Because pre-existing and commercial instruments were very limited for the analysis of the complex mixtures of highly oxidized species comprising real SOA, we have developed or co-developed several experimental techniques aimed at extracting more information out of ambient and laboratory air, and pioneered their application in field experiments. These include measurements of eddy covariance fluxes, metals, and fast (millisecond) processes with the Aerodyne Aerosol Mass Spectrometer (AMS), development and application of soft-ionization techniques, measurements of the volatility and gas/particle partitioning of organic species, and measurement of the SOA formation potential of ambient air in real-time. We have also pioneered many data analysis techniques for aerosol mass spectrometry data, including 2 and 3-dimensional factor analysis, techniques for constrained high-resolution ion fitting, and elemental analysis of bulk organics with detection limits 6 orders-of-magnitude better than commercial techniques. We have applied these techniques to characterize POA sources (such as biomass burning, motor vehicles, and cooking) and to analyze ambient air at ground sites and from several aircraft in major field studies such as MILAGRO, SOAR, AMAZE, ARCTAS, DAURE, FLAME-3, CalNex, BEACHON-RoMBAS, DC3, SEAC4RS, and SOAS. We have proposed a new paradigm (Jimenez et al., Science, 2009) that is consistent with worldwide measurements and in which OA and OA precursor gases evolve continuously by becoming increasingly oxidized, less volatile, and more hygroscopic, leading to the formation of oxygenated organic aerosol (OOA), with concentrations comparable to those of sulfate aerosol throughout the Northern Hemisphere. We have used computer box modeling and collaborated closely with several regional and global modelers for deeper analysis and interpretation of field study data. Upcoming projects include development of hyphenated soft-ionization techniques, laboratory research on SOA formation from the NO3 radical and of indoor air chemistry, and a field study in the Amazon. | In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group since Fall 2008, as well as of upcoming projects of interest to 1st year students. Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (POA, emitted in the particle phase) and secondary OA (SOA, formed by gas-to-particle conversion). Together with others in the community and contrary to the understanding at the time, we demonstrated in the mid-2000s that SOA dominates over POA at most locations. This paradigm shift has led to intense research on the sources, processing, properties, and fate of SOA. Because pre-existing and commercial instruments were very limited for the analysis of the complex mixtures of highly oxidized species comprising real SOA, we have developed or co-developed several experimental techniques aimed at extracting more information out of ambient and laboratory air, and pioneered their application in field experiments. These include measurements of eddy covariance fluxes, metals, and fast (millisecond) processes with the Aerodyne Aerosol Mass Spectrometer (AMS), development and application of soft-ionization techniques, measurements of the volatility and gas/particle partitioning of organic species, and measurement of the SOA formation potential of ambient air in real-time. We have also pioneered many data analysis techniques for aerosol mass spectrometry data, including 2 and 3-dimensional factor analysis, techniques for constrained high-resolution ion fitting, and elemental analysis of bulk organics with detection limits 6 orders-of-magnitude better than commercial techniques. We have applied these techniques to characterize POA sources (such as biomass burning, motor vehicles, and cooking) and to analyze ambient air at ground sites and from several aircraft in major field studies such as MILAGRO, SOAR, AMAZE, ARCTAS, DAURE, FLAME-3, CalNex, BEACHON-RoMBAS, DC3, SEAC4RS, and SOAS. We have proposed a new paradigm (Jimenez et al., Science, 2009) that is consistent with worldwide measurements and in which OA and OA precursor gases evolve continuously by becoming increasingly oxidized, less volatile, and more hygroscopic, leading to the formation of oxygenated organic aerosol (OOA), with concentrations comparable to those of sulfate aerosol throughout the Northern Hemisphere. We have used computer box modeling and collaborated closely with several regional and global modelers for deeper analysis and interpretation of field study data. Upcoming projects include development of hyphenated soft-ionization techniques, laboratory research on SOA formation from the NO3 radical and of indoor air chemistry, and a field study in the Amazon. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 21, 2013 </font> | ||
Demetrios Pagonis, First Year Graduate Student, | Demetrios Pagonis, First Year Graduate Student, | ||
| Line 1,137: | Line 4,977: | ||
The GC-MS measured a large number of primary and secondary anthropogenic and biogenic VOCs in the C2 to C11 range, with a time resolution of 30 minutes. Measured compounds of particular interest include isoprene, speciated monoterpenes, methylvinylketone (MVK), methacrolein, C2 to C11 alkanes, lightweight unsaturated hydrocarbons including ethene, propene, and acetylene, C6 to C9 aromatics, C1 to C7 oxygenated VOCS (alcohols, ketones, aldehydes), halogenated VOCs, acetonitrile, and several sulfur-containing compounds. A summary of these measurements will be presented. This summary will include characterization of various anthropogenic and biogenic sources sampled at the site, relationships of the most important VOCs to basic meteorological conditions, and diurnal profiles that illustrate shifts in photochemistry and emissions. These GCMS measurements will provide key information for constraints in models and to aid in the interpretation of data from other instruments. | The GC-MS measured a large number of primary and secondary anthropogenic and biogenic VOCs in the C2 to C11 range, with a time resolution of 30 minutes. Measured compounds of particular interest include isoprene, speciated monoterpenes, methylvinylketone (MVK), methacrolein, C2 to C11 alkanes, lightweight unsaturated hydrocarbons including ethene, propene, and acetylene, C6 to C9 aromatics, C1 to C7 oxygenated VOCS (alcohols, ketones, aldehydes), halogenated VOCs, acetonitrile, and several sulfur-containing compounds. A summary of these measurements will be presented. This summary will include characterization of various anthropogenic and biogenic sources sampled at the site, relationships of the most important VOCs to basic meteorological conditions, and diurnal profiles that illustrate shifts in photochemistry and emissions. These GCMS measurements will provide key information for constraints in models and to aid in the interpretation of data from other instruments. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 14, 2013 </font> | ||
Joost de Gouw, Cooperative Institute for Research in Environmental Sciences, University of Colorado at Boulder | Joost de Gouw, Cooperative Institute for Research in Environmental Sciences, University of Colorado at Boulder | ||
| Line 1,150: | Line 4,991: | ||
Atmospheric emissions associated with the use of energy affect the chemical composition of the atmosphere, and have led to such diverse societal issues as air quality degradation and global climate change. Over the pas decade, resource limitations and concerns over energy security and the environment have led to significant changes in the use of energy in the U.S. The domestic production of oil and natural gas has strongly increased as a result of advances in directional drilling and hydraulic fracturing. Also, ethanol made from corn now constitutes approximately 10% of the volume of gasoline sales in the U.S. In this presentation, we will look at some of the atmospheric implications of these changing energy sources and how they are affecting atmospheric chemistry, air quality and climate change. | Atmospheric emissions associated with the use of energy affect the chemical composition of the atmosphere, and have led to such diverse societal issues as air quality degradation and global climate change. Over the pas decade, resource limitations and concerns over energy security and the environment have led to significant changes in the use of energy in the U.S. The domestic production of oil and natural gas has strongly increased as a result of advances in directional drilling and hydraulic fracturing. Also, ethanol made from corn now constitutes approximately 10% of the volume of gasoline sales in the U.S. In this presentation, we will look at some of the atmospheric implications of these changing energy sources and how they are affecting atmospheric chemistry, air quality and climate change. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 7, 2013 </font> | ||
Daniel James Cziczo, | Daniel James Cziczo, | ||
| Line 1,163: | Line 5,005: | ||
The formation of clouds is dependent on the availability of aerosols to initiate the condensation of water vapor. In the case of cirrus clouds, ice nucleation can occur homogeneously at low temperature and high saturation on the vast majority of particles or heterogeneously at high temperature and low saturation on the small fraction of atmospheric aerosols that are efficient ice nuclei (IN). The critical properties that make for a good IN are slowly being established. This work focuses on those particles which have been shown to be important IN through in situ studies: mineral dust and metallic particles are the dominant source of residual particles, whereas sulfate/organic particles are underrepresented and elemental carbon and biological material are essentially absent. We are now undertaking ice nucleation studies using the Droplet Measurement Technologies SPectrometer for Ice Nuclei (SPIN) on mineral dust and metallic aerosol and report ice nucleation conditions. A comparison to the types that are not observed in cirrus and to the onset of homogeneous freezing is made. | The formation of clouds is dependent on the availability of aerosols to initiate the condensation of water vapor. In the case of cirrus clouds, ice nucleation can occur homogeneously at low temperature and high saturation on the vast majority of particles or heterogeneously at high temperature and low saturation on the small fraction of atmospheric aerosols that are efficient ice nuclei (IN). The critical properties that make for a good IN are slowly being established. This work focuses on those particles which have been shown to be important IN through in situ studies: mineral dust and metallic particles are the dominant source of residual particles, whereas sulfate/organic particles are underrepresented and elemental carbon and biological material are essentially absent. We are now undertaking ice nucleation studies using the Droplet Measurement Technologies SPectrometer for Ice Nuclei (SPIN) on mineral dust and metallic aerosol and report ice nucleation conditions. A comparison to the types that are not observed in cirrus and to the onset of homogeneous freezing is made. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 30, 2013 </font> | ||
Rainer Volkamer, | Rainer Volkamer, | ||
| Line 1,175: | Line 5,018: | ||
Discoveries about chemistry in the lower atmosphere are often driven by advances with analytical techniques to measure atmospheric composition. Oceans cover 70% of the Earth surface, yet the remote marine atmosphere remains one of the most poorly probed atmospheric environments on Earth. Ocean emissions of organic carbon molecules (gas- and aerosol phase) and halogens are relevant to climate discussions, because they modify oxidative capacity, i.e., the rate at which climate active gases such as ozone, and methane are removed from the atmosphere. They can further modify aerosols that influence Earth albedo. I will present optical spectroscopic instruments that my group has designed, assembled, and deployed over the past 6 years in laboratory studies of aerosol formation, and field measurements in the tropical atmosphere. These innovative instruments enable measurements of halogen oxide radicals (bromine and iodine oxide), and oxygenated small molecules, and allow us to study their chemical formation pathways, multiphase chemistry, distributions and relevance in the tropical atmosphere. We find that novel chemistry is operative at atmospheric interfaces (ocean surface, and aerosols). Marine organic carbon gases and halogens are particularly relevant in the tropical free troposphere, where most of the tropospheric ozone mass resides, 75% of the global methane destruction occurs, and mercury oxidation rates are accelerated at low temperatures. | Discoveries about chemistry in the lower atmosphere are often driven by advances with analytical techniques to measure atmospheric composition. Oceans cover 70% of the Earth surface, yet the remote marine atmosphere remains one of the most poorly probed atmospheric environments on Earth. Ocean emissions of organic carbon molecules (gas- and aerosol phase) and halogens are relevant to climate discussions, because they modify oxidative capacity, i.e., the rate at which climate active gases such as ozone, and methane are removed from the atmosphere. They can further modify aerosols that influence Earth albedo. I will present optical spectroscopic instruments that my group has designed, assembled, and deployed over the past 6 years in laboratory studies of aerosol formation, and field measurements in the tropical atmosphere. These innovative instruments enable measurements of halogen oxide radicals (bromine and iodine oxide), and oxygenated small molecules, and allow us to study their chemical formation pathways, multiphase chemistry, distributions and relevance in the tropical atmosphere. We find that novel chemistry is operative at atmospheric interfaces (ocean surface, and aerosols). Marine organic carbon gases and halogens are particularly relevant in the tropical free troposphere, where most of the tropospheric ozone mass resides, 75% of the global methane destruction occurs, and mercury oxidation rates are accelerated at low temperatures. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 23, 2013 </font> | ||
Paul Ziemann | Paul Ziemann | ||
| Line 1,198: | Line 5,042: | ||
Knowledge of aerosol particle composition is crucial in order to gain an understanding of health and environmental effects of atmospheric particulate matter, and requires measurements that can provide detailed information about chemical components. An Aerosol Time-of-Flight Mass Spectrometer (ATOFMS) is used for the semi-quantitative analysis of the composition of individual atmospheric particles upon laser-induced desorption and ionization. Integration of the spectroscopic technique of Laser-Induced Breakdown Spectroscopy (LIBS), in which light emitted by the elemental components of the particle upon irradiation with a laser is collected, should increase the quantitative data provided by this instrument. Due to the similarities between both techniques, integration of the two should be straightforward and requires only minor additions to the ATOFMS. This presentation will focus on the advantage of coupling these two techniques as well as the inherent physical and electronic challenges, including: plasma generation (excitation laser pulse energy), plasma dynamics in an electric field and vacuum conditions, emission detection (collection angle and distance from the light source), and software program design and implementation. These challenges continue to be systematically addressed in Dr. Deborah Gross’s Lab at Carleton College and are presented here as evidence of the range of issues that arise in producing a new single-particle analysis instrument. | Knowledge of aerosol particle composition is crucial in order to gain an understanding of health and environmental effects of atmospheric particulate matter, and requires measurements that can provide detailed information about chemical components. An Aerosol Time-of-Flight Mass Spectrometer (ATOFMS) is used for the semi-quantitative analysis of the composition of individual atmospheric particles upon laser-induced desorption and ionization. Integration of the spectroscopic technique of Laser-Induced Breakdown Spectroscopy (LIBS), in which light emitted by the elemental components of the particle upon irradiation with a laser is collected, should increase the quantitative data provided by this instrument. Due to the similarities between both techniques, integration of the two should be straightforward and requires only minor additions to the ATOFMS. This presentation will focus on the advantage of coupling these two techniques as well as the inherent physical and electronic challenges, including: plasma generation (excitation laser pulse energy), plasma dynamics in an electric field and vacuum conditions, emission detection (collection angle and distance from the light source), and software program design and implementation. These challenges continue to be systematically addressed in Dr. Deborah Gross’s Lab at Carleton College and are presented here as evidence of the range of issues that arise in producing a new single-particle analysis instrument. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 16, 2013 </font> | ||
Margaret Tolbert | Margaret Tolbert | ||
| Line 1,216: | Line 5,061: | ||
Scientists from the University of Colorado and Aktiv-Dry LLC have formulated dry powder vaccines effective against measles. We have developed and patented a system known as CO2-Assisted Nebulization with a Bubble Dryer® (CAN-BD) that stabilizes, micronizes, and dries these and various other biologicals and pharmaceuticals with great success. Based on our success with a variety of biopharmaceuticals, we have demonstrated that Aktiv-Dry’s CAN-BD system can be used to manufacture stable and active dry powders for inhaled vaccines and antibiotics. These dry inhalable powders can be administered through multi-dose or single-dose inhalers (such as Aktiv-Dry’s PuffHaler® or the BD Technologies’ Solovent) and are easy to handle and administer in situations in resource-poor countries. The measles live-attenuated, inhalable dry powder aerosolizable vaccine has been tested in Phase I clinical trials recently successfully completed in Pune, India; the 60 subjects were all dosed by inhaling a single breath of dry powder aerosol beginning in March 2012, and no serious adverse events have been reported to date. No serious adverse events were observed in the studies of the inhaled powder vaccine in macaques or in 60 human volunteers in a Phase I clinical trial. | Scientists from the University of Colorado and Aktiv-Dry LLC have formulated dry powder vaccines effective against measles. We have developed and patented a system known as CO2-Assisted Nebulization with a Bubble Dryer® (CAN-BD) that stabilizes, micronizes, and dries these and various other biologicals and pharmaceuticals with great success. Based on our success with a variety of biopharmaceuticals, we have demonstrated that Aktiv-Dry’s CAN-BD system can be used to manufacture stable and active dry powders for inhaled vaccines and antibiotics. These dry inhalable powders can be administered through multi-dose or single-dose inhalers (such as Aktiv-Dry’s PuffHaler® or the BD Technologies’ Solovent) and are easy to handle and administer in situations in resource-poor countries. The measles live-attenuated, inhalable dry powder aerosolizable vaccine has been tested in Phase I clinical trials recently successfully completed in Pune, India; the 60 subjects were all dosed by inhaling a single breath of dry powder aerosol beginning in March 2012, and no serious adverse events have been reported to date. No serious adverse events were observed in the studies of the inhaled powder vaccine in macaques or in 60 human volunteers in a Phase I clinical trial. | ||
| − | = | + | <br /> |
| + | <font size="4"> Wednesday, September 4, 2013 </font> | ||
'''Characterizing the influence of multi-generational chemical processing on organic particles''' | '''Characterizing the influence of multi-generational chemical processing on organic particles''' | ||
| Line 1,229: | Line 5,075: | ||
Reactions of organic compounds in the atmosphere with common oxidants (e.g. the hydroxyl radical and ozone) can lead to the formation of secondary organic aerosol (SOA) and can engender transformations within the particle-phase through heterogeneous reactions. The resulting specific chemical composition of the particles can influence their ability to take up water, activate into cloud droplets and absorb and scatter sunlight. Most organic compounds emitted into the atmosphere that are capable of forming SOA are subject to multi-generational oxidation, which simply means that they can react more than once, with each reaction having some probability of functionalizing or fragmenting the parent species. In this seminar, two aspects of this multi-generational ageing will be discussed: SOA models and SOA optical properties. Many models of SOA formation, especially those most commonly used to simulate regional and global SOA burdens do not account for such multi-generational ageing, instead parameterizing SOA formation as a one-step process. And those SOA models that do account for ageing tend to do so in a very ad hoc manner and with little validation (with chemically explicit models being the exception). The Statistical Oxidation Model (SOM) is a reduced complexity SOA model that explicitly accounts for multi-generational ageing in both the gas and particle phases and has been developed to capture the countering effects of fragmentation and functionalization. The basic framework of the SOM will be introduced along with an overview of next-generation developments of the model to make it amenable to use in 3D models. In the second part of this seminar, a series of SOA formation and ageing experiments will be discussed in which particle optical property measurements (light extinction and light absorption) have been made and correlated with particle composition. Our results demonstrate that SOA optical properties are not static and depend explicitly on the identity of the gas-phase precursor. | Reactions of organic compounds in the atmosphere with common oxidants (e.g. the hydroxyl radical and ozone) can lead to the formation of secondary organic aerosol (SOA) and can engender transformations within the particle-phase through heterogeneous reactions. The resulting specific chemical composition of the particles can influence their ability to take up water, activate into cloud droplets and absorb and scatter sunlight. Most organic compounds emitted into the atmosphere that are capable of forming SOA are subject to multi-generational oxidation, which simply means that they can react more than once, with each reaction having some probability of functionalizing or fragmenting the parent species. In this seminar, two aspects of this multi-generational ageing will be discussed: SOA models and SOA optical properties. Many models of SOA formation, especially those most commonly used to simulate regional and global SOA burdens do not account for such multi-generational ageing, instead parameterizing SOA formation as a one-step process. And those SOA models that do account for ageing tend to do so in a very ad hoc manner and with little validation (with chemically explicit models being the exception). The Statistical Oxidation Model (SOM) is a reduced complexity SOA model that explicitly accounts for multi-generational ageing in both the gas and particle phases and has been developed to capture the countering effects of fragmentation and functionalization. The basic framework of the SOM will be introduced along with an overview of next-generation developments of the model to make it amenable to use in 3D models. In the second part of this seminar, a series of SOA formation and ageing experiments will be discussed in which particle optical property measurements (light extinction and light absorption) have been made and correlated with particle composition. Our results demonstrate that SOA optical properties are not static and depend explicitly on the identity of the gas-phase precursor. | ||
| − | == Monday, May 6, 2013 | + | ==Spring 2013== |
| + | <br /> | ||
| + | <font size="4"> Monday, May 6, 2013 </font> | ||
'''How can we improve therapy for Tuberculosis?''' | '''How can we improve therapy for Tuberculosis?''' | ||
| Line 1,243: | Line 5,091: | ||
Tuberculosis [TB] is one of the leading killer infectious diseases today. Eradication of TB depends on the development of shorter and more effective treatment regimens, including novel treatment alternatives combined with classical TB therapies. At present patients with TB are treated daily with high concentrations of multidrug treatment administered by oral route, subcutaneous or intravenous injections. To improve TB control and treatment it is necessary to ease and shorten anti-TB drug regimens. One approach under study is the use of aerosolized drugs and use of drugs the target the host instead of the bacteria also known as host targeted therapy. In our laboratories we use an experimental murine model that allow for aerosolized anti-TB drug efficacy testing. The uses of aerosol delivery of anti-TB drugs which only option at present are to be used as injectables in addition to the use of lung immune modulators as adjuvants for TB chemotherapy will be discussed. | Tuberculosis [TB] is one of the leading killer infectious diseases today. Eradication of TB depends on the development of shorter and more effective treatment regimens, including novel treatment alternatives combined with classical TB therapies. At present patients with TB are treated daily with high concentrations of multidrug treatment administered by oral route, subcutaneous or intravenous injections. To improve TB control and treatment it is necessary to ease and shorten anti-TB drug regimens. One approach under study is the use of aerosolized drugs and use of drugs the target the host instead of the bacteria also known as host targeted therapy. In our laboratories we use an experimental murine model that allow for aerosolized anti-TB drug efficacy testing. The uses of aerosol delivery of anti-TB drugs which only option at present are to be used as injectables in addition to the use of lung immune modulators as adjuvants for TB chemotherapy will be discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 29, 2013 </font> | ||
'''High-Altitude Aircraft Measurements of the Tropical Tropopause Layer from the NASA ATTREX Campaign''' | '''High-Altitude Aircraft Measurements of the Tropical Tropopause Layer from the NASA ATTREX Campaign''' | ||
| Line 1,250: | Line 5,099: | ||
The NASA Airborne Tropical TRopopause EXperiment (ATTREX) is a five-year airborne science program focused on understanding physical processes occurring in the tropical tropopause layer (TTL, ~13-20 km), with an emphasis on understanding the control of the humidity of air entering the stratosphere. The long-duration Global Hawk Unmanned Aircraft System is used to make measurements of water vapor, clouds, meteorological conditions, numerous chemical tracers, radiative fluxes, and BrO. During February-March, 2013, six 24+ hour flights were conducted from Dryden Flight Research Center to the tropical eastern and central Pacific. Our primary mode of operation was to repeatedly ascend and descend through the TTL (between about 45 and 60 kft). Over 100 vertical profiles were obtained during the six flights, providing a unique, high spatial-resolution dataset of TTL composition. Some of the flights provided surveys of TTL composition, extending well into the southern hemisphere. Other flights targeted thin cirrus near the tropopause forming in anomalously cold regions. Examples of ATTREX measurements will be presented with an focus on TTL relative humidity and clouds. Plans for deploying the Global Hawk to Guam for western Pacific measurements in 2014 will also be discussed. | The NASA Airborne Tropical TRopopause EXperiment (ATTREX) is a five-year airborne science program focused on understanding physical processes occurring in the tropical tropopause layer (TTL, ~13-20 km), with an emphasis on understanding the control of the humidity of air entering the stratosphere. The long-duration Global Hawk Unmanned Aircraft System is used to make measurements of water vapor, clouds, meteorological conditions, numerous chemical tracers, radiative fluxes, and BrO. During February-March, 2013, six 24+ hour flights were conducted from Dryden Flight Research Center to the tropical eastern and central Pacific. Our primary mode of operation was to repeatedly ascend and descend through the TTL (between about 45 and 60 kft). Over 100 vertical profiles were obtained during the six flights, providing a unique, high spatial-resolution dataset of TTL composition. Some of the flights provided surveys of TTL composition, extending well into the southern hemisphere. Other flights targeted thin cirrus near the tropopause forming in anomalously cold regions. Examples of ATTREX measurements will be presented with an focus on TTL relative humidity and clouds. Plans for deploying the Global Hawk to Guam for western Pacific measurements in 2014 will also be discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 22, 2013 </font> | ||
'''An Overview of the OASIS 2009 Campaign, with Focus on Halogen, OVOC and Relative Nitrogen Chemistry''' | '''An Overview of the OASIS 2009 Campaign, with Focus on Halogen, OVOC and Relative Nitrogen Chemistry''' | ||
| Line 1,263: | Line 5,113: | ||
In light of these findings, we will discuss “the PAN conundrum,” a long-known discrepancy between predicted and observed PAN mixing ratios in the Arctic coastal boundary layer. We have been working to solve this problem using our detailed zero-D chemistry model. Preliminary results will be shown and discussed. | In light of these findings, we will discuss “the PAN conundrum,” a long-known discrepancy between predicted and observed PAN mixing ratios in the Arctic coastal boundary layer. We have been working to solve this problem using our detailed zero-D chemistry model. Preliminary results will be shown and discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 15, 2013 </font> | ||
''' Real-time organic aerosol formation and oxidative aging using a flow reactor in a ponderosa pine forest''' | ''' Real-time organic aerosol formation and oxidative aging using a flow reactor in a ponderosa pine forest''' | ||
| Line 1,290: | Line 5,141: | ||
formation pathways. | formation pathways. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 8, 2013 </font> | ||
'''Investigating and improving the freeze-thaw stability of vaccines with aluminum-containing adjuvants: Case studies with model HBsAg vaccines''' | '''Investigating and improving the freeze-thaw stability of vaccines with aluminum-containing adjuvants: Case studies with model HBsAg vaccines''' | ||
| Line 1,298: | Line 5,150: | ||
Vaccines with aluminum-containing adjuvants, the most prevalent class of adjuvant in childhood vaccines, are susceptible to reduced efficacy if frozen. Accidental freezing of such vaccines have been documented globally, including in the US. This seminar will focus on research related to the freeze-thaw stability of model hepatitis B vaccines. Efforts to both understand the causes of the freeze-thaw instability as well as approaches to improving the robustness of the vaccine following exposure to subzero temperatures will be discussed. | Vaccines with aluminum-containing adjuvants, the most prevalent class of adjuvant in childhood vaccines, are susceptible to reduced efficacy if frozen. Accidental freezing of such vaccines have been documented globally, including in the US. This seminar will focus on research related to the freeze-thaw stability of model hepatitis B vaccines. Efforts to both understand the causes of the freeze-thaw instability as well as approaches to improving the robustness of the vaccine following exposure to subzero temperatures will be discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Thursday, April 1, 2013 </font> | ||
Special Analytical & Environmental Chemistry Seminar | Special Analytical & Environmental Chemistry Seminar | ||
| Line 1,312: | Line 5,165: | ||
In this seminar I will present an aerosol model of a new type, the stochastic particle-resolved model PartMC-MOSAIC. This model tracks individual particles as they undergo chemical and physical transformations in the atmosphere. Hence aerosol impacts that depend on per-particle composition can be represented in detail. I will illustrate the usefulness of this new approach by focusing on the aging process of black carbon. Using PartMC-MOSAIC we are able to quantify the individual processes that contribute to the aging of black carbon and demonstrate the effect of aerosol mixing state on aging time scales, optical properties, and CCN activation properties. | In this seminar I will present an aerosol model of a new type, the stochastic particle-resolved model PartMC-MOSAIC. This model tracks individual particles as they undergo chemical and physical transformations in the atmosphere. Hence aerosol impacts that depend on per-particle composition can be represented in detail. I will illustrate the usefulness of this new approach by focusing on the aging process of black carbon. Using PartMC-MOSAIC we are able to quantify the individual processes that contribute to the aging of black carbon and demonstrate the effect of aerosol mixing state on aging time scales, optical properties, and CCN activation properties. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 18, 2013 </font> | ||
Ivan Ortega, University of Colorado, Boulder, Department of Chemistry and Biochemistry | Ivan Ortega, University of Colorado, Boulder, Department of Chemistry and Biochemistry | ||
| Line 1,321: | Line 5,175: | ||
Over the past few years the Atmospheric Trace Molecule Spectroscopy Laboratory (ATMOSpeclab) at CU Boulder has designed and deployed portable, high-sensitivity Multi Axis Differential Optical Absorption Spectroscopy (MAX-DOAS) instruments with a two-fold purpose: 1) to retrieve profile information of trace gases (i.e., NO2, HCHO, HONO, CHOCHO) and 2) to determine multi wavelength aerosol properties. Past MAX-DOAS measurements have extensively focused on the unique ability to derive information about trace gases; however, this technique can be expanded towards the retrieval of aerosol optical and microphysical properties. This talk presents the evaluation of a novel 2 dimensional MAX-DOAS instrument. The new instrument measures solar scattered light in the horizontal (varying azimuth angle), as well as the vertical plane (varying the elevation angle) which can be used to obtain effective aerosol microphysical properties, such as aerosol size distribution, phase function and refractive index; in addition to the trace gas and aerosol extinction profiles. The 2D MAX-DOAS was deployed and operated for 6 weeks at Cape Cod, MA as part of the Two Column Aerosol Project (TCAP). The TCAP deployment provides unique opportunities for comparisons with aerosol extinction profile measurements by the NASA Langley airborne High Spectral Resolution Lidar (HSRL), Aerosol Optical Depth by a co-located NOAA Multifilter Rotating Shadowband Radiometer (MFRSR) instrument, as well as in-situ measured optical properties aboard DoE’s G1 aircraft. A retrieval was developed to invert effective radius and complex refractive index of particles for monomodal logarithmic normal distribution derived from Mie-scattering calculations in the size range of 0.1-1um. Aerosol extinction retrieved from MAX-DOAS measurements can be compared with extinction predicted by Mie theory to test the assumption of particle homogeneity and sphericity. | Over the past few years the Atmospheric Trace Molecule Spectroscopy Laboratory (ATMOSpeclab) at CU Boulder has designed and deployed portable, high-sensitivity Multi Axis Differential Optical Absorption Spectroscopy (MAX-DOAS) instruments with a two-fold purpose: 1) to retrieve profile information of trace gases (i.e., NO2, HCHO, HONO, CHOCHO) and 2) to determine multi wavelength aerosol properties. Past MAX-DOAS measurements have extensively focused on the unique ability to derive information about trace gases; however, this technique can be expanded towards the retrieval of aerosol optical and microphysical properties. This talk presents the evaluation of a novel 2 dimensional MAX-DOAS instrument. The new instrument measures solar scattered light in the horizontal (varying azimuth angle), as well as the vertical plane (varying the elevation angle) which can be used to obtain effective aerosol microphysical properties, such as aerosol size distribution, phase function and refractive index; in addition to the trace gas and aerosol extinction profiles. The 2D MAX-DOAS was deployed and operated for 6 weeks at Cape Cod, MA as part of the Two Column Aerosol Project (TCAP). The TCAP deployment provides unique opportunities for comparisons with aerosol extinction profile measurements by the NASA Langley airborne High Spectral Resolution Lidar (HSRL), Aerosol Optical Depth by a co-located NOAA Multifilter Rotating Shadowband Radiometer (MFRSR) instrument, as well as in-situ measured optical properties aboard DoE’s G1 aircraft. A retrieval was developed to invert effective radius and complex refractive index of particles for monomodal logarithmic normal distribution derived from Mie-scattering calculations in the size range of 0.1-1um. Aerosol extinction retrieved from MAX-DOAS measurements can be compared with extinction predicted by Mie theory to test the assumption of particle homogeneity and sphericity. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 4, 2013 </font> | ||
Megan L. Melamed, | Megan L. Melamed, | ||
| Line 1,335: | Line 5,190: | ||
As of 2008, for the first time, the majority of the world’s population is living in urban areas [http://www.unfpa.org/pds/urbanization.htm], many in megacities (with populations over 10 million). Currently, urban population fractions are higher in developed than developing countries. However, with most of the world population growth expected to occur in developing countries, urbanization is increasing at a higher rate in developing countries. These dramatic increases in population and urbanization, especially in the developing world, have been accompanied by technological and economic growth and development, yielding changes in land use, energy use, and transportation. The resulting changes due to urbanization have dramatic impacts on anthropogenic and biogenic emissions and have notably altered local to global-scale atmospheric composition, increasing the importance of understanding the impacts of urbanization on atmospheric chemistry. In light of the importance of megacities on atmospheric chemistry, the International Global Atmospheric Chemistry (IGAC) Project and the World Meteorological Organization (WMO) released in January 2013 the first comprehensive assessment of Atmospheric Chemistry in Megacities. An overview of this assessment will be presented. | As of 2008, for the first time, the majority of the world’s population is living in urban areas [http://www.unfpa.org/pds/urbanization.htm], many in megacities (with populations over 10 million). Currently, urban population fractions are higher in developed than developing countries. However, with most of the world population growth expected to occur in developing countries, urbanization is increasing at a higher rate in developing countries. These dramatic increases in population and urbanization, especially in the developing world, have been accompanied by technological and economic growth and development, yielding changes in land use, energy use, and transportation. The resulting changes due to urbanization have dramatic impacts on anthropogenic and biogenic emissions and have notably altered local to global-scale atmospheric composition, increasing the importance of understanding the impacts of urbanization on atmospheric chemistry. In light of the importance of megacities on atmospheric chemistry, the International Global Atmospheric Chemistry (IGAC) Project and the World Meteorological Organization (WMO) released in January 2013 the first comprehensive assessment of Atmospheric Chemistry in Megacities. An overview of this assessment will be presented. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 25, 2013 </font> | ||
Prof. Waleed Abdalati, | Prof. Waleed Abdalati, | ||
| Line 1,345: | Line 5,201: | ||
For decades, NASA has been an iconic inspiration to the nation and the world, exploring our planet, the space environment, the solar system, and the universe. Success in these endeavors has been built on a foundation of science, technology and engineering that is producing such remarkable achievements as: landing a one-ton rover on the surface of Mars, developing a telescope that will peer 13.5 billion years into the past, and most importantly, providing comprehensive insights into the behavior and evolution of planet Earth. Enabling these capabilities, and the associated research, requires much more than technical and scientific advances, however. It involves a complex balancing of priorities and resources, as well as alignment among a diverse group of passionate stakeholders – all against a backdrop of political and policy turmoil. As chief scientist, it was my responsibility to help shape NASA’s direction, and work to achieve alignment among the interests and objectives of the scientific community, the White House, Congress and others. In this talk, I will discuss some of these challenges and the associated rewards as well as the role science plays at NASA, and the role NASA science plays in the broader national framework. | For decades, NASA has been an iconic inspiration to the nation and the world, exploring our planet, the space environment, the solar system, and the universe. Success in these endeavors has been built on a foundation of science, technology and engineering that is producing such remarkable achievements as: landing a one-ton rover on the surface of Mars, developing a telescope that will peer 13.5 billion years into the past, and most importantly, providing comprehensive insights into the behavior and evolution of planet Earth. Enabling these capabilities, and the associated research, requires much more than technical and scientific advances, however. It involves a complex balancing of priorities and resources, as well as alignment among a diverse group of passionate stakeholders – all against a backdrop of political and policy turmoil. As chief scientist, it was my responsibility to help shape NASA’s direction, and work to achieve alignment among the interests and objectives of the scientific community, the White House, Congress and others. In this talk, I will discuss some of these challenges and the associated rewards as well as the role science plays at NASA, and the role NASA science plays in the broader national framework. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 18, 2013 </font> | ||
Prof. Brian Majestic | Prof. Brian Majestic | ||
| Line 1,358: | Line 5,215: | ||
A new tool to determine the relative importance of these sources is Fe isotope analysis. Previous work has shown that crustal material has an isotopic composition equal to ~ 0.1 ‰. Therefore, isotopic deviations from 0.1 ‰ may indicate the presence of non-crustal Fe. In this talk, I will present Fe isotope data from the outskirts of Phoenix, AZ, a highly crustal environment as well as Bermuda, a pristine oceanic site. Bermuda is an ideal site to test this method because, during the summer months, the air masses reaching Bermuda originate from West Africa (crustal) and, during the winter months, the air masses originate from North America (more anthropogenic). Striking similarities between these two sites, as well as other Fe isotope studies, will be highlighted. | A new tool to determine the relative importance of these sources is Fe isotope analysis. Previous work has shown that crustal material has an isotopic composition equal to ~ 0.1 ‰. Therefore, isotopic deviations from 0.1 ‰ may indicate the presence of non-crustal Fe. In this talk, I will present Fe isotope data from the outskirts of Phoenix, AZ, a highly crustal environment as well as Bermuda, a pristine oceanic site. Bermuda is an ideal site to test this method because, during the summer months, the air masses reaching Bermuda originate from West Africa (crustal) and, during the winter months, the air masses originate from North America (more anthropogenic). Striking similarities between these two sites, as well as other Fe isotope studies, will be highlighted. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 11, 2013 </font> | ||
Professor Neil M. Donahue | Professor Neil M. Donahue | ||
| Line 1,373: | Line 5,231: | ||
Organic aerosols comprise a highly dynamic system of multiphase oxidation chemistry. A major element in the organic-aerosol lifecycle is the progressive oxidation of semi-volatile vapors. Oxidation influences the total aerosol mass by forming products with different vapor pressures than their precursor molecules, but the overall effect on the aerosol mass is nuanced. Oxidation (aging) can clearly enhance aerosol mass via formation of more heavily functionalized products, but it can also reduce the aerosol mass as carbon backbones are fragmented, especially later in the oxidation sequence. We have investigated a number of model systems as well as representative bulk aerosol systems to investigate this process, and developed a reduced chemical mechanism to describe it. In addition to influencing the overall mass, oxidation chemistry can produce small amounts of extremely highly oxidized organic vapors. These highly functionalized molecules can and do participate in the formation of molecular clusters (typically involving sulfuric acid in the atmosphere) and their subsequent growth to ultrafine particles and then to particles large enough to have significant health and climate effects. We have recently joined the CLOUD consortium at CERN to study this particle physics. | Organic aerosols comprise a highly dynamic system of multiphase oxidation chemistry. A major element in the organic-aerosol lifecycle is the progressive oxidation of semi-volatile vapors. Oxidation influences the total aerosol mass by forming products with different vapor pressures than their precursor molecules, but the overall effect on the aerosol mass is nuanced. Oxidation (aging) can clearly enhance aerosol mass via formation of more heavily functionalized products, but it can also reduce the aerosol mass as carbon backbones are fragmented, especially later in the oxidation sequence. We have investigated a number of model systems as well as representative bulk aerosol systems to investigate this process, and developed a reduced chemical mechanism to describe it. In addition to influencing the overall mass, oxidation chemistry can produce small amounts of extremely highly oxidized organic vapors. These highly functionalized molecules can and do participate in the formation of molecular clusters (typically involving sulfuric acid in the atmosphere) and their subsequent growth to ultrafine particles and then to particles large enough to have significant health and climate effects. We have recently joined the CLOUD consortium at CERN to study this particle physics. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, February 4, 2013 </font> | ||
Dick and Jean Bedell, Boulder, Colorado | Dick and Jean Bedell, Boulder, Colorado | ||
| Line 1,383: | Line 5,242: | ||
This presentation reflects observations of a fascinating country and culture by a Boulder couple. Dick and Jean Bedell have volunteered their medical services for several months each year over three decades. Through Rotary Clubs in India and United States, they helped establish a home for girls whose prostitute mothers died of AIDS, and a Hospice Centre staffed by 300 volunteers. They provide seminars for doctors, midwives and nurses in urban and rural facilities. Through Dr. Robert Sievers, they toured the amazing facility in Pune linked with his research on measles vaccine. Their presentation is informal with time for questions. | This presentation reflects observations of a fascinating country and culture by a Boulder couple. Dick and Jean Bedell have volunteered their medical services for several months each year over three decades. Through Rotary Clubs in India and United States, they helped establish a home for girls whose prostitute mothers died of AIDS, and a Hospice Centre staffed by 300 volunteers. They provide seminars for doctors, midwives and nurses in urban and rural facilities. Through Dr. Robert Sievers, they toured the amazing facility in Pune linked with his research on measles vaccine. Their presentation is informal with time for questions. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, January 28, 2013 </font> | ||
Roy (Lee) Mauldin, | Roy (Lee) Mauldin, | ||
| Line 1,396: | Line 5,256: | ||
Oxidation processes in Earth’s atmosphere are tightly connected to many environmental and human health issues and are essential drivers for biogeochemistry. Oxidation processes remove natural and anthropogenic trace gases and air pollutants from the atmosphere, and produce low-volatility vapours that control secondary aerosol formation and atmospheric production of cloud condensation nuclei. These atmospheric oxidation processes are typically thought to be dominated by three main oxidants: ozone, hydroxyl radical (OH) and nitrate radical. Field measurements however have shown discrepancies between measurements and models indicating the presence of other mechanisms. Our group has recently shown that products formed from the ozonolysis of biogenic alkenes (most likely stabilized Criegee radicals) have a substantial oxidizing capacity, with the ability to oxidized compounds such as SO2 or dimethyl sulphide. This new “X-chemistry” can have significant, or even dominant, contribution to formation of low-volatility inorganic (sulfuric acid) and, most strikingly, very highly oxidized condensable organic vapours. This new route to the formation of sulfuric acid and extremely low-volatility organic compounds can have a considerable impact to global budgets of cloud condensation nuclei. Our observations point toward a highly important, yet unexplored area of atmospheric chemistry and indicate the discovery of a new natural negative feedback mechanism that could partially counteract climate warming. | Oxidation processes in Earth’s atmosphere are tightly connected to many environmental and human health issues and are essential drivers for biogeochemistry. Oxidation processes remove natural and anthropogenic trace gases and air pollutants from the atmosphere, and produce low-volatility vapours that control secondary aerosol formation and atmospheric production of cloud condensation nuclei. These atmospheric oxidation processes are typically thought to be dominated by three main oxidants: ozone, hydroxyl radical (OH) and nitrate radical. Field measurements however have shown discrepancies between measurements and models indicating the presence of other mechanisms. Our group has recently shown that products formed from the ozonolysis of biogenic alkenes (most likely stabilized Criegee radicals) have a substantial oxidizing capacity, with the ability to oxidized compounds such as SO2 or dimethyl sulphide. This new “X-chemistry” can have significant, or even dominant, contribution to formation of low-volatility inorganic (sulfuric acid) and, most strikingly, very highly oxidized condensable organic vapours. This new route to the formation of sulfuric acid and extremely low-volatility organic compounds can have a considerable impact to global budgets of cloud condensation nuclei. Our observations point toward a highly important, yet unexplored area of atmospheric chemistry and indicate the discovery of a new natural negative feedback mechanism that could partially counteract climate warming. | ||
| − | == Monday, December 10, 2012 | + | ==Fall 2012== |
| + | <br /> | ||
| + | <font size="4"> Monday, December 10, 2012 </font> | ||
Callie Cole | Callie Cole | ||
| Line 1,406: | Line 5,268: | ||
Reactions involving organic molecules are important to astrochemistry and astrobiology alike. Several carbon- and nitrogen-containing anions (CN–, C3N–, and C5N–) as well as trans methyl formate have been spectroscopically detected in the interstellar medium (ISM), and this work focuses on relevant gas phase reactions involving these species. The tandem flowing afterglow-selected ion flow tube (FA-SIFT) was used to experimentally determine the rate constants and products of these reactions, and theoretical calculations were conducted to further understand their mechanisms. Additionally, an ion trap mass spectrometer has been modified to allow for the study of ion-neutral reactions. The building of this modification as well as future plans for this instrument with respect to astrochemistry will be discussed. | Reactions involving organic molecules are important to astrochemistry and astrobiology alike. Several carbon- and nitrogen-containing anions (CN–, C3N–, and C5N–) as well as trans methyl formate have been spectroscopically detected in the interstellar medium (ISM), and this work focuses on relevant gas phase reactions involving these species. The tandem flowing afterglow-selected ion flow tube (FA-SIFT) was used to experimentally determine the rate constants and products of these reactions, and theoretical calculations were conducted to further understand their mechanisms. Additionally, an ion trap mass spectrometer has been modified to allow for the study of ion-neutral reactions. The building of this modification as well as future plans for this instrument with respect to astrochemistry will be discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, November 26, 2012 </font> | ||
Siyuan Wang | Siyuan Wang | ||
| Line 1,416: | Line 5,279: | ||
Glyoxal has potentially large sources from remote to polluted environments. Glyoxal is highly water-soluble; it may undergo a series of aqueous-phase processes in aerosol liquid water and lead to the formation of highly oxidized, high-molecular-weight compounds. In the present work, the multi-phase behavior of glyoxal is probed from chamber experiments (PSI chamber, Switzerland) to ambient environments (sub-urban Hong Kong). Phase transfer and subsequent aqueous-phase processes are simulated using a box-model. In both cases glyoxal is found to form secondary organic material under humid condition, which may be affected by various factors such as aerosol acidity and sulfate. This modeling work provides reasonable interpretations for observations and insights into the complicated processes of such a simple molecule. | Glyoxal has potentially large sources from remote to polluted environments. Glyoxal is highly water-soluble; it may undergo a series of aqueous-phase processes in aerosol liquid water and lead to the formation of highly oxidized, high-molecular-weight compounds. In the present work, the multi-phase behavior of glyoxal is probed from chamber experiments (PSI chamber, Switzerland) to ambient environments (sub-urban Hong Kong). Phase transfer and subsequent aqueous-phase processes are simulated using a box-model. In both cases glyoxal is found to form secondary organic material under humid condition, which may be affected by various factors such as aerosol acidity and sulfate. This modeling work provides reasonable interpretations for observations and insights into the complicated processes of such a simple molecule. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, November 12, 2012 </font> | ||
Theodore Konstantinos Koenig | Theodore Konstantinos Koenig | ||
| Line 1,424: | Line 5,288: | ||
I will be presenting on work to determine whether water spin isomers can be enriched in the gas phase by selective adsorption. I will begin by briefly outlining the motivations and previous literature on the topic. I will then give information on the construction and testing of an instrument for the purpose of examining any effect by a variety of spectroscopies. Finally, initial FT-IR results will be presented to give a preliminary answer to whether selective adsorption does in fact occur. | I will be presenting on work to determine whether water spin isomers can be enriched in the gas phase by selective adsorption. I will begin by briefly outlining the motivations and previous literature on the topic. I will then give information on the construction and testing of an instrument for the purpose of examining any effect by a variety of spectroscopies. Finally, initial FT-IR results will be presented to give a preliminary answer to whether selective adsorption does in fact occur. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 29, 2012 </font> | ||
Christopher Kampf | Christopher Kampf | ||
| Line 1,444: | Line 5,309: | ||
Most studies investigating the formation of SOA from glyoxal so far employed simple box model and pseudo-lagrangian approaches, which lack a comprehensive description of emissions variability and transport phenomena. We investigate regional scale variability of glyoxal SOA using the online-coupled regional chemistry transport model WRF/Chem with a focus on the summer 2010 over California. In my talk I will highlight improvements made to the model to better represent glyoxal formation pathways, discuss model performance when compared against a variety of measurement data available from the CalNex and CARES field campaigns, and show how we extend it by two different methods to describe formation of SOA from glyoxal. Finally I will present first results of simulations made for a selected period in June 2010. | Most studies investigating the formation of SOA from glyoxal so far employed simple box model and pseudo-lagrangian approaches, which lack a comprehensive description of emissions variability and transport phenomena. We investigate regional scale variability of glyoxal SOA using the online-coupled regional chemistry transport model WRF/Chem with a focus on the summer 2010 over California. In my talk I will highlight improvements made to the model to better represent glyoxal formation pathways, discuss model performance when compared against a variety of measurement data available from the CalNex and CARES field campaigns, and show how we extend it by two different methods to describe formation of SOA from glyoxal. Finally I will present first results of simulations made for a selected period in June 2010. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 22, 2012 </font> | ||
Yong Liu | Yong Liu | ||
| Line 1,457: | Line 5,323: | ||
Despite recent extensive studies towards heterogeneous oxidation of unsaturated organics (mostly oleic acid) initiated by ozone, little is known about effects of environmental conditions such as ambient temperature and relative humidity; physical state; and oxidant concentration on heterogeneous reaction kinetics. In this work, we used linoleic acid as a proxy for atmospheric unsaturated organics to investigate its heterogeneous oxidation by O3 over a wide range of temperatures (258-314 K), relative humidities (0-80% RH) and ozone concentration (50 ppb -50ppm). Our experiments were carried out using a flow reactor coupled to an attenuated total reflection infrared spectrometer (ATR-IR). Pseudo-first order rate constants and overall reactive uptake coefficients were acquired from absorbance changes in peaks located near 1743 cm-1; 1710 cm-1; 1172 cm-1 and 1110 cm-1, which can be assigned to C=O in ester; C=O in acid; C-C and C-O stretching modes, respectively. Results showed that the kapp and γ increased with increasing temperatures. It was noted the temperature enhancement effect on the reaction kinetics was much more pronounced at lower temperatures. Such behavior can be explained by change in physical state of LA at lower temperatures. In addition, kapp and γ were enhanced by 2-fold as RH increased from 0 to 80%. Moreover, the effect of ozone concentration on the reaction kinetics was reported for the first time. kapp was found to display a Langmuir-Hinshelwood dependence on ozone concentration. Furthermore, yields and hygroscopic properties of reaction products were also investigated by FTIR spectroscopy. The intensity ratio of two C=O stretching bands, A1743/A1710, which was utilized as an indicator of the product yields, increased sharply with increasing temperatures in the lower temperature region (258-284 K), and then remained nearly constant in the higher temperature region (284-314 K). The product yields showed no significant variation with RH, for the intensity ratio of A1743/A1710 barely changed in the wide RH range of 0-80%. Water uptake studies showed that the LA thin film absorbed water with an increasing RH, and the hygroscopicity of the thin film was enhanced after ozone exposure. | Despite recent extensive studies towards heterogeneous oxidation of unsaturated organics (mostly oleic acid) initiated by ozone, little is known about effects of environmental conditions such as ambient temperature and relative humidity; physical state; and oxidant concentration on heterogeneous reaction kinetics. In this work, we used linoleic acid as a proxy for atmospheric unsaturated organics to investigate its heterogeneous oxidation by O3 over a wide range of temperatures (258-314 K), relative humidities (0-80% RH) and ozone concentration (50 ppb -50ppm). Our experiments were carried out using a flow reactor coupled to an attenuated total reflection infrared spectrometer (ATR-IR). Pseudo-first order rate constants and overall reactive uptake coefficients were acquired from absorbance changes in peaks located near 1743 cm-1; 1710 cm-1; 1172 cm-1 and 1110 cm-1, which can be assigned to C=O in ester; C=O in acid; C-C and C-O stretching modes, respectively. Results showed that the kapp and γ increased with increasing temperatures. It was noted the temperature enhancement effect on the reaction kinetics was much more pronounced at lower temperatures. Such behavior can be explained by change in physical state of LA at lower temperatures. In addition, kapp and γ were enhanced by 2-fold as RH increased from 0 to 80%. Moreover, the effect of ozone concentration on the reaction kinetics was reported for the first time. kapp was found to display a Langmuir-Hinshelwood dependence on ozone concentration. Furthermore, yields and hygroscopic properties of reaction products were also investigated by FTIR spectroscopy. The intensity ratio of two C=O stretching bands, A1743/A1710, which was utilized as an indicator of the product yields, increased sharply with increasing temperatures in the lower temperature region (258-284 K), and then remained nearly constant in the higher temperature region (284-314 K). The product yields showed no significant variation with RH, for the intensity ratio of A1743/A1710 barely changed in the wide RH range of 0-80%. Water uptake studies showed that the LA thin film absorbed water with an increasing RH, and the hygroscopicity of the thin film was enhanced after ozone exposure. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 15, 2012 </font> | ||
Katherine Primm | Katherine Primm | ||
| Line 1,475: | Line 5,342: | ||
To better assess and characterize the sensing properties of porous silicon (pSi), we have developed a strategy to create small spatially heterogeneous areas on a pSi surface that exhibit different degrees of surface oxidation by pin printing 9:1 H2O2:glycerol on the prepared pSi surface. The pSi surface chemistry and the heterogeneity of these pin printed regions were assessed using FT-IR microscopy and epi-luminescence microscopy, coupled with multispectral imaging. The results show that the pin printed areas are chemically heterogeneous across their ca. 300 μm diameter and the photoluminescence response to analyte depends on the analyte type and on the spatial position within the printed region (i.e., on the exact extent of oxide formation (Si-O-Si, O2SiH, O3SiH and Si-OH vs. SiH, SiH2 and SiH3) at the pSi surface). The combination of pin printing and multispectral microscopies allows us to create the entire range of pSi chemical compositions and simultaneously assess their performance all within a single 300 μm diameter printed feature. This can lead to more sophisticated pattern recognition schema to allow simultaneous analyte identification and quantification. | To better assess and characterize the sensing properties of porous silicon (pSi), we have developed a strategy to create small spatially heterogeneous areas on a pSi surface that exhibit different degrees of surface oxidation by pin printing 9:1 H2O2:glycerol on the prepared pSi surface. The pSi surface chemistry and the heterogeneity of these pin printed regions were assessed using FT-IR microscopy and epi-luminescence microscopy, coupled with multispectral imaging. The results show that the pin printed areas are chemically heterogeneous across their ca. 300 μm diameter and the photoluminescence response to analyte depends on the analyte type and on the spatial position within the printed region (i.e., on the exact extent of oxide formation (Si-O-Si, O2SiH, O3SiH and Si-OH vs. SiH, SiH2 and SiH3) at the pSi surface). The combination of pin printing and multispectral microscopies allows us to create the entire range of pSi chemical compositions and simultaneously assess their performance all within a single 300 μm diameter printed feature. This can lead to more sophisticated pattern recognition schema to allow simultaneous analyte identification and quantification. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 8, 2012 </font> | ||
Jay Kroll | Jay Kroll | ||
| Line 1,494: | Line 5,362: | ||
Direct Analysis in Real Time (DART) is an ambient ionization source for mass spectrometry (MS) that is utilized in a wide variety of applications including food science, forensics, homeland security, and environmental analysis. Introduced in 2005, DART-MS does not require sample preparation and allows the analysis of solid and liquid samples in their native state. This talk will discuss the development of novel methods of sampling with the DART source to enhance its capabilities. Such methods include rapid heating of samples of metal substrates, ambient ion transfer tubes for non-proximate sensing, and magnetic automated sampling systems for the analysis of powders. | Direct Analysis in Real Time (DART) is an ambient ionization source for mass spectrometry (MS) that is utilized in a wide variety of applications including food science, forensics, homeland security, and environmental analysis. Introduced in 2005, DART-MS does not require sample preparation and allows the analysis of solid and liquid samples in their native state. This talk will discuss the development of novel methods of sampling with the DART source to enhance its capabilities. Such methods include rapid heating of samples of metal substrates, ambient ion transfer tubes for non-proximate sensing, and magnetic automated sampling systems for the analysis of powders. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, October 1, 2012 </font> | ||
[http://chem.ucr.edu/index.php?main=faculty&facsort=profile&faculty=ziemann Paul Ziemann] | [http://chem.ucr.edu/index.php?main=faculty&facsort=profile&faculty=ziemann Paul Ziemann] | ||
| Line 1,526: | Line 5,395: | ||
projects for which I am recruiting CU graduate students to start in my group in June 2013. | projects for which I am recruiting CU graduate students to start in my group in June 2013. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 24, 2012 </font> | ||
Robert E. Sievers and team of 30 collaborators sponsored by FNIH and the | Robert E. Sievers and team of 30 collaborators sponsored by FNIH and the | ||
| Line 1,547: | Line 5,417: | ||
Reactive trace gases and aerosols are relevant components of tropospheric chemistry and climate. Bromine and iodine oxide radicals, and oxygenated VOC species modify HOx radical abundances, influence the reactive chemistry and lifetime of climate active gases (e.g., ozone, methane, dimethyl sulfide) and can trigger the atmospheric deposition of mercury to ecosystems. Reactive trace gases are also a source for secondary aerosol mass that modifies aerosol optical properties, and cloud interactions (Earth albedo). In this talk I will summarize research in the development of innovative optical spectroscopic instrumentation (in-situ and remote sensing) to measure trace gases and aerosols, their applications in field and laboratory measurements conducted by our group in 2012. These include ongoing data analysis and modeling of the TORERO, TCAP, WACS field projects, mechanistic studies in simulation chambers of secondary organic aerosol (SOA) formation, and heterogeneous chemistry of OVOC volatilization from surfaces coated with organics in flow tubes. Future opportunities exist as part of these ongoing data analysis and modeling activities, with simulation chamber experiments at facilities in Boulder and Switzerland (PSI 2013), and to further develop the University of Colorado Airborne Multi AXis Differential Optical Absorption Spectroscopy instrument (CU AMAX-DOAS) in preparation for field projects aboard research aircraft over the western tropical pacific ocean (CONTRAST 2014). | Reactive trace gases and aerosols are relevant components of tropospheric chemistry and climate. Bromine and iodine oxide radicals, and oxygenated VOC species modify HOx radical abundances, influence the reactive chemistry and lifetime of climate active gases (e.g., ozone, methane, dimethyl sulfide) and can trigger the atmospheric deposition of mercury to ecosystems. Reactive trace gases are also a source for secondary aerosol mass that modifies aerosol optical properties, and cloud interactions (Earth albedo). In this talk I will summarize research in the development of innovative optical spectroscopic instrumentation (in-situ and remote sensing) to measure trace gases and aerosols, their applications in field and laboratory measurements conducted by our group in 2012. These include ongoing data analysis and modeling of the TORERO, TCAP, WACS field projects, mechanistic studies in simulation chambers of secondary organic aerosol (SOA) formation, and heterogeneous chemistry of OVOC volatilization from surfaces coated with organics in flow tubes. Future opportunities exist as part of these ongoing data analysis and modeling activities, with simulation chamber experiments at facilities in Boulder and Switzerland (PSI 2013), and to further develop the University of Colorado Airborne Multi AXis Differential Optical Absorption Spectroscopy instrument (CU AMAX-DOAS) in preparation for field projects aboard research aircraft over the western tropical pacific ocean (CONTRAST 2014). | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 17, 2012 </font> | ||
Alison Craven | Alison Craven | ||
| Line 1,561: | Line 5,432: | ||
DOM also has also been shown to enhance the dissolution of soils, sediments and minerals, which could result in the release of toxic trace elements into aqueous systems. Coal ash contains high concentrations of toxic trace elements that may have the potential to be released into the water. Releases of coal ash to rivers and streams, such as that which occurred from the Kingston Fossil Plant in Kingston, TN, into the Emory and Clinch Rivers in December 2008, are a concern because of the potential for human health problems, as well as ecological effects. In order to understand the effect that DOM has on the release of trace elements from coal ash, a series of release experiments were performed using coal ash generated from the Kingston Fossil Plant as a function of DOM concentration, DOM aromaticity, and calcium concentration. Various DOM isolates and filtered site-water samples collected near the Kingston Fossil Plant were used to produce coal ash suspensions at a fixed ash:water ratio of 1:1000 and a near-neutral pH. The major and trace elemental composition of the solution, and specific trace metals (mercury, lead, copper, aluminum) and metalloids (arsenic and selenium) were measured. The results indicate that DOM enhances the release of mercury, lead, copper, and aluminum from coal ash. The concentration of mercury, copper and aluminum released from the coal ash was positively correlated with the SUVA254 (ultraviolet absorbance at 254 nm divided by the dissolved organic carbon concentration) of the DOM. The release of arsenic and selenium from the coal ash was not dependent on the DOM concentration or SUVA254. Calcium was shown to inhibit the release of mercury, copper and aluminum from the coal ash | DOM also has also been shown to enhance the dissolution of soils, sediments and minerals, which could result in the release of toxic trace elements into aqueous systems. Coal ash contains high concentrations of toxic trace elements that may have the potential to be released into the water. Releases of coal ash to rivers and streams, such as that which occurred from the Kingston Fossil Plant in Kingston, TN, into the Emory and Clinch Rivers in December 2008, are a concern because of the potential for human health problems, as well as ecological effects. In order to understand the effect that DOM has on the release of trace elements from coal ash, a series of release experiments were performed using coal ash generated from the Kingston Fossil Plant as a function of DOM concentration, DOM aromaticity, and calcium concentration. Various DOM isolates and filtered site-water samples collected near the Kingston Fossil Plant were used to produce coal ash suspensions at a fixed ash:water ratio of 1:1000 and a near-neutral pH. The major and trace elemental composition of the solution, and specific trace metals (mercury, lead, copper, aluminum) and metalloids (arsenic and selenium) were measured. The results indicate that DOM enhances the release of mercury, lead, copper, and aluminum from coal ash. The concentration of mercury, copper and aluminum released from the coal ash was positively correlated with the SUVA254 (ultraviolet absorbance at 254 nm divided by the dissolved organic carbon concentration) of the DOM. The release of arsenic and selenium from the coal ash was not dependent on the DOM concentration or SUVA254. Calcium was shown to inhibit the release of mercury, copper and aluminum from the coal ash | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, September 10, 2012 </font> | ||
Jose L. Jimenez | Jose L. Jimenez | ||
| Line 1,580: | Line 5,452: | ||
Depositional ice nucleation on atmospheric aerosols has been identified as a potential pathway for cirrus cloud formation. Upper tropospheric aerosols as well as sub-visible cirrus residues are known to be enhanced in both sulfates and organics. While solid ammonium sulfate is an efficient depositional ice nucleus, the role of organics remains largely uncertain. I will discuss recent experiments in our group examining ice nucleation one particle at a time using Raman microscopy. We will probe ice nucleation at low upper tropospheric temperatures on mixed particles containing organics with ammonium sulfate. Raman mapping will be used to probe the composition, phase and structure of the mixed particles. Ice nucleation experiments reveal that at low temperatures, some organic containing particles nucleate ice on the outside while others nucleate ice from the particle interior. | Depositional ice nucleation on atmospheric aerosols has been identified as a potential pathway for cirrus cloud formation. Upper tropospheric aerosols as well as sub-visible cirrus residues are known to be enhanced in both sulfates and organics. While solid ammonium sulfate is an efficient depositional ice nucleus, the role of organics remains largely uncertain. I will discuss recent experiments in our group examining ice nucleation one particle at a time using Raman microscopy. We will probe ice nucleation at low upper tropospheric temperatures on mixed particles containing organics with ammonium sulfate. Raman mapping will be used to probe the composition, phase and structure of the mixed particles. Ice nucleation experiments reveal that at low temperatures, some organic containing particles nucleate ice on the outside while others nucleate ice from the particle interior. | ||
| − | == Tuesday, July 17, 2012 | + | ==Spring 2012== |
| + | <br /> | ||
| + | <font size="4"> Tuesday, July 17, 2012 </font> | ||
[http://www.geo.cornell.edu/eas/PeoplePlaces/Faculty/mahowald/ Professor Nathalie Mahowald] | [http://www.geo.cornell.edu/eas/PeoplePlaces/Faculty/mahowald/ Professor Nathalie Mahowald] | ||
| Line 1,593: | Line 5,467: | ||
* [http://www.sciencemag.org/content/334/6057/794.abstract Science paper related to this talk] | * [http://www.sciencemag.org/content/334/6057/794.abstract Science paper related to this talk] | ||
| − | = | + | <br /> |
| + | <font size="4"> Tuesday, June 26, 2012 </font> | ||
Professor Jean-Francois Doussin | Professor Jean-Francois Doussin | ||
| Line 1,605: | Line 5,480: | ||
Secondary organic aerosols account for the majority of organic aerosols and play an important role in climate on both the global scale and regional scale. While significant progress has been made on the understanding of SOA formation, the transformations it undergoes during its atmospheric transit are still far from being understood. At the University of Paris East (France), a new indoor atmospheric simulation chamber has been built and its physical characteristics make it particularly well-suited to study SOA ageing processes involving oxidant, sunlight or clouds and their links with aerosol properties. In this talk, a brief description of this new tool, its analytical environment and its capabilities will be given. Then, results on on-going research involving biogenic species (such as a-pinene and isoprene) will be presented. In particular, results about ageing processes at the molecular level and their impact on aerosol properties as well as identification of field tracers for aged SOA will be shown. | Secondary organic aerosols account for the majority of organic aerosols and play an important role in climate on both the global scale and regional scale. While significant progress has been made on the understanding of SOA formation, the transformations it undergoes during its atmospheric transit are still far from being understood. At the University of Paris East (France), a new indoor atmospheric simulation chamber has been built and its physical characteristics make it particularly well-suited to study SOA ageing processes involving oxidant, sunlight or clouds and their links with aerosol properties. In this talk, a brief description of this new tool, its analytical environment and its capabilities will be given. Then, results on on-going research involving biogenic species (such as a-pinene and isoprene) will be presented. In particular, results about ageing processes at the molecular level and their impact on aerosol properties as well as identification of field tracers for aged SOA will be shown. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, May 14, 2012 </font> | ||
Lucy Carpenter, U York, UK | Lucy Carpenter, U York, UK | ||
| Line 1,617: | Line 5,493: | ||
Oxygenated volatile organic compounds (OVOCs) in the atmosphere are precursors to peroxy acetyl nitrate (PAN), affect the tropospheric ozone budget, and in the remote marine environment represent a significant sink of the hydroxyl radical (OH). The sparse observational database for these compounds, particularly in the tropics, contributes to a high uncertainty in their emissions and atmospheric significance. Here we show multi-annual measurements of acetone, methanol and acetaldehyde in the remote marine boundary layer made at the Cape Verde Atmospheric Observatory (CVAO) (16,848°N, 24.871°W), a subtropical marine boundary layer global GAW station situated on the island of São Vicente. Simulations of OVOCs at Cape Verde using the CAM-Chem global chemical transport model are markedly different from the observations in both concentration and variability and we explore reasons for this including oceanic and terrestrial primary and/or secondary OVOC sources. Finally, preliminary isoprene data from Cape Verde is discussed in the light of recent suggestions that marine isoprene emissions could contribute significantly to the sub-micron OC fraction of marine aerosol in the tropics. | Oxygenated volatile organic compounds (OVOCs) in the atmosphere are precursors to peroxy acetyl nitrate (PAN), affect the tropospheric ozone budget, and in the remote marine environment represent a significant sink of the hydroxyl radical (OH). The sparse observational database for these compounds, particularly in the tropics, contributes to a high uncertainty in their emissions and atmospheric significance. Here we show multi-annual measurements of acetone, methanol and acetaldehyde in the remote marine boundary layer made at the Cape Verde Atmospheric Observatory (CVAO) (16,848°N, 24.871°W), a subtropical marine boundary layer global GAW station situated on the island of São Vicente. Simulations of OVOCs at Cape Verde using the CAM-Chem global chemical transport model are markedly different from the observations in both concentration and variability and we explore reasons for this including oceanic and terrestrial primary and/or secondary OVOC sources. Finally, preliminary isoprene data from Cape Verde is discussed in the light of recent suggestions that marine isoprene emissions could contribute significantly to the sub-micron OC fraction of marine aerosol in the tropics. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, May 7, 2012 </font> | ||
Christa Hasenkopf | Christa Hasenkopf | ||
| Line 1,639: | Line 5,516: | ||
and outreach components of this project. | and outreach components of this project. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 30, 2012 </font> | ||
Gregory Schill | Gregory Schill | ||
| Line 1,649: | Line 5,527: | ||
Depositional ice nucleation on atmospheric aerosols has been identified as a potential pathway for cirrus cloud formation. Upper tropospheric aerosols as well as sub-visible cirrus residues are known to be enhanced in both sulfates and organics. While ammonium sulfate is known to be an efficient depositional ice nucleus, the role of organics remains largely uncertain. To explore this role, we used a Knudsen cell flow reactor and Raman microscopy to study the ice nucleation efficacy of crystalline organic films and ammonium sulfate particles coated by liquid or glassy organics respectively. In the Knudsen cell studies, it was found that the ice nucleation efficiency of a series of monocarboxylic acids films was dependent on their O:C ratio. For the Raman experiments, we find that if the organic coatings are liquid, water vapor diffuses through the shell and ice nucleates on the ammonium sulfate core. Here, the organics minimally affect the ice nucleation efficiency of ammonium sulfate. In contrast, if the coatings become glassy, ice instead nucleates on the organic shell. These glassy coatings can alter the ice nucleation efficiency of ammonium sulfate. | Depositional ice nucleation on atmospheric aerosols has been identified as a potential pathway for cirrus cloud formation. Upper tropospheric aerosols as well as sub-visible cirrus residues are known to be enhanced in both sulfates and organics. While ammonium sulfate is known to be an efficient depositional ice nucleus, the role of organics remains largely uncertain. To explore this role, we used a Knudsen cell flow reactor and Raman microscopy to study the ice nucleation efficacy of crystalline organic films and ammonium sulfate particles coated by liquid or glassy organics respectively. In the Knudsen cell studies, it was found that the ice nucleation efficiency of a series of monocarboxylic acids films was dependent on their O:C ratio. For the Raman experiments, we find that if the organic coatings are liquid, water vapor diffuses through the shell and ice nucleates on the ammonium sulfate core. Here, the organics minimally affect the ice nucleation efficiency of ammonium sulfate. In contrast, if the coatings become glassy, ice instead nucleates on the organic shell. These glassy coatings can alter the ice nucleation efficiency of ammonium sulfate. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 23, 2012 </font> | ||
<b>Known and Unexplored Organic Constituents in the Earth’s Atmosphere: Instrument Development to Enhance Exploration</b> | <b>Known and Unexplored Organic Constituents in the Earth’s Atmosphere: Instrument Development to Enhance Exploration</b> | ||
| Line 1,660: | Line 5,539: | ||
The complex chemical composition of atmospheric aerosols, particularly the organic carbon portion, presents unique measurement challenges. We developed the Thermal Desorption Aerosol Gas chromatograph (TAG) system for hourly in-situ speciation of a wide range of primary and secondary organic compounds in aerosols. This instrument combines a particle collector with thermal desorption followed by gas chromatography and mass spectrometric detection to provide separation, identification, and quantification of organic constituents at the molecular level. Observed compounds include alkanes, aldehydes, ketones, PAHs, monocarboxylic acids, and many more. The hourly time resolution measurements provided by TAG capture dynamic and frequent changes in aerosol composition. We have incorporated a two-dimensional chromatography (GC×GC) capability into TAG with a time of flight (TOF) MS detector. Two-dimensional chromatography provides two types of compound separation, most typically by volatility and polarity using two columns with different stationary phases connected in series separated by a modulator. The modulator periodically traps analytes eluting from the first column, and injects fractions of this effluent onto the second column in the form of narrow pulses providing additional separation for co-eluting peaks. The approach is especially useful for distinguishing polar compounds that would otherwise be buried in the unresolved complex mixture (UCM). We are developing a semivolatile collection system that allows simultaneous measurement of chemically specific semivolatile organics in the gas and particle phases, enabling in-situ analysis of speciated organic partitioning in the real atmosphere. We have also developed and deployed a combined TAG-AMS (Aerosol Mass Spectrometer) instrument for simultaneous measurements of the total and speciated aerosol composition. This talk will review our recent developments (TAG, 2DTAG, SVTAG, TAG-AMS), and present new observations of speciated semi-volatile separations between the gas and particle phases, 2DTAG and TAG-AMS observations in ambient air and controlled chamber source oxidation studies. New experiments using soft ionization techniques to more fully separate the UCM and to identify more of the organic species in aerosols will also be presented. | The complex chemical composition of atmospheric aerosols, particularly the organic carbon portion, presents unique measurement challenges. We developed the Thermal Desorption Aerosol Gas chromatograph (TAG) system for hourly in-situ speciation of a wide range of primary and secondary organic compounds in aerosols. This instrument combines a particle collector with thermal desorption followed by gas chromatography and mass spectrometric detection to provide separation, identification, and quantification of organic constituents at the molecular level. Observed compounds include alkanes, aldehydes, ketones, PAHs, monocarboxylic acids, and many more. The hourly time resolution measurements provided by TAG capture dynamic and frequent changes in aerosol composition. We have incorporated a two-dimensional chromatography (GC×GC) capability into TAG with a time of flight (TOF) MS detector. Two-dimensional chromatography provides two types of compound separation, most typically by volatility and polarity using two columns with different stationary phases connected in series separated by a modulator. The modulator periodically traps analytes eluting from the first column, and injects fractions of this effluent onto the second column in the form of narrow pulses providing additional separation for co-eluting peaks. The approach is especially useful for distinguishing polar compounds that would otherwise be buried in the unresolved complex mixture (UCM). We are developing a semivolatile collection system that allows simultaneous measurement of chemically specific semivolatile organics in the gas and particle phases, enabling in-situ analysis of speciated organic partitioning in the real atmosphere. We have also developed and deployed a combined TAG-AMS (Aerosol Mass Spectrometer) instrument for simultaneous measurements of the total and speciated aerosol composition. This talk will review our recent developments (TAG, 2DTAG, SVTAG, TAG-AMS), and present new observations of speciated semi-volatile separations between the gas and particle phases, 2DTAG and TAG-AMS observations in ambient air and controlled chamber source oxidation studies. New experiments using soft ionization techniques to more fully separate the UCM and to identify more of the organic species in aerosols will also be presented. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 9, 2012 </font> | ||
Sunil Baidar | Sunil Baidar | ||
| Line 1,672: | Line 5,552: | ||
Here we describe the CU AMAX-DOAS instrument, give an overview of the profile retrieval algorithm, and present some results from the CalNex and CARES campaigns. Comparison of measured NO2 vertical columns with CMAQ and WRF-Chem model simulation results will also be presented. Comparisons show strong evidence for decreasing NOx emissions to be widespread in California. | Here we describe the CU AMAX-DOAS instrument, give an overview of the profile retrieval algorithm, and present some results from the CalNex and CARES campaigns. Comparison of measured NO2 vertical columns with CMAQ and WRF-Chem model simulation results will also be presented. Comparisons show strong evidence for decreasing NOx emissions to be widespread in California. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 2, 2012 </font> | ||
Lia Rebits | Lia Rebits | ||
| Line 1,682: | Line 5,563: | ||
Despite the drastic decline in tuberculosis-related mortality due to the development of a vaccine and the discovery of effective anti-mycobacterial antibiotics during the middle part of the twentieth century, there has been a resurgence of the disease since the 1980’s primarily due to the advent of HIV/AIDS and drug-resistant Mycobacterium tuberculosis strains. The need for improved diagnostic tests that are capable of greater specificity, sensitivity, and rapidity than the current tests is apparent and such tests would contribute significantly to the number of lives saved annually. A DNA aptamer-based biosensor has the potential to meet these challenges; aptamers have proven to have similar dissociation constants to antibodies but provide additional benefits such as increased stability, ease of synthesis and a greater amenability to labeling. The SELEX process will be used to selectively evolve DNA aptamers that bind specifically to antigens particular to Mycobacterium tuberculosis. Aptamers discovered in this way may be used to selectively remove the mycobacterium or antigen from a biological matrix, as well as serve as the detection molecule via conjugation with a reporter in a similar fashion as that of a sandwich-ELISA test. | Despite the drastic decline in tuberculosis-related mortality due to the development of a vaccine and the discovery of effective anti-mycobacterial antibiotics during the middle part of the twentieth century, there has been a resurgence of the disease since the 1980’s primarily due to the advent of HIV/AIDS and drug-resistant Mycobacterium tuberculosis strains. The need for improved diagnostic tests that are capable of greater specificity, sensitivity, and rapidity than the current tests is apparent and such tests would contribute significantly to the number of lives saved annually. A DNA aptamer-based biosensor has the potential to meet these challenges; aptamers have proven to have similar dissociation constants to antibodies but provide additional benefits such as increased stability, ease of synthesis and a greater amenability to labeling. The SELEX process will be used to selectively evolve DNA aptamers that bind specifically to antigens particular to Mycobacterium tuberculosis. Aptamers discovered in this way may be used to selectively remove the mycobacterium or antigen from a biological matrix, as well as serve as the detection molecule via conjugation with a reporter in a similar fashion as that of a sandwich-ELISA test. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 5, 2012 </font> | ||
Sarah Brooks | Sarah Brooks | ||
| Line 1,693: | Line 5,575: | ||
Emissions of biogenic volatile organic compounds (VOCs) lead to formation of secondary organic aerosol, contributing to a major fraction of the global tropospheric aerosol loading. The extent to which VOC oxidation products condense onto preexisting primary aerosols and modify their cloud-nucleating properties, however, remain highly uncertain. In chamber studies we show that water-soluble organic acids produced from the reaction between α-pinene and ozone rapidly accumulate onto preexisting soot particles. In less than 30 minutes, initially hydrophobic aerosols are converted to aerosols containing a mass fraction of 80-90% organics which activate efficiently as cloud condensation nuclei under representative atmospheric conditions. Our results imply that the microphysical properties of aerosols present in continental air masses are largely controlled by the composition and thickness of coatings (~30 nm in 30 minutes) formed during aerosol aging processes, rather than by the size or composition of original primary particles. In addition, chemical changes in aerosols impact their ice nucleating ability. Our results suggest that atmospheric models underestimate the impacts of chemical transformations of aerosols leading to inaccurate predictions of the indirect effects of aerosols on climate. | Emissions of biogenic volatile organic compounds (VOCs) lead to formation of secondary organic aerosol, contributing to a major fraction of the global tropospheric aerosol loading. The extent to which VOC oxidation products condense onto preexisting primary aerosols and modify their cloud-nucleating properties, however, remain highly uncertain. In chamber studies we show that water-soluble organic acids produced from the reaction between α-pinene and ozone rapidly accumulate onto preexisting soot particles. In less than 30 minutes, initially hydrophobic aerosols are converted to aerosols containing a mass fraction of 80-90% organics which activate efficiently as cloud condensation nuclei under representative atmospheric conditions. Our results imply that the microphysical properties of aerosols present in continental air masses are largely controlled by the composition and thickness of coatings (~30 nm in 30 minutes) formed during aerosol aging processes, rather than by the size or composition of original primary particles. In addition, chemical changes in aerosols impact their ice nucleating ability. Our results suggest that atmospheric models underestimate the impacts of chemical transformations of aerosols leading to inaccurate predictions of the indirect effects of aerosols on climate. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Feb. 20, 2012 </font> | ||
Informal Seminar by Anna Wonaschuetz, University of Arizona (Group of Armin Sooroshian) | Informal Seminar by Anna Wonaschuetz, University of Arizona (Group of Armin Sooroshian) | ||
| Line 1,714: | Line 5,597: | ||
of Southern Arizona. | of Southern Arizona. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Feb. 13, 2012 </font> | ||
Susan Doughty, Ph.D., J.D. | Susan Doughty, Ph.D., J.D. | ||
| Line 1,728: | Line 5,612: | ||
It is important for everyone to have an awareness of Intellectual Property (IP) since the legal and financial stakes for missteps are potentially serious and costly. A summary of the types of Intellectual Property protection available as well as a case-study on the intersection of IP with University researchers will be provided. | It is important for everyone to have an awareness of Intellectual Property (IP) since the legal and financial stakes for missteps are potentially serious and costly. A summary of the types of Intellectual Property protection available as well as a case-study on the intersection of IP with University researchers will be provided. | ||
| − | = | + | <br /> |
| + | <font size="4"> Tuesday, Jan. 17, 2012 </font> | ||
[http://www.cmu.edu/me/people/allen-robinson.html Prof. Allen L. Robinson] | [http://www.cmu.edu/me/people/allen-robinson.html Prof. Allen L. Robinson] | ||
| Line 1,740: | Line 5,625: | ||
Allen Robinson: Dr. Allen Robinson is a Professor in the Departments of Mechanical Engineering and Engineering and Public Policy at Carnegie Mellon University. His research examines the impact of emissions from combustion systems on urban, regional and global air quality. He received his Ph.D. from the University of California at Berkeley in Mechanical Engineering in 1996 and his B.S. in Civil Engineering from Stanford University in 1990. He was awarded the Carnegie Institute of Technology Outstanding Research Award in 2010, the Ahrens Career Development Chair in Mechanical Engineering in 2005 and the George Tallman Ladd Outstanding Young Faculty Award in 2000. | Allen Robinson: Dr. Allen Robinson is a Professor in the Departments of Mechanical Engineering and Engineering and Public Policy at Carnegie Mellon University. His research examines the impact of emissions from combustion systems on urban, regional and global air quality. He received his Ph.D. from the University of California at Berkeley in Mechanical Engineering in 1996 and his B.S. in Civil Engineering from Stanford University in 1990. He was awarded the Carnegie Institute of Technology Outstanding Research Award in 2010, the Ahrens Career Development Chair in Mechanical Engineering in 2005 and the George Tallman Ladd Outstanding Young Faculty Award in 2000. | ||
| − | = | + | <br /> |
| + | <font size="4"> Friday, Jan. 13, 2012 </font> | ||
[http://chem.ucr.edu/index.php?main=faculty&facsort=profile&faculty=ziemann Prof. Paul Ziemann], University of California-Riverside | [http://chem.ucr.edu/index.php?main=faculty&facsort=profile&faculty=ziemann Prof. Paul Ziemann], University of California-Riverside | ||
| Line 1,748: | Line 5,634: | ||
Much of what is currently understood about the chemical and physical processes involved in the atmospheric oxidation of organic gases and particles and the formation of secondary organic aerosol (SOA) has been obtained from experiments carried out in environmental (smog) chambers. Achieving a level of understanding from such studies that is sufficient for extrapolating results to the atmosphere and for developing accurate models of SOA formation requires the acquisition of detailed information on a variety of gas and particle properties and processes, such as gas and particle phase reaction kinetics, gas and particle composition, particle phase, compound vapor pressures, and gas-particle-wall interactions. Obtaining such data is a challenge, but in this talk I will show results of studies carried out in our laboratory on the OH radical-initiated reactions of alkanes that are approaching this goal. I will demonstrate that by using a diverse array of measurement techniques including mass spectrometry, gas and liquid chromatography, spectrophotometry (with and without compound derivatization), traditional elemental analysis, pycnometry, temperature-programmed thermal desorption, and scanning mobility particle sizing it is possible to develop quantitative chemical reaction mechanisms of organic gas and aerosol chemistry and models of SOA formation. Doing so, however, requires a more comprehensive approach to chamber studies that attempts to account more fully for the effects of walls. | Much of what is currently understood about the chemical and physical processes involved in the atmospheric oxidation of organic gases and particles and the formation of secondary organic aerosol (SOA) has been obtained from experiments carried out in environmental (smog) chambers. Achieving a level of understanding from such studies that is sufficient for extrapolating results to the atmosphere and for developing accurate models of SOA formation requires the acquisition of detailed information on a variety of gas and particle properties and processes, such as gas and particle phase reaction kinetics, gas and particle composition, particle phase, compound vapor pressures, and gas-particle-wall interactions. Obtaining such data is a challenge, but in this talk I will show results of studies carried out in our laboratory on the OH radical-initiated reactions of alkanes that are approaching this goal. I will demonstrate that by using a diverse array of measurement techniques including mass spectrometry, gas and liquid chromatography, spectrophotometry (with and without compound derivatization), traditional elemental analysis, pycnometry, temperature-programmed thermal desorption, and scanning mobility particle sizing it is possible to develop quantitative chemical reaction mechanisms of organic gas and aerosol chemistry and models of SOA formation. Doing so, however, requires a more comprehensive approach to chamber studies that attempts to account more fully for the effects of walls. | ||
| − | == Monday, Nov. 28, 2011 | + | ==Fall 2011== |
| + | <br /> | ||
| + | <font size="4"> Monday, Nov. 28, 2011 </font> | ||
Reddy Yatavelli and Harald Stark | Reddy Yatavelli and Harald Stark | ||
| Line 1,758: | Line 5,646: | ||
Organic acids are important constituents of aerosol particles. We will present preliminary analysis of organic acids in gas and aerosol particles measured in a ponderosa pine forest during July - August 2011 as part of the BEACHON-RoMBAS field campaign. We will also introduce the new measurement technique, based on chemical ionization, high-resolution time-of-flight mass spectrometry, utilizing a micro-orifice volatilization impactor [MOVI-ToF-CIMS], that was used to collect this data. Acetate anion (CH3C(O)O-, 59 Th) was chosen as the reagent ion for detection of organic acids. The high-resolution mass spectra, in combination with the temperature-dependent desorption information, can be used to identify specific compounds and estimate their gas/particle partitioning | Organic acids are important constituents of aerosol particles. We will present preliminary analysis of organic acids in gas and aerosol particles measured in a ponderosa pine forest during July - August 2011 as part of the BEACHON-RoMBAS field campaign. We will also introduce the new measurement technique, based on chemical ionization, high-resolution time-of-flight mass spectrometry, utilizing a micro-orifice volatilization impactor [MOVI-ToF-CIMS], that was used to collect this data. Acetate anion (CH3C(O)O-, 59 Th) was chosen as the reagent ion for detection of organic acids. The high-resolution mass spectra, in combination with the temperature-dependent desorption information, can be used to identify specific compounds and estimate their gas/particle partitioning | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Nov. 14, 2011 </font> | ||
Juliane L. Fry | Juliane L. Fry | ||
| Line 1,770: | Line 5,659: | ||
Biogenic hydrocarbons, such as isoprene (C5), monoterpenes (C10) and sesquiterpenes (C15), react rapidly with nitrate radical (NO3) to form low-volatility products. We hypothesize therefore that nitrate oxidation may be an important source of secondary organic aerosol in pollution-impacted forest environments. In this talk I will present new data from the BEACHON-RoMBAS field campaign at the Manitou Forest Observatory in the Colorado front range (July/August 2011) relevant to this hypothesis. I will also describe a set of experiments underway as a collaborative project with NCAR’s Atmospheric Chemistry Division and NOAA’s Chemical Sciences Division to explore nitrate-initiated aerosol formation in laboratory chamber and flow tube experiments. | Biogenic hydrocarbons, such as isoprene (C5), monoterpenes (C10) and sesquiterpenes (C15), react rapidly with nitrate radical (NO3) to form low-volatility products. We hypothesize therefore that nitrate oxidation may be an important source of secondary organic aerosol in pollution-impacted forest environments. In this talk I will present new data from the BEACHON-RoMBAS field campaign at the Manitou Forest Observatory in the Colorado front range (July/August 2011) relevant to this hypothesis. I will also describe a set of experiments underway as a collaborative project with NCAR’s Atmospheric Chemistry Division and NOAA’s Chemical Sciences Division to explore nitrate-initiated aerosol formation in laboratory chamber and flow tube experiments. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Nov. 7, 2011 </font> | ||
Nick, Wagner | Nick, Wagner | ||
| Line 1,782: | Line 5,672: | ||
Nighttime chemistry accounts for up to half of the NOx removal from the atmosphere and produces atomic chlorine in the early morning through photolysis of ClNO2. NOx is removed through the production and heterogeneous loss of N2O5. The heterogeneous loss of N2O5 typically produces nitric acid which is removed through deposition. However, it has recently been discovered that in the presence of aerosol chloride, the N2O5 uptake produces ClNO2. The rate N2O5 heterogeneous loss been the subject of several laboratory studies; however, it has rarely been studied using ambient measurements. In this work, N2O5 uptake coefficients are determined from ambient wintertime measurements at the BAO tower in Erie, CO. The uptake coefficients are determined using an iterative box model. These uptake coefficients were found to be anti-correlated with the nitrate fraction of aerosol confirming suppression of uptake by aerosol nitrate. Additionally, a plume of chloride was observed in measurements of aerosol chloride, HCl and ClNO2. The N2O5 uptake coefficient was enhanced in this plume due to the competition between aerosol chloride and nitrate to react with N2O5. | Nighttime chemistry accounts for up to half of the NOx removal from the atmosphere and produces atomic chlorine in the early morning through photolysis of ClNO2. NOx is removed through the production and heterogeneous loss of N2O5. The heterogeneous loss of N2O5 typically produces nitric acid which is removed through deposition. However, it has recently been discovered that in the presence of aerosol chloride, the N2O5 uptake produces ClNO2. The rate N2O5 heterogeneous loss been the subject of several laboratory studies; however, it has rarely been studied using ambient measurements. In this work, N2O5 uptake coefficients are determined from ambient wintertime measurements at the BAO tower in Erie, CO. The uptake coefficients are determined using an iterative box model. These uptake coefficients were found to be anti-correlated with the nitrate fraction of aerosol confirming suppression of uptake by aerosol nitrate. Additionally, a plume of chloride was observed in measurements of aerosol chloride, HCl and ClNO2. The N2O5 uptake coefficient was enhanced in this plume due to the competition between aerosol chloride and nitrate to react with N2O5. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Oct. 17, 2011 </font> | ||
Ryan Davis | Ryan Davis | ||
| Line 1,800: | Line 5,691: | ||
Industrial processes and combustion sources release aerosols into the atmosphere that often contain components that are harmful to human health and the environment. In order to understand the sources and dynamics of these aerosol emissions, single-particle mass spectra of ambient aerosols were collected in Milwaukee, Wisconsin using ATOFMS during the summer of 2010. The ATOFMS data provided temporal trends of particulate metals, which can be correlated with wind direction and local source information to pinpoint their origins. Additional insight was gained into the particle emissions and dynamics in the local area by looking at the temporal behavior of particle classes. | Industrial processes and combustion sources release aerosols into the atmosphere that often contain components that are harmful to human health and the environment. In order to understand the sources and dynamics of these aerosol emissions, single-particle mass spectra of ambient aerosols were collected in Milwaukee, Wisconsin using ATOFMS during the summer of 2010. The ATOFMS data provided temporal trends of particulate metals, which can be correlated with wind direction and local source information to pinpoint their origins. Additional insight was gained into the particle emissions and dynamics in the local area by looking at the temporal behavior of particle classes. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Oct. 3, 2011 </font> | ||
Margaret A. Tolbert | Margaret A. Tolbert | ||
| Line 1,818: | Line 5,710: | ||
The Volkamer group develops optical spectroscopic instruments for use in laboratory experiments, and field studies to measure reactive trace gases and radicals that are relevant to tropospheric ozone, mercury cyling, and the formation of secondary organic aerosol (SOA). This talk gives an overview about group activities in the recent past and near future. The TORERO field experiment will study the Tropical Ocean Troposphere Exchange of Reactive halogen species and Oxygenated VOC by means of a comprehensive chemical payload of instruments centered around the CU Airborne Multi-AXis DOAS instrument aboard the NSF/NCAR GV aircraft in the area of Gallapagos Islands. It combines aircraft, ship and island based measurements, to probe chemical gases that form by destroying heat trapping ozone and can form new aerosols that cool climate. The group also participates in the Ganges Valley Aerosol Experiment (GVAX) in India, deploying the innovative CU Cavity Enhanced DOAS instrument, and a novel 2D scanning ground-based MAX-DOAS instrument. Future applications at the planned simulation chamber facility as part of the Center for Atmospheric Chemistry are briefly discussed. | The Volkamer group develops optical spectroscopic instruments for use in laboratory experiments, and field studies to measure reactive trace gases and radicals that are relevant to tropospheric ozone, mercury cyling, and the formation of secondary organic aerosol (SOA). This talk gives an overview about group activities in the recent past and near future. The TORERO field experiment will study the Tropical Ocean Troposphere Exchange of Reactive halogen species and Oxygenated VOC by means of a comprehensive chemical payload of instruments centered around the CU Airborne Multi-AXis DOAS instrument aboard the NSF/NCAR GV aircraft in the area of Gallapagos Islands. It combines aircraft, ship and island based measurements, to probe chemical gases that form by destroying heat trapping ozone and can form new aerosols that cool climate. The group also participates in the Ganges Valley Aerosol Experiment (GVAX) in India, deploying the innovative CU Cavity Enhanced DOAS instrument, and a novel 2D scanning ground-based MAX-DOAS instrument. Future applications at the planned simulation chamber facility as part of the Center for Atmospheric Chemistry are briefly discussed. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, Sep. 19, 2011 </font> | ||
Bob Sievers | Bob Sievers | ||
| Line 1,836: | Line 5,729: | ||
Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (emitted in the particle phase) and secondary OA (formed from chemical reactions of gas-phase species). In this talk I will summarize research in OA by our group over the last year, including a summary of the recent CalNex-LA and BEACHON-RoMBAS field projects in Pasadena (CA) and Woodland Springs (CO) respectively, direct measurements of the SOA formation and photochemical evolution of ambient air and collaborative work with modelers to constrain the OA budget. I will also discuss our new MOVI-CIMS instrument which allows molecular-level analysis of OA with high sensitivity and mass resolution and some first results from BEACHON-RoMBAS. Finally, I will introduce future field projects in India, Thailand, Southeast US, and the Amazon, and the outlook for the new smog chamber facility in Cristol Bldg. | Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (emitted in the particle phase) and secondary OA (formed from chemical reactions of gas-phase species). In this talk I will summarize research in OA by our group over the last year, including a summary of the recent CalNex-LA and BEACHON-RoMBAS field projects in Pasadena (CA) and Woodland Springs (CO) respectively, direct measurements of the SOA formation and photochemical evolution of ambient air and collaborative work with modelers to constrain the OA budget. I will also discuss our new MOVI-CIMS instrument which allows molecular-level analysis of OA with high sensitivity and mass resolution and some first results from BEACHON-RoMBAS. Finally, I will introduce future field projects in India, Thailand, Southeast US, and the Amazon, and the outlook for the new smog chamber facility in Cristol Bldg. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, August 29, 2011 </font> | ||
Thomas J. Wenzel | Thomas J. Wenzel | ||
| Line 1,847: | Line 5,741: | ||
The use of crown ethers, calix[4]resorcinarenes and ionic cyclodextrin derivatives as enantioselective NMR shift reagents will be described. The cyclodextrins and calix[4]resorcinarenes are unusual because they are water-soluble reagents. A crown ether system that is useful for the analysis of secondary amines will be described. The beneficial learning that occurs when undergraduates conduct research was a major factor in causing me to restructure the way I teach my courses. The use of collaborative in-class learning activities and semester-long laboratory projects will be described, as will efforts to enact similar curricular changes at a consortium of over 20 schools. | The use of crown ethers, calix[4]resorcinarenes and ionic cyclodextrin derivatives as enantioselective NMR shift reagents will be described. The cyclodextrins and calix[4]resorcinarenes are unusual because they are water-soluble reagents. A crown ether system that is useful for the analysis of secondary amines will be described. The beneficial learning that occurs when undergraduates conduct research was a major factor in causing me to restructure the way I teach my courses. The use of collaborative in-class learning activities and semester-long laboratory projects will be described, as will efforts to enact similar curricular changes at a consortium of over 20 schools. | ||
| − | = | + | <br /> |
| + | <font size="4"> Friday, August 19, 2011 </font> | ||
<b>SPECIAL SEMINAR</b> | <b>SPECIAL SEMINAR</b> | ||
| Line 1,859: | Line 5,754: | ||
Alzheimer’s disease (AD) is the result of a progressive aggregation of ß-amyloid peptide (ß‐AP) leading to the formation of extracellular fibrillar that deposits in selected areas of the brain. Polyphenols are efficient fibril inhibitors in AD by destabilizing beta‐amyloid fibrils. Our research aims to develop a drug carrier system that targets the brain via the nasal route to improve the treatment of Alzheimer’s disease (AD) by formulating well‐defined micron‐sized particles (10‐50µm) using supercritical carbon dioxide technology. Chitosan is a natural polymer that has a great potential for nasal delivery system facilitating the passage of hydrophilic molecules through the nasal mucosa. The technique of choice is the Carbon Dioxide Assisted Nebulization with a Bubble Dryer® (CAN-BD) since chitosan is soluble in water that would facilitate the micronization of oleuropein into chitosan. | Alzheimer’s disease (AD) is the result of a progressive aggregation of ß-amyloid peptide (ß‐AP) leading to the formation of extracellular fibrillar that deposits in selected areas of the brain. Polyphenols are efficient fibril inhibitors in AD by destabilizing beta‐amyloid fibrils. Our research aims to develop a drug carrier system that targets the brain via the nasal route to improve the treatment of Alzheimer’s disease (AD) by formulating well‐defined micron‐sized particles (10‐50µm) using supercritical carbon dioxide technology. Chitosan is a natural polymer that has a great potential for nasal delivery system facilitating the passage of hydrophilic molecules through the nasal mucosa. The technique of choice is the Carbon Dioxide Assisted Nebulization with a Bubble Dryer® (CAN-BD) since chitosan is soluble in water that would facilitate the micronization of oleuropein into chitosan. | ||
| − | == Monday, May 23, 2011 | + | ==Spring 2011== |
| + | <br /> | ||
| + | <font size="4">Monday, May 23, 2011</font> | ||
Ph.D. Defense, Daniel Bon, CIRES Aud., 23-May-11, 2 pm | Ph.D. Defense, Daniel Bon, CIRES Aud., 23-May-11, 2 pm | ||
| Line 1,875: | Line 5,772: | ||
PIT-MS instrument data from the TexAQS 2006 campaign in Houston, Texas are used to evaluate an industrial emissions inventory. The complexity of measured VOCs in industrial plumes is significantly larger than described in the inventory. For many VOCs, emission fluxes inferred from the measurements are significantly higher than in the inventory. | PIT-MS instrument data from the TexAQS 2006 campaign in Houston, Texas are used to evaluate an industrial emissions inventory. The complexity of measured VOCs in industrial plumes is significantly larger than described in the inventory. For many VOCs, emission fluxes inferred from the measurements are significantly higher than in the inventory. | ||
| − | = | + | <br /> |
| + | <font size="4">Thursday, May 12, 2011</font> | ||
[http://www.chem.utoronto.ca/staff/ABBATT/home.htm Prof. Jon Abbatt], Univ. of Toronto | [http://www.chem.utoronto.ca/staff/ABBATT/home.htm Prof. Jon Abbatt], Univ. of Toronto | ||
| Line 1,883: | Line 5,781: | ||
Abstract: Although the kinetics and mechanisms of gas phase reactions in the atmosphere are relatively well defined, the processes that occur via aerosol particles are much less well understood. In particular, a general issue persists of the degree to which condensed phase material in the atmosphere is subject to oxidative processes. Such multiphase chemistry may either occur at the surfaces of particles or films in the atmosphere, or in the bulk phase. This seminar will present three examples of multiphase chemistry, moving from a system for which we feel we understand the kinetics well, i.e. bromide oxidation by ozone in ice and water, to progressively less well understood systems, i.e. PAH heterogeneous reactions and the oxidation of SOA materials by hydroxyl radicals. In the case of SOA oxidation, a combination of experiments are presented using both laboratory surrogates for atmospheric SOA, and experiments with field samples. The potential atmospheric impacts of this work will be presented. | Abstract: Although the kinetics and mechanisms of gas phase reactions in the atmosphere are relatively well defined, the processes that occur via aerosol particles are much less well understood. In particular, a general issue persists of the degree to which condensed phase material in the atmosphere is subject to oxidative processes. Such multiphase chemistry may either occur at the surfaces of particles or films in the atmosphere, or in the bulk phase. This seminar will present three examples of multiphase chemistry, moving from a system for which we feel we understand the kinetics well, i.e. bromide oxidation by ozone in ice and water, to progressively less well understood systems, i.e. PAH heterogeneous reactions and the oxidation of SOA materials by hydroxyl radicals. In the case of SOA oxidation, a combination of experiments are presented using both laboratory surrogates for atmospheric SOA, and experiments with field samples. The potential atmospheric impacts of this work will be presented. | ||
| − | = | + | <br /> |
| + | <font size="4"> Thursday, April 28, 2011 </font> | ||
Thesis defense for Ingrid Ulbrich | Thesis defense for Ingrid Ulbrich | ||
| Line 1,896: | Line 5,795: | ||
Atmospheric aerosol has impacts on health, visibility, ecosystems, and climate. The organic component of submicron aerosol is a complex mixture of tens of thousands of compounds, and it is still challenging to quantify the direct sources of organic aerosol. Organic aerosol can also form from a variety of secondary reactions in the atmosphere, which are poorly understood. Real-time instrumental techniques, including the Aerosol Mass Spectrometer (AMS), which can quantitatively measure aerosol composition with high time and size resolution, and some chemical resolution, produce large volumes of data that contain rich information about aerosol sources and processes. This thesis work seeks to extract the underlying information that describes organic aerosol sources and processes by applying factor analytical techniques to organic aerosol datasets from the AMS. We have developed a custom, open-source software tool to compare factorization solutions, their residuals, and tracer-factor correlations. The application of existing mathematical techniques to these new datasets requires careful characterization of the precision in the data and the factorization models' behavior with these specialized datasets. We explore this behavior with synthetic datasets modeled on AMS data. The synthetic data factorization has predictable behaviors when solved with "too many" factors. These behaviors then guide the choice of solution for real aerosol datasets. The factor analyses of real aerosol datasets are useful for identifying aerosol types related to sources (e.g., urban combustion and biomass burning) and secondary atmospheric processes (e.g., semivolatile and low-volatility oxidized organic aerosol). We have also factored three-dimensional datasets of size-resolved aerosol composition data to explore the variability of aerosol size distributions as the aerosol undergoes processing in an urban atmosphere. This study provides evidence that primary particles are coated with condensed secondary aerosol during photochemical processing, shifting the size distribution of the primary particles to larger sizes. Application of these three-dimensional factorization techniques to other complex aerosol composition datasets (e.g., that use thermal desorption or chromatography for further chemical separation) has the potential to yield additional insights about aerosol sources and processes. | Atmospheric aerosol has impacts on health, visibility, ecosystems, and climate. The organic component of submicron aerosol is a complex mixture of tens of thousands of compounds, and it is still challenging to quantify the direct sources of organic aerosol. Organic aerosol can also form from a variety of secondary reactions in the atmosphere, which are poorly understood. Real-time instrumental techniques, including the Aerosol Mass Spectrometer (AMS), which can quantitatively measure aerosol composition with high time and size resolution, and some chemical resolution, produce large volumes of data that contain rich information about aerosol sources and processes. This thesis work seeks to extract the underlying information that describes organic aerosol sources and processes by applying factor analytical techniques to organic aerosol datasets from the AMS. We have developed a custom, open-source software tool to compare factorization solutions, their residuals, and tracer-factor correlations. The application of existing mathematical techniques to these new datasets requires careful characterization of the precision in the data and the factorization models' behavior with these specialized datasets. We explore this behavior with synthetic datasets modeled on AMS data. The synthetic data factorization has predictable behaviors when solved with "too many" factors. These behaviors then guide the choice of solution for real aerosol datasets. The factor analyses of real aerosol datasets are useful for identifying aerosol types related to sources (e.g., urban combustion and biomass burning) and secondary atmospheric processes (e.g., semivolatile and low-volatility oxidized organic aerosol). We have also factored three-dimensional datasets of size-resolved aerosol composition data to explore the variability of aerosol size distributions as the aerosol undergoes processing in an urban atmosphere. This study provides evidence that primary particles are coated with condensed secondary aerosol during photochemical processing, shifting the size distribution of the primary particles to larger sizes. Application of these three-dimensional factorization techniques to other complex aerosol composition datasets (e.g., that use thermal desorption or chromatography for further chemical separation) has the potential to yield additional insights about aerosol sources and processes. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 25, 2011 </font> | ||
Frank N Keutsch | Frank N Keutsch | ||
| Line 1,910: | Line 5,810: | ||
We have developed robust instrumentation for the ultra-sensitive measurement of BVOC oxidation products as tracers of BVOC oxidation by combining spectroscopic techniques that are new for field instrumentation with novel lasers. We propose that glyoxal is ideally suited as tracer of OH-driven BVOC oxidation and present rural measurements of glyoxal. We discuss these measurements within the context of the discrepancy between modeled and measured OH radical concentrations. We also present results from a study using the first formaldehyde flux measurements via eddy correlation obtained during the BEACHON-ROCS 2010 campaign at Manitou Forest. Different scenarios that can explain the unexpectedly large daytime fluxes of formaldehyde out of the forest canopy are discussed with respect to implications for the proposed unknown, highly-reactive BVOCs | We have developed robust instrumentation for the ultra-sensitive measurement of BVOC oxidation products as tracers of BVOC oxidation by combining spectroscopic techniques that are new for field instrumentation with novel lasers. We propose that glyoxal is ideally suited as tracer of OH-driven BVOC oxidation and present rural measurements of glyoxal. We discuss these measurements within the context of the discrepancy between modeled and measured OH radical concentrations. We also present results from a study using the first formaldehyde flux measurements via eddy correlation obtained during the BEACHON-ROCS 2010 campaign at Manitou Forest. Different scenarios that can explain the unexpectedly large daytime fluxes of formaldehyde out of the forest canopy are discussed with respect to implications for the proposed unknown, highly-reactive BVOCs | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 18, 2011 </font> | ||
Prof. Jesse H. Kroll | Prof. Jesse H. Kroll | ||
| Line 1,924: | Line 5,825: | ||
The multigenerational oxidative processing (“aging”) of atmospheric organic aerosol is poorly understood, despite its likely influence on particle loadings and properties. In these experiments, n-alkanes are exposed to elevated levels of OH, in order to access the equivalent of several days’ worth of atmospheric oxidation. Measurements of the abundance and oxidation state of particulate organic carbon demonstrate the important role of fragmentation (C-C bond-breaking) reactions as oxidation progresses. Such reactions are highly efficient pathways for the formation of oxidized carbon; however, the corresponding changes to carbon number also tend to offset any decreases in volatility from the addition of functional groups. Thus particulate organics have increasingly smaller carbon numbers as they become more oxidized, with the continual escape of some fraction of the carbon to the gas phase during aging. | The multigenerational oxidative processing (“aging”) of atmospheric organic aerosol is poorly understood, despite its likely influence on particle loadings and properties. In these experiments, n-alkanes are exposed to elevated levels of OH, in order to access the equivalent of several days’ worth of atmospheric oxidation. Measurements of the abundance and oxidation state of particulate organic carbon demonstrate the important role of fragmentation (C-C bond-breaking) reactions as oxidation progresses. Such reactions are highly efficient pathways for the formation of oxidized carbon; however, the corresponding changes to carbon number also tend to offset any decreases in volatility from the addition of functional groups. Thus particulate organics have increasingly smaller carbon numbers as they become more oxidized, with the continual escape of some fraction of the carbon to the gas phase during aging. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 11, 2011 </font> | ||
J’aime Manion | J’aime Manion | ||
| Line 1,942: | Line 5,844: | ||
In a modern world where the media and fringe groups seem to be touting the supposed dangers of vaccination, the scientific observations remain that many lives are saved and epidemics prevented through the use of vaccines. In an effort to improve the safety of vaccinations in the developing world the Sievers group is currently researching the production of inhaled, needle-free vaccines. This talk will cover the design of a needle-free dry powder HPV vaccine, collaboration with an overseas vaccine producer (Serum Institute of India) in the production of a needle-free Measles vaccine and tests of uniform activity throughout the particle size ranges of the aforementioned vaccine. | In a modern world where the media and fringe groups seem to be touting the supposed dangers of vaccination, the scientific observations remain that many lives are saved and epidemics prevented through the use of vaccines. In an effort to improve the safety of vaccinations in the developing world the Sievers group is currently researching the production of inhaled, needle-free vaccines. This talk will cover the design of a needle-free dry powder HPV vaccine, collaboration with an overseas vaccine producer (Serum Institute of India) in the production of a needle-free Measles vaccine and tests of uniform activity throughout the particle size ranges of the aforementioned vaccine. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, April 4, 2011 </font> | ||
Charles S. Henry | Charles S. Henry | ||
| Line 1,954: | Line 5,857: | ||
Ambient aerosols have significant impacts on both our environment and human health. From an environmental perspective, aerosols are one of the most significant unknowns for predicting the impact of human activity on the earth's climate. From a health perspective, aerosols are known to contribute to a wide range of diseases and cause significant changes in mortality and morbidity, however, the mechanisms for these health effects are not clear. It is clear for both environmental and health effects that knowing chemical composition is important in developing a better understanding of the impact of aerosol. This talk will focus on new microfluidic tools our lab has create to measure the chemical composition of aerosols. Microfluidic devices are attractive as an alternative to traditional aerosol analyzers because they can provide fast measurement times, high sensitivity, are compatible with a range of measurement chemistries, and can be relatively inexpensive. This talk will focus on recent results from our laboratory where microfluidic tools were used to make measurements of key chemical components of aerosols. | Ambient aerosols have significant impacts on both our environment and human health. From an environmental perspective, aerosols are one of the most significant unknowns for predicting the impact of human activity on the earth's climate. From a health perspective, aerosols are known to contribute to a wide range of diseases and cause significant changes in mortality and morbidity, however, the mechanisms for these health effects are not clear. It is clear for both environmental and health effects that knowing chemical composition is important in developing a better understanding of the impact of aerosol. This talk will focus on new microfluidic tools our lab has create to measure the chemical composition of aerosols. Microfluidic devices are attractive as an alternative to traditional aerosol analyzers because they can provide fast measurement times, high sensitivity, are compatible with a range of measurement chemistries, and can be relatively inexpensive. This talk will focus on recent results from our laboratory where microfluidic tools were used to make measurements of key chemical components of aerosols. | ||
| − | = | + | <br /> |
| + | <font size="4"> Monday, March 28, 2011 </font> | ||
Dr. Erika Dawson | Dr. Erika Dawson | ||
| Line 1,966: | Line 5,870: | ||
This talk will describe my experiences at InDevR during the development of our two existing product lines, the ViroCyt Virus Counter and ampliPHOX Colorimetric Detection Technology. Starting with two separate technologies originally developed at CU, InDevR has advanced the original ideas into two unique instruments that are currently in different stages of development. I will share some recent data comparing the Virus Counter to standard virus quantification methods for applications in bioprocessing and vaccine development. I will also describe how ampliPHOX has been utilized to quickly and inexpensively detect and characterize influenza viruses using the FluChip. | This talk will describe my experiences at InDevR during the development of our two existing product lines, the ViroCyt Virus Counter and ampliPHOX Colorimetric Detection Technology. Starting with two separate technologies originally developed at CU, InDevR has advanced the original ideas into two unique instruments that are currently in different stages of development. I will share some recent data comparing the Virus Counter to standard virus quantification methods for applications in bioprocessing and vaccine development. I will also describe how ampliPHOX has been utilized to quickly and inexpensively detect and characterize influenza viruses using the FluChip. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, March 14, 2011</font> | ||
<b>Highly Concentrated Dispersions of Stable Submicron Therapeutic Protein Particles For Subcutaneous Injection</b> | <b>Highly Concentrated Dispersions of Stable Submicron Therapeutic Protein Particles For Subcutaneous Injection</b> | ||
| Line 1,978: | Line 5,883: | ||
Protein and peptide therapeutics address needs in a wide range of diseases including cancer, infectious and inflammatory diseases. Despite their successes, administration of these therapeutics is limited by the lack of efficacious alternatives to intraveneous delivery of the required 100-500 mg doses. Here, we report a novel strategy compatible with subcutaneous injection that preserves IgG activity: aqueous-based, dispersions of submicron particles at 300 mg/ml IgG concentrations with apparent viscosities of below 50 cP. These dispersions may be prepared by generation of IgG particles, formed with thin film freezing (TFF) or a novel rapid spiral wound in-situ freezing technology (SWIFT) that preserve protein stability by limiting the protein’s exposure to liquid/gas and liquid/ ice interfaces. Dispersions of these particles in a solution containing PEG300 and n-methyl-2-pyrrolidone in buffer at the protein isoelectric point restricted protein solubility to <40 mg/ml and prevented complete particle dissolution. The low apparent viscosities of these dispersions result from the low viscosity of the initial solution and the low intrinsic viscosities ([η]). Additionally, antibody stability against denaturation and aggregation (as measured by HPLC SEC and ELISA) was enhanced by low solvation and decreased protein mobility of the solid state. High protein stability and biological activity is maintained upon subcutaneous delivery into mice(Jennifer Maynard laboratory, UT). The ability to form active, highly concentrated dispersions of therapeutic proteins with low viscosities, offers novel opportunities in subcutaneous injection for more effective treatment of disease. | Protein and peptide therapeutics address needs in a wide range of diseases including cancer, infectious and inflammatory diseases. Despite their successes, administration of these therapeutics is limited by the lack of efficacious alternatives to intraveneous delivery of the required 100-500 mg doses. Here, we report a novel strategy compatible with subcutaneous injection that preserves IgG activity: aqueous-based, dispersions of submicron particles at 300 mg/ml IgG concentrations with apparent viscosities of below 50 cP. These dispersions may be prepared by generation of IgG particles, formed with thin film freezing (TFF) or a novel rapid spiral wound in-situ freezing technology (SWIFT) that preserve protein stability by limiting the protein’s exposure to liquid/gas and liquid/ ice interfaces. Dispersions of these particles in a solution containing PEG300 and n-methyl-2-pyrrolidone in buffer at the protein isoelectric point restricted protein solubility to <40 mg/ml and prevented complete particle dissolution. The low apparent viscosities of these dispersions result from the low viscosity of the initial solution and the low intrinsic viscosities ([η]). Additionally, antibody stability against denaturation and aggregation (as measured by HPLC SEC and ELISA) was enhanced by low solvation and decreased protein mobility of the solid state. High protein stability and biological activity is maintained upon subcutaneous delivery into mice(Jennifer Maynard laboratory, UT). The ability to form active, highly concentrated dispersions of therapeutic proteins with low viscosities, offers novel opportunities in subcutaneous injection for more effective treatment of disease. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, March 7, 2011</font> | ||
<b>Optical constants of model SOA formed by cloud processing</b> | <b>Optical constants of model SOA formed by cloud processing</b> | ||
| Line 1,997: | Line 5,903: | ||
The role of heterogeneous chemistry as a source of Secondary Organic Aerosol (SOA) remains difficult to quantify. SOA is the portion of organic aerosol that forms in the atmosphere as a result of atmospheric transformations. Glyoxal is a building block for SOA formation as a result of heterogeneous chemistry. Measurements from the MCMA-2003 campaign in Mexico City showed that the gas phase glyoxal concentrations were lower by a factor of two to three than predicted by the Master Chemical Mechanism, and that this missing gas phase glyoxal was equivalent to the formation of about 5 μg/m3 of organic aerosol mass over the course of eight hours. Numerous recent laboratory studies have found that glyoxal can form significant amounts of SOA due to uptake, by both dark (equilibrium and irreversible) processes and by rapid photochemical uptake to aerosols. These processes have recently been reviewed, and a model framework has been developed based on these laboratory experiments to numerically describe glyoxal processing in aqueous aerosol particles. Here this model is applied to predict SOA formation due to glyoxal processing in Mexico City. We present an initial case study where the model was constrained with measurements from Mexico City during MCMA 2003 in a first attempt to bridge between laboratory and field observations, and an early assessment of our understanding of the processes and parameters that determine the amount of SOA formed from glyoxal. | The role of heterogeneous chemistry as a source of Secondary Organic Aerosol (SOA) remains difficult to quantify. SOA is the portion of organic aerosol that forms in the atmosphere as a result of atmospheric transformations. Glyoxal is a building block for SOA formation as a result of heterogeneous chemistry. Measurements from the MCMA-2003 campaign in Mexico City showed that the gas phase glyoxal concentrations were lower by a factor of two to three than predicted by the Master Chemical Mechanism, and that this missing gas phase glyoxal was equivalent to the formation of about 5 μg/m3 of organic aerosol mass over the course of eight hours. Numerous recent laboratory studies have found that glyoxal can form significant amounts of SOA due to uptake, by both dark (equilibrium and irreversible) processes and by rapid photochemical uptake to aerosols. These processes have recently been reviewed, and a model framework has been developed based on these laboratory experiments to numerically describe glyoxal processing in aqueous aerosol particles. Here this model is applied to predict SOA formation due to glyoxal processing in Mexico City. We present an initial case study where the model was constrained with measurements from Mexico City during MCMA 2003 in a first attempt to bridge between laboratory and field observations, and an early assessment of our understanding of the processes and parameters that determine the amount of SOA formed from glyoxal. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, February 14, 2011</font> | ||
<b>Roles of Subvisible Particles in Protein Aggregation Pathways</b> | <b>Roles of Subvisible Particles in Protein Aggregation Pathways</b> | ||
| Line 2,010: | Line 5,917: | ||
Typically, protein aggregates are arbitrarily divided in subclasses: 1) soluble aggregates are oligomers that are found in solution and include species ranging in size from dimers to those large enough to elute in the void volume of a size exclusion chromatography (SEC) column; 2) insoluble aggregates are those that can be separated from the native protein and soluble aggregates by centrifugation or filtration; and 3) particles are aggregates that are usually too large to be analyzed by SEC and are at too low of a mass percent to be quantified by mass loss due to filtration or centrifugation. Particles typically are counted for quantification, and the size range examined is from about 1 micron to 125 microns. The connections between all of these species in the aggregation pathways of proteins are poorly understood. However, recent studies in which all three types were quantified during pharmaceutically relevant stresses (e.g., freeze-thawing, agitation and agitation in the presence of silicone oil) have shown that the first detectable aggregate type is subvisible particles. These can be quantified at mass percent far below the limit of detection for loss of monomer. Thus, particle counting provides the most sensitive method to date to observe and quantify protein aggregation. Also, it appears that when there is sufficient mass of aggregate such that insoluble aggregates can be quantified due to loss of monomer, these aggregates are composed of agglomerates of subvisible particles. Therefore, subvisible particles are fundamentally important components on the protein aggregation pathway. Finally, when particles are formed during a unit operation such a filling of vials, the particles can foster enhanced protein aggregation and particle formation during subsequent stress (e.g., agitation after vial filling). | Typically, protein aggregates are arbitrarily divided in subclasses: 1) soluble aggregates are oligomers that are found in solution and include species ranging in size from dimers to those large enough to elute in the void volume of a size exclusion chromatography (SEC) column; 2) insoluble aggregates are those that can be separated from the native protein and soluble aggregates by centrifugation or filtration; and 3) particles are aggregates that are usually too large to be analyzed by SEC and are at too low of a mass percent to be quantified by mass loss due to filtration or centrifugation. Particles typically are counted for quantification, and the size range examined is from about 1 micron to 125 microns. The connections between all of these species in the aggregation pathways of proteins are poorly understood. However, recent studies in which all three types were quantified during pharmaceutically relevant stresses (e.g., freeze-thawing, agitation and agitation in the presence of silicone oil) have shown that the first detectable aggregate type is subvisible particles. These can be quantified at mass percent far below the limit of detection for loss of monomer. Thus, particle counting provides the most sensitive method to date to observe and quantify protein aggregation. Also, it appears that when there is sufficient mass of aggregate such that insoluble aggregates can be quantified due to loss of monomer, these aggregates are composed of agglomerates of subvisible particles. Therefore, subvisible particles are fundamentally important components on the protein aggregation pathway. Finally, when particles are formed during a unit operation such a filling of vials, the particles can foster enhanced protein aggregation and particle formation during subsequent stress (e.g., agitation after vial filling). | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, February 7, 2011</font> | ||
<b>Atmospheric Spectroscopy</b> | <b>Atmospheric Spectroscopy</b> | ||
| Line 2,021: | Line 5,929: | ||
An overview of the spectroscopy of the Earth’s atmosphere with the Atmospheric Chemistry Experiment (ACE) satellite will be presented. The primary instrument is a high-resolution infrared Fourier Transform Spectrometer (FTS) operating from 2 to 13 microns that measures the vertical distribution of trace gases, and the meteorological variables of temperature and pressure. Secondary instruments include a UV/Visible spectrometer called MAESTRO and two filtered solar imagers. Although now in its eighth year, the ACE satellite is still operating nominally. Ground-based atmospheric solar absorption spectra recorded with a copy of the ACE-FTS will also be discussed. The analysis of our spectra requires new laboratory measurements as will be illustrated with satellite retrievals of a wide range of organic molecules associated with air pollution. | An overview of the spectroscopy of the Earth’s atmosphere with the Atmospheric Chemistry Experiment (ACE) satellite will be presented. The primary instrument is a high-resolution infrared Fourier Transform Spectrometer (FTS) operating from 2 to 13 microns that measures the vertical distribution of trace gases, and the meteorological variables of temperature and pressure. Secondary instruments include a UV/Visible spectrometer called MAESTRO and two filtered solar imagers. Although now in its eighth year, the ACE satellite is still operating nominally. Ground-based atmospheric solar absorption spectra recorded with a copy of the ACE-FTS will also be discussed. The analysis of our spectra requires new laboratory measurements as will be illustrated with satellite retrievals of a wide range of organic molecules associated with air pollution. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, January 31, 2011</font> | ||
<b>Segregating Primary Biopolymer Associations with Airborne Particulate Matter: | <b>Segregating Primary Biopolymer Associations with Airborne Particulate Matter: | ||
| Line 2,032: | Line 5,941: | ||
While generally considered oligotrophic, the atmosphere carries microcellular hallmarks of life – both in primary and weathered forms. In this context, bioaerosols are defined as a generic class of airborne particulate matter comprised either in whole or in part of macromolecular compounds of biological origin. The contribution of the most common primary biopolymers — DNA, lipids, carbohydrates and proteins — to the pool of atmospheric organic carbon remains relatively unknown, as is their potential to weather and participate in secondary aerosol formation. We report here, phylogenetic characterization of atmospheric DNA pools juxtaposed to carbohydrate, proteins and lipids, as normalized by size segregated organic carbon mass. | While generally considered oligotrophic, the atmosphere carries microcellular hallmarks of life – both in primary and weathered forms. In this context, bioaerosols are defined as a generic class of airborne particulate matter comprised either in whole or in part of macromolecular compounds of biological origin. The contribution of the most common primary biopolymers — DNA, lipids, carbohydrates and proteins — to the pool of atmospheric organic carbon remains relatively unknown, as is their potential to weather and participate in secondary aerosol formation. We report here, phylogenetic characterization of atmospheric DNA pools juxtaposed to carbohydrate, proteins and lipids, as normalized by size segregated organic carbon mass. | ||
| − | == Monday, November 29, 2010 | + | ==Fall 2010== |
| + | <br /> | ||
| + | <font size="4"> Monday, November 29, 2010</font> | ||
<b>Ground based FTIR and MAXDOAS column observations of atmospheric trace gases</b> | <b>Ground based FTIR and MAXDOAS column observations of atmospheric trace gases</b> | ||
| Line 2,046: | Line 5,957: | ||
An overview of measurements with a MAXDOAS instrument deployed at the Caltech supersite in Pasadena, CA this year will be presented in the second part of this talk. Validation of real time slant column densities of NO2 will be presented from the period of May to June 2010 in Pasadena, CA as part of CALNEX campaign. Using a geometric approximation NO2 vertical column densities are retrieved. The degree of mixing in the boundary layer is assessed by comparison of the NO2 vertical column densities with NO2 mixing ratios as measured from a cavity enhanced DOAS instrument located on top of Millikan Library, and independent measurements of mixing height by ceilometers. | An overview of measurements with a MAXDOAS instrument deployed at the Caltech supersite in Pasadena, CA this year will be presented in the second part of this talk. Validation of real time slant column densities of NO2 will be presented from the period of May to June 2010 in Pasadena, CA as part of CALNEX campaign. Using a geometric approximation NO2 vertical column densities are retrieved. The degree of mixing in the boundary layer is assessed by comparison of the NO2 vertical column densities with NO2 mixing ratios as measured from a cavity enhanced DOAS instrument located on top of Millikan Library, and independent measurements of mixing height by ceilometers. | ||
| − | = | + | <br /> |
| − | + | <font size="4">Monday, November 15, 2010</font> | |
<b>Isotopic Labeling of Early Earth Haze Analogs</b> | <b>Isotopic Labeling of Early Earth Haze Analogs</b> | ||
| Line 2,059: | Line 5,970: | ||
Now that the possibility of haze has been demonstrated, some questions remain: What is its composition? How is it made? To answer these questions, we label each of the carbon sources with 13C and use an aerosol mass spectrometer (AMS) to observe the haze fragments generated by electron impact ionization. We aim to use the information generated by the AMS, coupled with isotopic labeling, to elucidate the chemical pathway(s) by which the haze is made. In this talk, we share preliminary results from these experiments, demonstrate the feasibility of the proposed methods in elucidating the pathway(s), and touch on future work. | Now that the possibility of haze has been demonstrated, some questions remain: What is its composition? How is it made? To answer these questions, we label each of the carbon sources with 13C and use an aerosol mass spectrometer (AMS) to observe the haze fragments generated by electron impact ionization. We aim to use the information generated by the AMS, coupled with isotopic labeling, to elucidate the chemical pathway(s) by which the haze is made. In this talk, we share preliminary results from these experiments, demonstrate the feasibility of the proposed methods in elucidating the pathway(s), and touch on future work. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, November 1, 2010</font> | ||
<b>Levoglucosan, S-Phenylmercapturic Acid, and S-Benzylmercapturic Acid as Urinary Biomarkers of Wood Smoke Exposure</b> | <b>Levoglucosan, S-Phenylmercapturic Acid, and S-Benzylmercapturic Acid as Urinary Biomarkers of Wood Smoke Exposure</b> | ||
| Line 2,077: | Line 5,989: | ||
Molecularly imprinted polymers (MIP) have been shown to preferentially bind molecules with specific shape or functional groups. This technology is discussed as it relates to the development of a nicotine sensor. Qualitative results are shown using an Atomic Force Microscope and FTIR spectra, and progress is made towards quantitative analysis using a GC. | Molecularly imprinted polymers (MIP) have been shown to preferentially bind molecules with specific shape or functional groups. This technology is discussed as it relates to the development of a nicotine sensor. Qualitative results are shown using an Atomic Force Microscope and FTIR spectra, and progress is made towards quantitative analysis using a GC. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, October 25, 2010</font> | ||
<b>Copper Binding by Dissolved Organic Matter using Competitive Ligand Exchange- Solid Phase Extraction and Application to the Biotic Ligand Model</b> | <b>Copper Binding by Dissolved Organic Matter using Competitive Ligand Exchange- Solid Phase Extraction and Application to the Biotic Ligand Model</b> | ||
| Line 2,088: | Line 6,001: | ||
Much work has been done to measure the conditional stability constants for the copper-dissolved organic matter complex, but the methods used to measure these stability constants have “detection window” limitations that are not always recognized by those incorporating the stability constants into toxicity models. In this study, the competitive ligand exchange-solid phase extraction (CLE-SPE) method was used to measure the stability constants at pH 6.5 and 0.01 ionic strength over a wide range of Cu:dissolved organic matter concentration ratios. A comparison of the CLE-SPE stability constants with those measured by other methods revealed that the most important factor to determine the binding strength is the ratio of Cu:dissolved organic matter concentration, even for dramatically different environmental and experimental conditions. The Cu:dissolved organic matter concentration ratio is important because as the ratio decreases, the binding constant increases due to the heterogeneity of binding sites present in dissolved organic matter. Results obtained from this study can be used in the BLM to more accurately predict the role of dissolved organic matter in copper speciation. | Much work has been done to measure the conditional stability constants for the copper-dissolved organic matter complex, but the methods used to measure these stability constants have “detection window” limitations that are not always recognized by those incorporating the stability constants into toxicity models. In this study, the competitive ligand exchange-solid phase extraction (CLE-SPE) method was used to measure the stability constants at pH 6.5 and 0.01 ionic strength over a wide range of Cu:dissolved organic matter concentration ratios. A comparison of the CLE-SPE stability constants with those measured by other methods revealed that the most important factor to determine the binding strength is the ratio of Cu:dissolved organic matter concentration, even for dramatically different environmental and experimental conditions. The Cu:dissolved organic matter concentration ratio is important because as the ratio decreases, the binding constant increases due to the heterogeneity of binding sites present in dissolved organic matter. Results obtained from this study can be used in the BLM to more accurately predict the role of dissolved organic matter in copper speciation. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, October 4, 2010</font> | ||
<b>Eddy-covariance measurements with time-of-flight mass spectrometry: | <b>Eddy-covariance measurements with time-of-flight mass spectrometry: | ||
| Line 2,108: | Line 6,022: | ||
Submicron atmospheric aerosols impact climate and human health, but their sources and composition are poorly understood. To address this knowledge gap, high-resolution time-of-flight aerosol mass spectrometry (AMS) and other advanced instrumentation were deployed during the CalNex field campaign in May and June 2010 to characterize the composition of aerosols in the Los Angeles area. Utilizing AMS, the concentrations for both organic and non-refractory inorganic (sulfate, nitrate, ammonium, chloride) submicron aerosols were quantified at the Pasadena ground site 15 km NE of downtown Los Angeles. Nitrate aerosols appear to dominate in the cooler mornings, but their concentration is reduced in the afternoon when organic aerosols (OA) increase and dominate. The diurnal variations in concentration are strongly influenced by vertical dilution from the rising planetary boundary layer in the afternoon. The concentrations of oxygenated OA (OOA), and hydrocarbon-like OA (HOA) are estimated, and it is found that OOA is consistently the largest type of OA present (~75% of the total OA concentration). This result indicates that the air mass over the site has undergone substantial chemical aging. The correlations between OOA and Ox (O3 + NO2) concentrations, as well as between HOA, CO and black carbon concentrations are consistent with previous studies. Ultimately, the goal of this research will be to test the accuracy of recently-proposed SOA models by combining the characterization of SOA with measurements of precursor and oxidant concentrations carried out concurrently during CalNex. | Submicron atmospheric aerosols impact climate and human health, but their sources and composition are poorly understood. To address this knowledge gap, high-resolution time-of-flight aerosol mass spectrometry (AMS) and other advanced instrumentation were deployed during the CalNex field campaign in May and June 2010 to characterize the composition of aerosols in the Los Angeles area. Utilizing AMS, the concentrations for both organic and non-refractory inorganic (sulfate, nitrate, ammonium, chloride) submicron aerosols were quantified at the Pasadena ground site 15 km NE of downtown Los Angeles. Nitrate aerosols appear to dominate in the cooler mornings, but their concentration is reduced in the afternoon when organic aerosols (OA) increase and dominate. The diurnal variations in concentration are strongly influenced by vertical dilution from the rising planetary boundary layer in the afternoon. The concentrations of oxygenated OA (OOA), and hydrocarbon-like OA (HOA) are estimated, and it is found that OOA is consistently the largest type of OA present (~75% of the total OA concentration). This result indicates that the air mass over the site has undergone substantial chemical aging. The correlations between OOA and Ox (O3 + NO2) concentrations, as well as between HOA, CO and black carbon concentrations are consistent with previous studies. Ultimately, the goal of this research will be to test the accuracy of recently-proposed SOA models by combining the characterization of SOA with measurements of precursor and oxidant concentrations carried out concurrently during CalNex. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, September 27, 2010</font> | ||
<b>Update on RASEI, Solar Fuels, and Critical Areas of Electrochemical Energy Research</b> | <b>Update on RASEI, Solar Fuels, and Critical Areas of Electrochemical Energy Research</b> | ||
| Line 2,126: | Line 6,041: | ||
Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (emitted in the particle phase) and secondary OA (formed from chemical reactions of gas-phase species). In this talk I will summarize research in OA by our group over the last year, including a summary of the recent CalNex-LA field project in Pasadena (CA), research on the SOA formation and photochemical evolution of biomass burning emissions, on SOA model/measurement comparisons in different environments, and on collaborative work with global modelers to constraint the global OA budget. I will also discuss upcoming projects including characterization and use of semicontinuous GC-MS and chemical ionization mass spec. to analyze OA, and field projects in the Rocky Mountains, India, and Southeast Asia. | Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (emitted in the particle phase) and secondary OA (formed from chemical reactions of gas-phase species). In this talk I will summarize research in OA by our group over the last year, including a summary of the recent CalNex-LA field project in Pasadena (CA), research on the SOA formation and photochemical evolution of biomass burning emissions, on SOA model/measurement comparisons in different environments, and on collaborative work with global modelers to constraint the global OA budget. I will also discuss upcoming projects including characterization and use of semicontinuous GC-MS and chemical ionization mass spec. to analyze OA, and field projects in the Rocky Mountains, India, and Southeast Asia. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, September 20, 2010</font> | ||
<b>Nanoscale Therapeutics in the Treatment of Multidrug Resistant Pathogens</b> | <b>Nanoscale Therapeutics in the Treatment of Multidrug Resistant Pathogens</b> | ||
| Line 2,144: | Line 6,060: | ||
Ten percent of all pre-school deaths in India are measles-related. We have developed a Measles Vaccine, Dry Powder (MVDP) and PuffHaler® a novel dry powder inhaler for respiratory delivery to overcome the safety and vaccine wastage problems associated with the current injectable vaccine. MVDP was manufactured from bulk liquid, live attenuated, measles vaccine using CAN-BD® to yield a stable, dry powder, with fine particles suitable for aerosolization and delivery to the pulmonary tract by inhalation. This was achieved by substituting myo-inositol for sorbitol as a stabilizing sugar that is less hygroscopic. Contact with water is detrimental to the stability of measles vaccine and the microparticles are reconstituted only when they deposit in the moist surfaces of the respiratory tract. There is no need for purified water to be carried to remote locations. The particles dried by CAN-BD are homogeneous; the vaccine viral activity distribution in the 1 to 5 micron aerodynamic diameter microparticles is the same as the mass distribution, within experimental error. The shelf life is excellent at 2 to 8 °C with only about 0.5 log loss of activity after more than 2 years. At 25 °C, there is approximately 0.8 log loss after 6 months of storage. The immunogenicity and protective efficacy of a primary dose of MVDP delivered was tested in non-anesthetized, free breathing, Rhesus macaques. MVDP induced MV-specific humoral and T-cell responses at least as robust as subcutaneous measles vaccine and completely protected macaques from infection with wild type measles virus without adverse effects. Phase I clinical trials are now scheduled. | Ten percent of all pre-school deaths in India are measles-related. We have developed a Measles Vaccine, Dry Powder (MVDP) and PuffHaler® a novel dry powder inhaler for respiratory delivery to overcome the safety and vaccine wastage problems associated with the current injectable vaccine. MVDP was manufactured from bulk liquid, live attenuated, measles vaccine using CAN-BD® to yield a stable, dry powder, with fine particles suitable for aerosolization and delivery to the pulmonary tract by inhalation. This was achieved by substituting myo-inositol for sorbitol as a stabilizing sugar that is less hygroscopic. Contact with water is detrimental to the stability of measles vaccine and the microparticles are reconstituted only when they deposit in the moist surfaces of the respiratory tract. There is no need for purified water to be carried to remote locations. The particles dried by CAN-BD are homogeneous; the vaccine viral activity distribution in the 1 to 5 micron aerodynamic diameter microparticles is the same as the mass distribution, within experimental error. The shelf life is excellent at 2 to 8 °C with only about 0.5 log loss of activity after more than 2 years. At 25 °C, there is approximately 0.8 log loss after 6 months of storage. The immunogenicity and protective efficacy of a primary dose of MVDP delivered was tested in non-anesthetized, free breathing, Rhesus macaques. MVDP induced MV-specific humoral and T-cell responses at least as robust as subcutaneous measles vaccine and completely protected macaques from infection with wild type measles virus without adverse effects. Phase I clinical trials are now scheduled. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, September 13, 2010</font> | ||
<b>Sulfur Particles on Early Earth </b> | <b>Sulfur Particles on Early Earth </b> | ||
| Line 2,158: | Line 6,075: | ||
The climate relevance of biologically active ocean upwelling regions has primarily been studied in terms of the air-sea partitioning of long-lived greenhouse gases (e.g., CO2, CH4, N2O etc), and the release of the reactive gas DMS, which can form aerosols as a result of atmospheric transformations. Considerably less attention has been paid to open ocean sources of other reactive gases that, like DMS, can form aerosols. Such molecules are glyoxal (CHOCHO) and iodine oxide (IO). Glyoxal is an indicator for oxidative hydrocarbon chemistry, and a building block for secondary organic aerosol (SOA). SOA modifies the hygroscopic properties of organic aerosols, and potentially also adds to the growth of small particles to sizes that can more easily activate to form cloud droplets. Iodine oxide (IO) can nucleate new particles, and/or add to the growth of pre-existing particles. Due to the very high solubility of the glyoxal molecule, concentrations in excess of 100ppt over the open ocean like we found over the Pacific Ocean require an airborne source mechanism (Sinreich et al., 2010). We have investigated the source mechanism further during a ship campaign in 2009, as well as a first research flight aboard the NSF/NCAR GV research aircraft (HIAPER). Both campaigns give clues about the sources of both gases over the remote tropical Pacific Ocean, and reveal a surprising impact on the composition of the free troposphere. | The climate relevance of biologically active ocean upwelling regions has primarily been studied in terms of the air-sea partitioning of long-lived greenhouse gases (e.g., CO2, CH4, N2O etc), and the release of the reactive gas DMS, which can form aerosols as a result of atmospheric transformations. Considerably less attention has been paid to open ocean sources of other reactive gases that, like DMS, can form aerosols. Such molecules are glyoxal (CHOCHO) and iodine oxide (IO). Glyoxal is an indicator for oxidative hydrocarbon chemistry, and a building block for secondary organic aerosol (SOA). SOA modifies the hygroscopic properties of organic aerosols, and potentially also adds to the growth of small particles to sizes that can more easily activate to form cloud droplets. Iodine oxide (IO) can nucleate new particles, and/or add to the growth of pre-existing particles. Due to the very high solubility of the glyoxal molecule, concentrations in excess of 100ppt over the open ocean like we found over the Pacific Ocean require an airborne source mechanism (Sinreich et al., 2010). We have investigated the source mechanism further during a ship campaign in 2009, as well as a first research flight aboard the NSF/NCAR GV research aircraft (HIAPER). Both campaigns give clues about the sources of both gases over the remote tropical Pacific Ocean, and reveal a surprising impact on the composition of the free troposphere. | ||
| − | == Tuesday, April 27, 2010 | + | ==Spring 2010== |
| + | <br /> | ||
| + | <font size="4">Tuesday, April 27, 2010</font> | ||
<b>Laboratory Studies of Titan Tholins</b> | <b>Laboratory Studies of Titan Tholins</b> | ||
| Line 2,171: | Line 6,090: | ||
Aerodyne quadrupole aerosol mass spectrometer (Q-AMS) of tholins formed at a range of pressures. | Aerodyne quadrupole aerosol mass spectrometer (Q-AMS) of tholins formed at a range of pressures. | ||
| − | = | + | <br /> |
| + | <font size="4">Tuesday, April 13, 2010</font> | ||
<b>DOAS Measurements of Halogen Oxides</b> | <b>DOAS Measurements of Halogen Oxides</b> | ||
| Line 2,179: | Line 6,099: | ||
Halogens species are of outmost importance to the atmosphere due to their potential to destroy ozone and the DOAS (differential optical absorption spectroscopy) method is a powerful tool to measure halogen oxides like IO, BrO, but also OClO. After a brief introduction to the longpath-DOAS and multi-axes-DOAS techniques, this talk will present results from several field studies focusing mainly on the observations of iodine and bromine oxides and their impact on the marine boundary layer. This will be followed by a brief excursion to the stratosphere and the chlorine chemistry of the polar ozone hole. The third part of the talk will give an outlook on the future deployment of an airborne multi-axis (AMAX) DOAS instrument in the CalNex campaign in California this summer. | Halogens species are of outmost importance to the atmosphere due to their potential to destroy ozone and the DOAS (differential optical absorption spectroscopy) method is a powerful tool to measure halogen oxides like IO, BrO, but also OClO. After a brief introduction to the longpath-DOAS and multi-axes-DOAS techniques, this talk will present results from several field studies focusing mainly on the observations of iodine and bromine oxides and their impact on the marine boundary layer. This will be followed by a brief excursion to the stratosphere and the chlorine chemistry of the polar ozone hole. The third part of the talk will give an outlook on the future deployment of an airborne multi-axis (AMAX) DOAS instrument in the CalNex campaign in California this summer. | ||
| − | = | + | <br /> |
| + | <font size="4">Tuesday, April 6, 2010</font> | ||
<b>Single Particle Studies of Aerosol Hygroscopicity, Aging and Mass | <b>Single Particle Studies of Aerosol Hygroscopicity, Aging and Mass | ||
| Line 2,203: | Line 6,124: | ||
evaporation of water will be described and first measurements reported. | evaporation of water will be described and first measurements reported. | ||
| − | = | + | <br /> |
| + | <font size="4">Tuesday, March 30, 2010</font> | ||
<b> Development and Application of a Metastable Atom Bombardment (MAB) Source for Penning Ionization Time-of-Flight Aerosol Mass Spectrometry </b> | <b> Development and Application of a Metastable Atom Bombardment (MAB) Source for Penning Ionization Time-of-Flight Aerosol Mass Spectrometry </b> | ||
| Line 2,213: | Line 6,135: | ||
The Aerodyne time-of-flight aerosol mass spectrometer (ToF-AMS) utilizes thermal vaporization followed by electron ionization (EI) to convert aerosol components to gas-phase ions. The method enables quantification of chemical classes, but the extensive fragmentation caused by EI limits the specificity of both chemical analysis and source identification by factor analysis. To better identify the molecular components of aerosols, we have constructed a metastable atom bombardment (MAB) ionization source that can be interfaced to standard ToF-AMS hardware. A beam of metastable rare gas atoms is produced by a low-voltage DC discharge and focused toward the vaporization plume, yielding Penning Ionization of the analyte molecules. By changing gases, the excited energies of the metastables can be adjusted between 20.61 eV (He) and 9.92 eV (Kr). Source parameters, including pressures, current, geometry, and materials, were optimized for He, Ar, and Kr. Instrument sensitivity and induced fragmentation was characterized for each using lab-generated oleic acid particles. The demonstrated sensitivities are 0.1% of EI (3% of the SNR of EI in the V-mode, comparable to the Q-AMS SNR), which is sufficient for ambient monitoring. A metastable flux of 2.6e14 sr-1sec-1 has been achieved. The MAB-AMS has been deployed to the FLAME-3 campaign at the USDA Fire Sciences Laboratory in Missoula, MT, and used to sample smoke from open burning of different biomass samples. | The Aerodyne time-of-flight aerosol mass spectrometer (ToF-AMS) utilizes thermal vaporization followed by electron ionization (EI) to convert aerosol components to gas-phase ions. The method enables quantification of chemical classes, but the extensive fragmentation caused by EI limits the specificity of both chemical analysis and source identification by factor analysis. To better identify the molecular components of aerosols, we have constructed a metastable atom bombardment (MAB) ionization source that can be interfaced to standard ToF-AMS hardware. A beam of metastable rare gas atoms is produced by a low-voltage DC discharge and focused toward the vaporization plume, yielding Penning Ionization of the analyte molecules. By changing gases, the excited energies of the metastables can be adjusted between 20.61 eV (He) and 9.92 eV (Kr). Source parameters, including pressures, current, geometry, and materials, were optimized for He, Ar, and Kr. Instrument sensitivity and induced fragmentation was characterized for each using lab-generated oleic acid particles. The demonstrated sensitivities are 0.1% of EI (3% of the SNR of EI in the V-mode, comparable to the Q-AMS SNR), which is sufficient for ambient monitoring. A metastable flux of 2.6e14 sr-1sec-1 has been achieved. The MAB-AMS has been deployed to the FLAME-3 campaign at the USDA Fire Sciences Laboratory in Missoula, MT, and used to sample smoke from open burning of different biomass samples. | ||
| − | = | + | <br /> |
| + | <font size="4">Tuesday, March 16, 2010</font> | ||
[http://cloudbase.phy.umist.ac.uk/people/coe/coe.htm Hugh Coe], Prof. of Atmospheric Composition, University of Manchester, UK | [http://cloudbase.phy.umist.ac.uk/people/coe/coe.htm Hugh Coe], Prof. of Atmospheric Composition, University of Manchester, UK | ||
| Line 2,223: | Line 6,146: | ||
The time dependent measurements obtained by aerosol mass spectrometry enable the chemical transformation time scales of aerosol material to be explored. In particular, factor analyses of the observed organic mass spectral fingerprint demonstrates the continuum nature of chemical processing. Ammonium nitrate is an important aerosol component in NW Europe. It exists in a temperature dependent equilibrium which favours particulate formation in the moist, cool upper portion of the boundary layer. We have shown that this results in aerosol optical depths inferred from ground based measurements are biased low by up to 50% by comparison with AERONET, aircraft measurements, and lidar data during periods of high AOD in anticyclonic conditions. The ubiquitous nature of black carbon (BC) was also demonstrated and how this is processed by secondary particulate will be shown using single particle soot photometry. These topics will all be explored and comparison with global model studies will also be discussed. In contrast, tropical environemnts are dominated by transport of aerosol into the region, by primary production of biological material in the coarse mode aerosol or by secondary organic aerosol produced within the region by photochemical oxidation of precursors. These will presented and the properties of secondary organic material compared with other tropical sites and with global models. | The time dependent measurements obtained by aerosol mass spectrometry enable the chemical transformation time scales of aerosol material to be explored. In particular, factor analyses of the observed organic mass spectral fingerprint demonstrates the continuum nature of chemical processing. Ammonium nitrate is an important aerosol component in NW Europe. It exists in a temperature dependent equilibrium which favours particulate formation in the moist, cool upper portion of the boundary layer. We have shown that this results in aerosol optical depths inferred from ground based measurements are biased low by up to 50% by comparison with AERONET, aircraft measurements, and lidar data during periods of high AOD in anticyclonic conditions. The ubiquitous nature of black carbon (BC) was also demonstrated and how this is processed by secondary particulate will be shown using single particle soot photometry. These topics will all be explored and comparison with global model studies will also be discussed. In contrast, tropical environemnts are dominated by transport of aerosol into the region, by primary production of biological material in the coarse mode aerosol or by secondary organic aerosol produced within the region by photochemical oxidation of precursors. These will presented and the properties of secondary organic material compared with other tropical sites and with global models. | ||
| − | = | + | <br /> |
| + | <font size="4">Tuesday, March 9, 2010</font> | ||
[http://info.aero.jussieu.fr/weblib/web/userspages/page.jsp?composantId=kmelia363&pubId=3167 Nathalie Carrasco], [http://www.latmos.ipsl.fr/ Laboratoire Atmosphères, Milieux, Observations Spatiales, IPSL, Verrières le Buisson, France] | [http://info.aero.jussieu.fr/weblib/web/userspages/page.jsp?composantId=kmelia363&pubId=3167 Nathalie Carrasco], [http://www.latmos.ipsl.fr/ Laboratoire Atmosphères, Milieux, Observations Spatiales, IPSL, Verrières le Buisson, France] | ||
| Line 2,235: | Line 6,159: | ||
Several techniques have been developed to simulate lab analogs, called tholins, of Titan’s haze. Most efficient methods presently known involve plasma techniques. Such a plasma device, named PAMPRE, has been developed in LATMOS, using a RF plasma discharge (13.6 MHz) in a stationary flux of nitrogen and methane gaz mixture, at various temperature and pressure conditions. Recent results will be presented concerning in situ mass spectrometry analysis of the gaseous phase and ex-situ chemical and morphological analysis of the tholins. | Several techniques have been developed to simulate lab analogs, called tholins, of Titan’s haze. Most efficient methods presently known involve plasma techniques. Such a plasma device, named PAMPRE, has been developed in LATMOS, using a RF plasma discharge (13.6 MHz) in a stationary flux of nitrogen and methane gaz mixture, at various temperature and pressure conditions. Recent results will be presented concerning in situ mass spectrometry analysis of the gaseous phase and ex-situ chemical and morphological analysis of the tholins. | ||
| − | = | + | <br /> |
| + | <font size="4">Tuesday, March 2, 2010</font> | ||
<b> Dry powder formulation development for nasal vaccination</b> | <b> Dry powder formulation development for nasal vaccination</b> | ||
| Line 2,246: | Line 6,171: | ||
I will briefly talk about some aspects of the mucosal immune system, which are important for the nasal vaccination strategy. I will then focus on the nose as delivery location, introduce a nasal cast model for the determination of formulation deposition in the different parts of the nose and show some delivery devices I have been characterizing. Further on I will shortly review the vaccine delivery systems described in literature and will then talk about our formulation strategies. Besides I will introduce a rapid in vitro test to assess the respiratory toxicity of both excipients and complete formulations. | I will briefly talk about some aspects of the mucosal immune system, which are important for the nasal vaccination strategy. I will then focus on the nose as delivery location, introduce a nasal cast model for the determination of formulation deposition in the different parts of the nose and show some delivery devices I have been characterizing. Further on I will shortly review the vaccine delivery systems described in literature and will then talk about our formulation strategies. Besides I will introduce a rapid in vitro test to assess the respiratory toxicity of both excipients and complete formulations. | ||
| − | = | + | <br /> |
| + | <font size="4">Tuesday, February 16, 2010</font> | ||
<b>Observations of iodine oxide and reactive gaseous mercury at a | <b>Observations of iodine oxide and reactive gaseous mercury at a | ||
| Line 2,257: | Line 6,183: | ||
An increasing body of evidence suggests that atmospheric halogens, marked largely by the presence of BrO and IO, are ubiquitous components of the lower troposphere in coastal and oceanic areas. The role of halogen chemistry in mercury oxidation in coastal regions remains unknown. Simulations of atmospheric mercury oxidation suggest that chemistry initiated by atomic Br may have been underestimated. Synergistic effects of the iodine compounds can be expected and little is known about the possible direct role I atoms may play to oxidize mercury. Finally, other reactive trace gases, such as CH2O and C2H2O2, can suppress the oxidation of Hg by converting bromine-radicals into chemically inert reservoir species. Here we present data from several months of MAX-DOAS measurements of halogen oxides, O4, CH2O and C2H2O2 in parallel with measurements of speciated mercury (Hg0, Hg2+) that are currently being conducted along the U.S. Gulf Coast. | An increasing body of evidence suggests that atmospheric halogens, marked largely by the presence of BrO and IO, are ubiquitous components of the lower troposphere in coastal and oceanic areas. The role of halogen chemistry in mercury oxidation in coastal regions remains unknown. Simulations of atmospheric mercury oxidation suggest that chemistry initiated by atomic Br may have been underestimated. Synergistic effects of the iodine compounds can be expected and little is known about the possible direct role I atoms may play to oxidize mercury. Finally, other reactive trace gases, such as CH2O and C2H2O2, can suppress the oxidation of Hg by converting bromine-radicals into chemically inert reservoir species. Here we present data from several months of MAX-DOAS measurements of halogen oxides, O4, CH2O and C2H2O2 in parallel with measurements of speciated mercury (Hg0, Hg2+) that are currently being conducted along the U.S. Gulf Coast. | ||
| − | = | + | <br /> |
| + | <font size="4">Tuesday, February 9, 2010</font> | ||
<b>Light Emitting Diode Cavity Enhanced Differential Optical | <b>Light Emitting Diode Cavity Enhanced Differential Optical | ||
| Line 2,269: | Line 6,196: | ||
The combination of Cavity Enhanced Absorption Spectroscopy (CEAS) with broad-band light sources (e.g. Light-Emitting Diodes, LEDs) lends itself to the application of cavity enhanced DOAS (CE-DOAS) to perform sensitive and selective point measurements of multiple trace gases with a single instrument. In contrast to other broad-band CEAS techniques, CE-DOAS relies only on the measurement of relative intensity changes, i.e., does not require knowledge of the light intensity in the absence of trace gases and aerosols (I0) and is therefore insensitive to lamp drifts and/or the presence of aerosols. We have built a prototype LED-CE-DOAS instrument in the blue spectral range (420-490nm) to measure nitrogen dioxide (NO2), glyoxal (CHOCHO), methylglyoxal (CH3COCHO), iodine monoxide (IO), water (H2O) and oxygen dimers (O4). Aerosol extinction is retrieved at two wavelengths by means of observing water and O4 and measuring pressure, temperature and relative humidity independently. The instrument components are presented, and the approach to measure aerosol extinction is demonstrated by means of a set of experiments where laboratory generated monodisperse aerosols are added to the cavity. The aerosol extinction cross section agrees well with Mie calculations, demonstrating that our setup enables measurements of the above gases in open cavity mode. | The combination of Cavity Enhanced Absorption Spectroscopy (CEAS) with broad-band light sources (e.g. Light-Emitting Diodes, LEDs) lends itself to the application of cavity enhanced DOAS (CE-DOAS) to perform sensitive and selective point measurements of multiple trace gases with a single instrument. In contrast to other broad-band CEAS techniques, CE-DOAS relies only on the measurement of relative intensity changes, i.e., does not require knowledge of the light intensity in the absence of trace gases and aerosols (I0) and is therefore insensitive to lamp drifts and/or the presence of aerosols. We have built a prototype LED-CE-DOAS instrument in the blue spectral range (420-490nm) to measure nitrogen dioxide (NO2), glyoxal (CHOCHO), methylglyoxal (CH3COCHO), iodine monoxide (IO), water (H2O) and oxygen dimers (O4). Aerosol extinction is retrieved at two wavelengths by means of observing water and O4 and measuring pressure, temperature and relative humidity independently. The instrument components are presented, and the approach to measure aerosol extinction is demonstrated by means of a set of experiments where laboratory generated monodisperse aerosols are added to the cavity. The aerosol extinction cross section agrees well with Mie calculations, demonstrating that our setup enables measurements of the above gases in open cavity mode. | ||
| − | = | + | <br /> |
| + | <font size="4">Tuesday, February 2, 2010</font> | ||
<b>Environmental Analysis of Water and Food Samples by | <b>Environmental Analysis of Water and Food Samples by | ||
| Line 2,282: | Line 6,210: | ||
The analysis of pharmaceuticals and pesticides in water and food is one of the hot topics in environmental analysis. Recently the Associated Press published an article that much of the drinking water of the US is affected by pharmaceuticals coming from our wastewater. This article was backed up by numerous scientific studies since the late 1990s showing pharmaceuticals occurring in surface waters of the US. We have established a Laboratory for Environmental Mass Spectrometry soon to be a Center for Environmental Mass Spectrometry that uses an array of state of the art MS, including LC/time-of-flight MS, triple quadrupole MS with Jetspray, LC/MS ion trap, and other instrumentation to examine questions such as pharmaceuticals in drinking water and pesticides in food. Our seminar will discuss our new Center of Environmental MS, instrumentation, and applications to pharmaceuticals in drinking water and pesticides in food. | The analysis of pharmaceuticals and pesticides in water and food is one of the hot topics in environmental analysis. Recently the Associated Press published an article that much of the drinking water of the US is affected by pharmaceuticals coming from our wastewater. This article was backed up by numerous scientific studies since the late 1990s showing pharmaceuticals occurring in surface waters of the US. We have established a Laboratory for Environmental Mass Spectrometry soon to be a Center for Environmental Mass Spectrometry that uses an array of state of the art MS, including LC/time-of-flight MS, triple quadrupole MS with Jetspray, LC/MS ion trap, and other instrumentation to examine questions such as pharmaceuticals in drinking water and pesticides in food. Our seminar will discuss our new Center of Environmental MS, instrumentation, and applications to pharmaceuticals in drinking water and pesticides in food. | ||
| − | = | + | <br /> |
| + | <font size="4">Friday, January 22, 2010 (note special day)</font> | ||
Pedro Campuzano-Jost | Pedro Campuzano-Jost | ||
| Line 2,292: | Line 6,221: | ||
<b>Abstract</b> | <b>Abstract</b> | ||
| − | Trapping organic aerosol ions for molecular identification: Some lessons learned | + | '''Trapping organic aerosol ions for molecular identification: Some lessons learned''' |
The incapability of off-line techniques to resolve a significant part of the organic fraction of atmospheric aerosols has resulted in a stronger focus in recent years on developing real time aerosol mass spectrometers that are able to identify not just compound classes, but specific molecules. The approach chosen in most cases has been reducing fragmentation by use of soft-ionization techniques. While use of soft ionization can greatly simplify the mass spectra of complex mixtures, it also tends to erase the chemical information provided by the fragmentation pattern. Therefore, the ability to isolate and obtain tandem MS spectra of individual molecular ions is key to be able to id individual compounds. | The incapability of off-line techniques to resolve a significant part of the organic fraction of atmospheric aerosols has resulted in a stronger focus in recent years on developing real time aerosol mass spectrometers that are able to identify not just compound classes, but specific molecules. The approach chosen in most cases has been reducing fragmentation by use of soft-ionization techniques. While use of soft ionization can greatly simplify the mass spectra of complex mixtures, it also tends to erase the chemical information provided by the fragmentation pattern. Therefore, the ability to isolate and obtain tandem MS spectra of individual molecular ions is key to be able to id individual compounds. | ||
| Line 2,304: | Line 6,233: | ||
- A new field capable ITMS that combines a novel aerosol beam alignment design with a mass spectrometer with dual polarity detection. Ionization occurs by laser ablation at 266 nm. This instrument is currently being deployed to the top of Whistler Mountain, BC to study long range transport of Asian dust. | - A new field capable ITMS that combines a novel aerosol beam alignment design with a mass spectrometer with dual polarity detection. Ionization occurs by laser ablation at 266 nm. This instrument is currently being deployed to the top of Whistler Mountain, BC to study long range transport of Asian dust. | ||
| − | == | + | ==Fall 2009== |
| + | <br /> | ||
| + | |||
| + | <font size="4">Monday, November 30, 2009</font> | ||
| + | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium | CIRES Auditorium | ||
| Line 2,315: | Line 6,248: | ||
<b>Abstract</b> | <b>Abstract</b> | ||
| − | The desire to develop novel, more efficient heterogeneous catalysts is a vital area of research in renewable energy applications. Due to the huge compositional and structural parameter space under which solid state materials can exist, it is nearly impossible to develop new heterogeneous catalysts a-priori. Therefore, many research groups have turned to combinatorial methods to identify new materials. While methods of synthesizing combinatorial arrays of bulk materials are well established, combinatorial arrays of nano-materials have been more elusive. Here we present a novel synthesis methodology for arrays of nanoparticles on solid supports using nanoparticles as synthetic precursors. The combinatorial arrays are generated by utilizing multidirectional nanoparticle gradients created by exploiting the kinetics of electrostatic adhesion of nanoparticles to a solid support. Subsequent reaction of the solid supports at elevated temperatures causes adjacent particles to alloy yielding nanoparticles with a gradient of compositions. These reactions have been demonstrated to take place on solid supports in both gaseous and liquid environments. Nanoparticle arrays adsorbed onto indium tin oxide (ITO) coated glass have been shown to alloy in a similar fashion. The arrays on ITO glass were subsequently fabricated into working electrodes for an electrochemical cell. The electrodes were then demonstrated as a catalyst screening tool for methanol fuel cell catalysts. | + | The desire to develop novel, more efficient heterogeneous catalysts is a vital area of research in renewable energy applications. Due to the huge compositional and structural parameter space under which solid state materials can exist, it is nearly impossible to develop new heterogeneous catalysts a-priori. Therefore, many research groups have turned to combinatorial methods to identify new materials. While methods of synthesizing combinatorial arrays of bulk materials are well established, combinatorial arrays of nano-materials have been more elusive. Here we present a novel synthesis methodology for arrays of nanoparticles on solid supports using nanoparticles as synthetic precursors. The combinatorial arrays are generated by utilizing multidirectional nanoparticle gradients created by exploiting the kinetics of electrostatic adhesion of nanoparticles to a solid support. Subsequent reaction of the solid supports at elevated temperatures causes adjacent particles to alloy yielding nanoparticles with a gradient of compositions. These reactions have been demonstrated to take place on solid supports in both gaseous and liquid environments. Nanoparticle arrays adsorbed onto indium tin oxide (ITO) coated glass have been shown to alloy in a similar fashion. The arrays on ITO glass were subsequently fabricated into working electrodes for an electrochemical cell. The electrodes were then demonstrated as a catalyst screening tool for methanol fuel cell catalysts. |
| + | |||
| + | <br /> | ||
| + | <font size="4">Monday, November 2, 2009</font> | ||
| − | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium | CIRES Auditorium | ||
| Line 2,343: | Line 6,278: | ||
It has been discovered that the right amount of S-nitrosothiol (RSNO) species in blood prevents the activation of clotting agents. A new RSNO detection technique, based on an electrochemical sensor, rapidly measures the level of RSNOs in whole blood. Use of this S-nitrosothiol sensor during surgical procedures, like Extra Corporeal Life Support (ECLS), could provide physicians with a novel method of monitoring a patient’s health. This seminar is an opportunity to describe the methods of the S-nitrosothiol biosensor’s assembly and in vivo testing. | It has been discovered that the right amount of S-nitrosothiol (RSNO) species in blood prevents the activation of clotting agents. A new RSNO detection technique, based on an electrochemical sensor, rapidly measures the level of RSNOs in whole blood. Use of this S-nitrosothiol sensor during surgical procedures, like Extra Corporeal Life Support (ECLS), could provide physicians with a novel method of monitoring a patient’s health. This seminar is an opportunity to describe the methods of the S-nitrosothiol biosensor’s assembly and in vivo testing. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, October 26, 2009</font> | ||
| + | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium | CIRES Auditorium | ||
| Line 2,356: | Line 6,293: | ||
Analytical chemistry is an essential tool in drinking water and wastewater regulation. Wet Chemistry specifically refers to analytical chemistry performed in aqueous solution. It is therefore easily performed in the same matrix as wastewater and drinking water. This presentation will cover three wastewater testing methods: EPA 420.1, which tests for all phenols/phenolic compounds; EPA 365.3 dissolved, which tests for the orthophosphate ion in a filtered solution; and SM 3500-Cr B, which tests for hexavalent chromium in natural or treated water. Each of these tests is colorimetric, and the chemistry of their respective color-producing reaction will be discussed, as well as typical matrix interferences. | Analytical chemistry is an essential tool in drinking water and wastewater regulation. Wet Chemistry specifically refers to analytical chemistry performed in aqueous solution. It is therefore easily performed in the same matrix as wastewater and drinking water. This presentation will cover three wastewater testing methods: EPA 420.1, which tests for all phenols/phenolic compounds; EPA 365.3 dissolved, which tests for the orthophosphate ion in a filtered solution; and SM 3500-Cr B, which tests for hexavalent chromium in natural or treated water. Each of these tests is colorimetric, and the chemistry of their respective color-producing reaction will be discussed, as well as typical matrix interferences. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, October 19, 2009</font> | ||
| + | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium | CIRES Auditorium | ||
| Line 2,372: | Line 6,311: | ||
Atmospheric particles play an important role in climate forcing; they are also strongly associated with adverse human health effects. Organic aerosols account for a large fraction of ambient fine particle mass, but their sources and transformation processes are still poorly understood. Motor vehicles, wood stoves, and other combustion systems are major sources of organic aerosols. This talk discusses recent field, laboratory, and modeling results on organic particle emissions from combustion systems. The results reveal a dynamic picture in which low-volatility organics evaporate, oxidize, and recondense as they are transported away from the source. This new picture alters our understanding of the contribution of combustion sources to urban and regional pollution and brings chemical transport model predictions into better agreement with field observations. The talk concludes with a discussion of the implications of these recent findings on human exposures and the design of regulations to control organic aerosols. | Atmospheric particles play an important role in climate forcing; they are also strongly associated with adverse human health effects. Organic aerosols account for a large fraction of ambient fine particle mass, but their sources and transformation processes are still poorly understood. Motor vehicles, wood stoves, and other combustion systems are major sources of organic aerosols. This talk discusses recent field, laboratory, and modeling results on organic particle emissions from combustion systems. The results reveal a dynamic picture in which low-volatility organics evaporate, oxidize, and recondense as they are transported away from the source. This new picture alters our understanding of the contribution of combustion sources to urban and regional pollution and brings chemical transport model predictions into better agreement with field observations. The talk concludes with a discussion of the implications of these recent findings on human exposures and the design of regulations to control organic aerosols. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, October 12, 2009</font> | ||
| + | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium | CIRES Auditorium | ||
| Line 2,393: | Line 6,334: | ||
The homonuclear diatomic metal ion Mo2- was studied using negative ion photoelectron spectroscopy. This bare, homonuclear group 6 transition metal dimer allows the study of multiple metal-metal bonding free of ligand effects. The photoelectron spectra, obtained at 488 nm with an instrumental resolution of about 6 meV (50 cm-1), provide measurements of the electron affinities, vibrational frequencies for both the anion and the neutral states, and bond length changes upon electron detachment. The Mo2- spectra displays a transition to the multiply-bonded 1Σg+(dπ)4(dδ)4(dσ)2(sσ)2 ground state of the neutral molecule. In the anion, the extra electron occupies the vacant antibonding sσ* orbital, giving a 2Σu+ ground state. | The homonuclear diatomic metal ion Mo2- was studied using negative ion photoelectron spectroscopy. This bare, homonuclear group 6 transition metal dimer allows the study of multiple metal-metal bonding free of ligand effects. The photoelectron spectra, obtained at 488 nm with an instrumental resolution of about 6 meV (50 cm-1), provide measurements of the electron affinities, vibrational frequencies for both the anion and the neutral states, and bond length changes upon electron detachment. The Mo2- spectra displays a transition to the multiply-bonded 1Σg+(dπ)4(dδ)4(dσ)2(sσ)2 ground state of the neutral molecule. In the anion, the extra electron occupies the vacant antibonding sσ* orbital, giving a 2Σu+ ground state. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, October 5, 2009</font> | ||
| + | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium | CIRES Auditorium | ||
| Line 2,418: | Line 6,361: | ||
Aerosols play major roles in climate forcing and the hydrological cycle, and also on human health effects, visibility degradation, and deposition of acids, toxics, and nutrients to ecosystems. Organic aerosols (OA) account for a large fraction (~50%) of ambient submicron aerosol mass, but their sources and transformation processes are still poorly understood. I will summarize recent findings about the sources, processing and budget of OA arising from multiple field and lab studies, as well as comparisons to 1D and 3D models. OA from all sources evolves in the atmosphere by becoming more oxygenated and hygroscopic and less volatile, and partially losing the chemical signature of the original source and gaining a common signature of atmospheric oxidation. Secondary OA (SOA) formation in urban areas is greatly underestimated by traditional models, in contrast with biogenic SOA formed under pristine conditions which is reasonably predicted. New models close the gap and even exceed the observed SOA in urban areas, but it is not clear whether this is for the right reasons. Further progress necessitates more complete high-quality measurements at one location as well as new measurement & analysis techniques, and we are preparing for two such campaigns in Los Angeles, CA in 2010 and Manitou Springs, CO in 2011. | Aerosols play major roles in climate forcing and the hydrological cycle, and also on human health effects, visibility degradation, and deposition of acids, toxics, and nutrients to ecosystems. Organic aerosols (OA) account for a large fraction (~50%) of ambient submicron aerosol mass, but their sources and transformation processes are still poorly understood. I will summarize recent findings about the sources, processing and budget of OA arising from multiple field and lab studies, as well as comparisons to 1D and 3D models. OA from all sources evolves in the atmosphere by becoming more oxygenated and hygroscopic and less volatile, and partially losing the chemical signature of the original source and gaining a common signature of atmospheric oxidation. Secondary OA (SOA) formation in urban areas is greatly underestimated by traditional models, in contrast with biogenic SOA formed under pristine conditions which is reasonably predicted. New models close the gap and even exceed the observed SOA in urban areas, but it is not clear whether this is for the right reasons. Further progress necessitates more complete high-quality measurements at one location as well as new measurement & analysis techniques, and we are preparing for two such campaigns in Los Angeles, CA in 2010 and Manitou Springs, CO in 2011. | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, September 28, 2009</font> | ||
| + | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium | CIRES Auditorium | ||
University of Colorado, Boulder | University of Colorado, Boulder | ||
| − | |||
<b>Water and photon mediated chemistry relevant to atmospheric aerosol formation</b> | <b>Water and photon mediated chemistry relevant to atmospheric aerosol formation</b> | ||
| Line 2,449: | Line 6,393: | ||
- Development of a detailed model to represent SOA formation via the aerosol aqueous phase | - Development of a detailed model to represent SOA formation via the aerosol aqueous phase | ||
| − | = | + | <br /> |
| + | <font size="4">Monday, September 21, 2009</font> | ||
| + | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium | CIRES Auditorium | ||
| Line 2,475: | Line 6,421: | ||
Lithium ion batteries (LIBs) are emerging as the dominant power source for portable electronics. Improvement in their capacity lifetime during charge-discharge cycles must be achieved before LIBs can be used for plug-in-hybrid and electric vehicles. LiCoO2 and graphite are common cathode and anode materials, respectively, for LIBs. The capacity loss of LIBs is linked to electrode deterioration caused by interfacial reactions with the electrolyte, dissolution of the metal oxide cathodes and structural instabilities caused by volume expansion during lithium intercalation. | Lithium ion batteries (LIBs) are emerging as the dominant power source for portable electronics. Improvement in their capacity lifetime during charge-discharge cycles must be achieved before LIBs can be used for plug-in-hybrid and electric vehicles. LiCoO2 and graphite are common cathode and anode materials, respectively, for LIBs. The capacity loss of LIBs is linked to electrode deterioration caused by interfacial reactions with the electrolyte, dissolution of the metal oxide cathodes and structural instabilities caused by volume expansion during lithium intercalation. | ||
| − | Atomic layer deposition (ALD) is based on the strategy of sequential, self-limiting surface reactions to deposit inorganic materials. ALD can deposit thin, conformal films on cathode and anode materials in LIBs to provide chemical protection, restrict dissolution and stabilize structural changes. Our recent work has demonstrated that ultrathin Al2O3 ALD films can improve the capacity stability of LiCoO2 cathodes and graphite anodes. Additional work is developing new protective coatings that will serve as an artificial solid-electrolyte interphase (SEI) for further stability improvement. | + | Atomic layer deposition (ALD) is based on the strategy of sequential, self-limiting surface reactions to deposit inorganic materials. ALD can deposit thin, conformal films on cathode and anode materials in LIBs to provide chemical protection, restrict dissolution and stabilize structural changes. Our recent work has demonstrated that ultrathin Al2O3 ALD films can improve the capacity stability of LiCoO2 cathodes and graphite anodes. Additional work is developing new protective coatings that will serve as an artificial solid-electrolyte interphase (SEI) for further stability improvement. |
| + | |||
| + | <br /> | ||
| + | <font size="4">Monday, September 14, 2009 </font> | ||
| − | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium | CIRES Auditorium | ||
| Line 2,517: | Line 6,465: | ||
In addition to visualizing biomolecule interactions in cells, we are working on ways to mimic and disrupt such interactions for disease treatment. We view this largely as a materials problem as well, and have been learning how to design metal nanoclusters to effectively interact with cellular biomolecules. A few lessons learned in our studies of cell and nuclear targeting and the prevention of viral infection will be described. | In addition to visualizing biomolecule interactions in cells, we are working on ways to mimic and disrupt such interactions for disease treatment. We view this largely as a materials problem as well, and have been learning how to design metal nanoclusters to effectively interact with cellular biomolecules. A few lessons learned in our studies of cell and nuclear targeting and the prevention of viral infection will be described. | ||
| − | = | + | <br /> |
| + | <font size="4">Thursday, September 10, 2009 </font> | ||
| + | |||
4:00 p.m. | 4:00 p.m. | ||
CIRES Auditorium (note change of location) | CIRES Auditorium (note change of location) | ||
| Line 2,534: | Line 6,484: | ||
In the seminar, I will discuss several cases where entrainment plays a major role in the evolution of atmospheric compounds. By analyzing observational evidence or performing numerical experiments by the large eddy simulation technique and a conceptual (mixed-layer theory) model, we are able to find the contribution of entrainment to the diurnal variability of carbon dioxide or isoprene. Particular emphasis is placed on the need to maintain a balance in dynamic processes and the specific characteristic of each atmospheric compound. | In the seminar, I will discuss several cases where entrainment plays a major role in the evolution of atmospheric compounds. By analyzing observational evidence or performing numerical experiments by the large eddy simulation technique and a conceptual (mixed-layer theory) model, we are able to find the contribution of entrainment to the diurnal variability of carbon dioxide or isoprene. Particular emphasis is placed on the need to maintain a balance in dynamic processes and the specific characteristic of each atmospheric compound. | ||
| + | |||
| + | * | ||
Latest revision as of 17:16, 29 October 2024
This page contains the abstracts for upcoming and past Analytical & Environmental Chemistry Seminars at CU. Please post newer seminars at the top, but do not erase the abstracts from old seminars.
A shortcut to this page is: http://tinyurl.com/anylsem
Contents
- 1 4 November 2024
- 2 28 October 2024
- 3 7 October 2024
- 4 30 September 2024
- 5 23 September 2024
- 6 16 September 2024
- 7 Tuesday, 27 August 2024
- 8 Thursday, 15 August 2024
- 9 Spring 2024
- 10 Fall 2023
- 11 Summer 2023
- 12 Spring 2023
- 13 Fall 2022
- 14 Summer 2022
- 15 Spring 2022
- 16 Fall 2021
- 17 Spring 2021
- 18 Fall 2020
- 19 Summer 2020
- 20 Spring 2020
- 21 Fall 2019
- 22 Summer 2019
- 23 Spring 2019
- 24 Fall 2018
- 25 Summer 2018
- 26 Spring 2018
- 27 Fall 2017
- 28 Spring 2017
- 29 Fall 2016
- 30 Spring 2016
- 31 Fall 2015
- 32 Spring 2015
- 33 Fall 2014
- 34 Spring 2014
- 35 Fall 2013
- 36 Spring 2013
- 37 Fall 2012
- 38 Spring 2012
- 39 Fall 2011
- 40 Spring 2011
- 41 Fall 2010
- 42 Spring 2010
- 43 Fall 2009
4 November 2024
Freedom from Fluorocarbons: 2D Perovskites as the Next Generation of Refrigerants
Leo Bloxham,
CU ANYL 1st year
Phase change materials (PCMs) absorb, store, and release thermal energy as latent heat when transitioning between different physical states. Currently, hydrofluorocarbons (HFCs) are the most common PCM used for refrigeration purposes. Since HFCs are known greenhouse gasses, any alternative PCM which does not release gas into the atmosphere is of very high interest. Two-dimensional (2D) perovskites with n-alkylammonium cations often undergo a solid-solid phase transition upon changes in pressure or temperature, during which the organic cations undergo an order-to-disorder partial melting transition. Here, we demonstrate tunability of this phase transition by various alloying methods, including blending organic cations of varying lengths and blending the central metal cations. We see that blending organic cations significantly decreases the phase transition temperature of Cu and Mn-based 2D perovskites. The magnitude of this decrease depends on the relative lengths of the two n-alkylammonium cations, as well as the molar ratio of each organic cation included. Furthermore, we found that blending Cu2+ and Mn2+ has a slight effect on the phase transition temperature due to similar lattice spacing between the inorganic layers in DA2CuCl4 and DA2MnCl4. Our study suggests new design principles for tuning structural phase transitions in 2D perovskites through alloying organic and metal cations, proving 2D perovskites to be strong candidates for the next generation of refrigerants.
and
Rotational Spectroscopy of Water-Ketone Clusters
Natalie Couch,
CU ANYL 1st year
Gas phase microwave spectroscopy is a powerful tool for investigating intermolecular interactions such as hydrogen bonding. These weak interactions are difficult to investigate in solution, where, for example, hydrogen bonds are constantly being broken and reformed. In the ultrahigh vacuum (~10-6 Torr) inside my undergraduate lab’s microwave spectrometer, clusters of molecules connected via hydrogen bonds exist long enough for us to probe their rotational transitions. The microwave region of the electromagnetic spectrum corresponds to energy differences between clusters’ rotational states, which give precise information on the structure of the cluster. In this presentation, I will discuss interactions between water and 2-methylcyclopentanone, a ketone that acts as a hydrogen bond acceptor. I will describe my experimental setup to acquire spectra of these clusters and show some preliminary results.
28 October 2024
The Airborne and Remote sensing Methane and Air Pollutant Surveys (AiRMAPS) and Initial Results from Colorado and Utah
Steven S. Brown,
Tropospheric Chemistry and Atmospheric Remote Sensing Program Lead,
NOAA Chemical Sciences Laboratory, Boulder, CO
Adjoint Professor of Chemistry,
University of Colorado, Boulder, CO
The Airborne and Remote Sensing Methane and Air Pollutant Surveys (AiRMAPS) is a 3-5 year initiative led by the NOAA Office of Oceanic and Atmospheric Research (OAR) and National Environmental and Satellite Data Information Service (NESDIS) to comprehensively characterize U.S. emissions and impacts of methane and co-emitted air pollutants. The 2024 AiRMAPS campaign consisted of two separate deployments in Colorado (June 28 - July 14) and Utah (July 15 – August 18). The Colorado campaign included a NOAA Twin Otter equipped with in-situ instruments and a Doppler lidar for 3-dimensional winds for boundary layer mass balance, a NASA-supported B-200 with the AVIRIS-3 imaging spectrometer for detailed methane mapping, three mobile laboratories driving at surface level, and images from the GHGSat spaceborne spectrometer. The Colorado campaign surveyed oil and gas, agricultural, landfill and urban emissions in the Denver-Julesburg basin and Denver metro area. The Utah campaign consisted of mass balance flights with a NOAA Twin Otter, three mobile laboratories and surface networks, mapping overflights of the NSF G-V aircraft with the MethaneAir instrument, and satellite overpasses of MethaneSat. This campaign included detailed surveys of the Salt Lake City urban area, and coordinated aircraft measurements in the Uinta Basin oil and gas producing region. This presentation will outline the goals of and strategy for AiRMAPS and present initial results from the 2024 campaigns in Colorado and Utah.
and
Towards the ideal CIMS
Henning Finkenzeller,
Postdoctoral researcher at University of Helsinki, INAR/Physics, Finland, and Karsa Ltd, Helsinki, Finland
Chemical Ionization Mass Spectrometry has arguably enabled a “molecular revolution” in atmospheric sciences and has become an analytical workhorse in the field. Still, achieving sensitivity that covers the spectrum of chemically diverse trace gases has required combining multiple CIMS instruments with different ionization schemes which differ regarding both the reagent ions and the IMR pressure. For example, Proton Transfer Mass Spectrometry (PTR-MS) achieves sensitivity to VOCs employing a rather unselective ionization at low pressure, while clustering with NO3- at high pressure ionizes highly oxygenated compounds.
In my presentation, I will present how multi-pressure chemical ionization synergizes the advantages of different ionization chemistries within a single instrument to attain sensitivity to a broad range of compounds. Additionally, I show exploratory measurements with novel reagent ions.
7 October 2024
Chemistry of Volatile Organic Compounds in the Atmosphere
Joost de Gouw,
ANYL Faculty, CU Boulder
Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products.
In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments.
Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments to better understand their sources and fate. One particular study involves the impact of the Marshall Fire in Boulder County on indoor air in homes that were near the burnt area. Second, we are working on the emissions and chemistry of VOCs in urban air. One study that will be highlighted involved field measurements in Los Angeles in the Summer of 2022. Third, we are working on the indoor air chemistry induced by air cleaners such as germicidal UV lamps that are effective at inactivating airborne viruses. Finally, we are also using data from satellite remote sensing instruments to measure the pollutants from oil and natural gas production, in urban air and from wildfires.
and
Understanding aerosols for indoor and outdoor air quality and climate
Jose-Luis Jimenez,
ANYL Faculty, CU Boulder
Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly summarize ongoing projects in our group, with more emphasis on projects of interest to first year PhD students.
We frequently perform ambient aerosol composition measurements. We recently deployed an AMS in the NASA DC8 aircraft during the ASIA-AQ campaign in Spring 2024, investigating air pollution in South Korea, the Philippines, Taiwan, and Thailand; we also continue the analysis of data from previous aircraft campaigns. Since May 2023 we have been operating continuous measurements of aerosol composition in Denver with multiple instruments (ACSM, SMPS, Aethalometer, XAct) as part of the NSF ASCENT long-term monitoring program, which we are going to continue until at least 2030. We also collected data to characterize the precursors responsible for SOA formation in the Los Angeles area in Summer of 2022. I will summarize recent results from ongoing analyses of these projects.
Indoor air chemistry and airborne disease transmission has also been a focus over the last decade. Over the last 3 years we have been investigating the formation and/or removal of air pollutants by different “air cleaner” technologies, with a special focus on germicidal ultraviolet light (GUV). I will present results from recent experimental and modeling projects, including an analysis of GUV tradeoffs across all possible wavelengths, and the characterization of a catalyst to remove ozone from indoor air.
Dual frequency combs (DFC) are new light sources with unique properties for analytical spectroscopy. The mid-IR contains important chemical information about organic functional groups, but analysis of aerosols suspended in air in this region with FTIR was considered infeasible, due to interferences from narrow absorption lines of abundant gases such as CO2. As part of the collaborative IARPA SAURON project led by CU-Boulder Engineering, we are working to test the aerosol measurement capabilities of new DFC systems.
30 September 2024
Multiphase laboratory studies of wildfire smoke plume aging under wet and dry conditions
Prof. David O. De Haan,
Department of Chemistry & Biochemistry, University of San Diego
"Field studies have shown that when wildfire smoke plumes age under dry conditions in the atmosphere, approximately half of the total brown carbon (BrC) absorption tends to fade with time due to photobleaching. Cloud-processed smoke plumes, however, do not fade noticeably, but the chemical or physical transformations behind this effect are unknown. To address this gap, we burned four types of biomass samples under simulated smoldering conditions (400 C) and fractionated the smoke for a series of chamber experiments. In one set of experiments, whole smoke, the VOC fraction, and denuded smoke where oxidized by OH, ozone, or NO3 under wet and dry conditions in a steel cloud chamber. In a second set of experiments, smoke POA, denuded POA, and water-soluble gas components of smoke were collected and re-aerosolized at constant rates into an oxidation flow reactor (OFR) for integrated OH exposure of 2 – 9 d of atmospheric oxidation. Observations of aerosol bounce indicated that aerosol particles had viscosities of > 1 Pa s even before photooxidation. No bleaching at 405 nm and little chemical reactivity was observed when smoke aerosol was exposed to the highest levels of OH radicals under dry conditions in the OFR. These observations suggest that diffusion is limited in smoke OA and that OH reactivity is limited to smoke aerosol surfaces. Ozone exposure, on the other hand, oxidized smoke OA on a 12-min timescale in the steel chamber., especially under dry conditions. "
23 September 2024
Environmental aquatic chemistry and source water protection
Prof. Fernando Rosario-Ortiz,
Environmental Engineering, CU Boulder
"Source water protection is an important consideration for the continued production of potable water in the US and abroad. Source waters can be impacted by contamination from anthropogenic sources as well as natural perturbations such as from wildland and urban fires.
The presence of organic contaminants in source waters is a concern for potable water systems. These organic contaminants can be removed via advanced oxidation processes, as well as via sunlight driven photochemical processes. In this later case, reactions are promoted via the formation of reactive intermediates. These reactive intermediates include short lived radical species that are formed from photochemical processes within dissolved organic matter. In my group, we study the formation of these reactive intermediates.
We also study how fires impact source water quality and ultimately potable water production. In this respect, we look at how compounds are formed, mobilized and degraded as well as how treatment systems can remove them. Lastly, we also study how compounds mobilized after fires can impact the formation of regulated disinfection byproducts."
and
Particle Formation and Growth in New Chemical Regimes
Prof. Ellie Browne,
CU ANYL Faculty
"Understanding the mechanisms through which aerosol particles form and grow is critical for constraining a planet’s energy budget, however, our knowledge of the key processes governing particle formation and growth in diverse environments remains weak. In the coming decades, our knowledge of particle formation and growth will continue to be challenged as changing climate and anthropogenic emissions alter the chemical regimes of modern-day atmospheric chemistry on Earth and measurements from, for instance, the James Webb Space Telescope and NASA’s Dragonfly mission offer increasingly chemically resolved insights into planetary atmospheres much different from our own.
In this talk I will highlight our projects on particle formation and growth in new and/or understudied chemical regimes. I will briefly discuss our recent advances in three different areas: how reactive nitrogen chemistry is important in agricultural regions, how the oxidation of volatile methyl siloxanes contributes to SOA in urban regions, and how organosulfur compounds affect gas- and particle-phase composition in anoxic planetary atmospheres."
16 September 2024
Chemistry at Aqueous Interfaces: Iodine, Ammonia, and Organics in the Anthropocene
Prof. Rainer Volkamer
CU ANYL Faculty
"Anthropogenic enhancements of natural processes modify atmospheric chemistry in the urban, remote marine, forested, and upper atmosphere. For example, the deposition of ozone at the air-sea interface leads to marine iodine emissions that have tripled in recent decades due to increases in pollution ozone over oceans, affecting particle formation, tropospheric and stratospheric ozone. In the upper troposphere, high concentrations of ammonia, NH3, drive the formation of the Asian Tropopause Aerosol Layer (ATAL), suggesting efficient transport through convective clouds despite the extremely high water solubility (Henry’s Law) of this gas. Can we measure the ice retention efficiency of NH3 experimentally in the laboratory? What are the implications for cloud processing for other water soluble oxygenated VOCs, like glyoxal and fatty acids? The Volkamer group is interested in studying the fundamentals of atmospheric processes that affect radiation in the atmosphere. We develop advanced instrumentation (optical spectroscopy and mass spectrometry) to measure small molecules and condensable vapors relevant to particle formation, ozone, public health and climate discussions. Our molecular perspective and focus on fundamentals aims to advance atmospheric models, and has helped satellites measure new species (e.g., glyoxal, HONO, NH3). Opportunities for graduate research in field measurements, laboratory experiments, and chemical calculations are discussed (i.e., CLOUD/CERN; TI3GER2; AIRMAPS; TEMPO)."
Tuesday, 27 August 2024
Iodine activation and recycling driven by Fe(III)-carboxylate photochemistry
Lucia Iezzi,
Paul Scherrer Institut
2:00 PM
Ekeley S274
"Iodine represents a globally important sink for ozone, influences the oxidative capacity of the troposphere and can efficiently nucleate particles. Nevertheless there is still lack of information on reactive iodine precursor gas fluxes. Recent field measurements have found indication of active chemical recycling of iodine from the particulate phase back to gas phase. This recycling may be driven by still not well understood reductive processes involving particulate iodate. In our work we used Coated Wall Flow Tube experiments to explore the role of Fe(III)-carboxylate photochemistry for iodine activation and recycling, and found that the coupling between ROS, iron and iodine species can efficiently reduce iodate and give unexpected feedbacks to the Fe(II)/(III) redox cycling."
Thursday, 15 August 2024
Utilizing the Properties of Functional Groups to Understand Indoor, High NOx, and Low NOx Atmospheric Chemistry
Anna Ziola,
Ziemann group
ANYL Dissertation Defense
2:00pm
CASE E240
https://cuboulder.zoom.us/j/96404067692 (Meeting ID: 964 0406 7692)
"Over 1000 Tg of volatile organic compounds (VOCs) are emitted into the atmosphere every year from both biogenic (Guenther et al., 2012) and anthropogenic (Huang et al., 2023) sources, an equivalent weight of about 200,000 school buses per day. Depending on the structure of these compounds and conditions in which they exist, they can remain in the atmosphere for seconds to years. This thesis investigates the fate of selected volatile organic compounds in three types of conditions: low NOx, high NOx, and indoor environments.
The first study was inspired by the significant decrease of nitrogen oxides (NOx) in the U.S. in the last few decades. This reduction has led to the oxidation of VOCs – crucial for the formation of ozone and fine particles – occurring in urban areas under conditions historically associated with remote rural locations. To gain a better understanding of VOC oxidation mechanisms, I investigated the fate of two model linear alkenes that were chosen due to the high abundance of alkenes in atmospheric emissions and the simplicity of the selected compounds. By reacting 1-pentene and 3-butenoic acid with OH radicals in an environmental chamber under low NOx conditions, monitoring the gas- and particle-phase products (and 3-butenoic acid) using real-time mass spectrometry and offline techniques, and kinetics modeling, I was able to elucidate the oxidation mechanisms of these compounds. This allowed for a better understanding of the effect of a carboxyl group on reaction branching ratios and product yields.
In the second study, I investigated the OH radical-initiated oxidation of a model ether compound under high NOx conditions. Ether groups are commonly found in more functionalized volatile chemical products (VCPs) – anthropogenic sources of VOCs that come from cleaning products, personal care products, adhesives, and elsewhere (Seltzer et al., 2021). Although it is known that ether groups affect reaction kinetics, nitrate yields, and SOA yields, the mechanism and degree to which a single ether group impacts the products formed from a large ether has not been determined or quantified. Therefore, for this study I reacted dioctyl ether (a symmetric C16 compound with a central ether group) with OH radicals under high NOx conditions; identified and quantified the gas- and particle-phase products using mass spectrometry, gas chromatography, liquid chromatography, and spectrophotometric techniques; and developed a quantitative reaction mechanism to explain their formation which could be used in models.
In the final chapter I investigated the fate of carboxylic acids and ozone in simulated indoor conditions. Humans spend the majority of their life indoors, yet we have limited knowledge about the chemistry that occurs in these spaces. One major process that impacts the composition of indoor air is surface chemistry. Indoor spaces can have dozens of different surfaces, each with unique properties and compositions. Here, I investigated the mechanisms by which carboxylic acids and ozone interact with various wood species and wood coatings, including lacquer and shellac. An iodide chemical ionization mass spectrometer (I-CIMS) and ozone monitor were used to measure uptake coefficients and deposition velocities in uncoated and coated tubes, an attenuated total reflectance-Fourier transform infrared spectrometer was used to measure diffusion coefficients of carboxylic acids in wood and coatings, and a multi-layer model was used to extract compound diffusion coefficients and material absorbance capacities from experiments. The results were then used to develop a conceptual model for describing the interactions of carboxylic acids wood and coatings."
Spring 2024
10 May 2024
Mountaintop DOAS observations of trace gases in the remote atmosphere: Relevance for ozone and mercury oxidation
Christopher F. Lee,
Volkamer group,
ANYL Dissertation Defense
"Although ozone is a ground-level pollutant, ozone in the stratosphere absorbs solar radiation and therefore reduces the amount of harmful ultraviolet light reaching the Earth’s surface. Mercury is a potent neurotoxin and global environmental hazard which enters the ecosystem following oxidation and subsequent deposition from the atmosphere. While ozone chemistry is relatively well-understood, atmospheric mercury chemistry is highly uncertain.
The chemistry of atmospheric halogens and their impact on ozone and mercury oxidation are investigated using Differential Optical Absorption Spectroscopy (DOAS) measurements of trace gases in conjunction with other measurements at mountaintop observatories on remote islands in the northern and southern hemisphere tropics (5 years of observations), and over the central continental United States (1.5 years of observations). Total ozone (O3) and stratospheric nitrogen dioxide (NO2) vertical columns are presented for the entire measurement period at all three sites. Other trace gases, including iodine monoxide (IO), bromine monoxide (BrO), sulfur dioxide (SO2), formaldehyde (HCHO), and water vapor (H2O) are presented for case study periods.
The first ground-based observations of IO radicals in the continental troposphere are presented, alongside co-located measurements of gas-phase elemental and oxidized mercury. The observed levels of tropospheric IO were up to three times higher than predicted by the GEOS-Chem global chemical transport model. Furthermore, the observed levels of oxidized mercury were up to ten times higher than predicted by the same model, which considers only bromine and hydroxyl radicals as major mercury oxidants. The role of iodine as a mercury oxidant was evaluated using a chemical box model of gas-phase mercury chemistry which included iodine, bromine, and hydroxyl radicals as mercury oxidants.
Observations of the 2023 Hunga Tonga volcanic plume in the stratosphere over the southern hemisphere site are presented. The eruption injected an enormous amount of water vapor into the stratosphere, increasing the total amount of stratospheric water vapor by 10% and resulting in a 5% reduction of ozone in the tropical stratosphere. A suite of ground-based, balloon-borne, and satellite observations revealed that the stratospheric ozone depletion was likely due to chlorine reservoir species being efficiently converted into reactive chlorine radical species as a result of the water vapor injection. Zenith-sky DOAS measurements of stratospheric NO2 and BrO constrained the role of NOx and BrOx in the observed ozone depletion."
22 April 2024
A partial story of an aircraft-based laminar gas sampling inlet
Da Yang,
Volkamer group
"Aircraft-based measurements allow for large spatial-scale characterization of atmospheric aerosol and gas, but these measurements, under high-speed flow conditions, complicate efforts to maintain sample integrity through the inlet transport process. Of particular concern is the role of turbulence in driving loss of gas-phase species and aerosol particles. While a significant amount of research has gone into understanding aerosol sampling efficiency for aircraft inlets, a similar research investment has not been made for gas sampling. To study the gas sampling loss of aircraft-based measurements, we used computational simulations and wind tunnel experiments to analyze the gas sampling performance of a laminar gas inlet developed and used in the Ti3GER project. We conducted measurements of H2SO4 in a high-speed wind tunnel. The gas transmission efficiency of H2SO4 through different sampling lines was measured using CIMS, and the experimental results are being compared to simulations of flow and mass diffusion modelling in the sampling line. Both experimental data and simulation results show the gas transmission efficiency increases with an increased sampling flow rate, including turbulent flow. In this presentation, I would like to give a short introduction to my work on studying aircraft-based sampling systems and focus on sharing the information I learned from gas-phase H2SO4 measurement results."
and
Characterisation of Br-CIMS response under different humidity level and its application in CLOUD 16
Yandong Tong,
Volkamer group
"Chemical ionisation mass spectrometry (CIMS) exhibits high sensitivity and versatility in gaseous species, therefore, it has been widely used in atmospheric chemistry studies, especially in new particle formation and secondary organic aerosol formation studies. Among the different ionisation methods, CIMS using bromide (Br-) as reagent ion has been recently developed, optimised, and characterised, which is suitable to detect various organic and inorganic species, e.g., iodine and its oxidation products. However, Br-CIMS shows humidity dependence in many studies. In this study, we developed a new water regulation system for our CIMS and implemented it in laboratory experiments and in the CLOUD 16 campaign at CERN in Geneva, Switzerland. By taking advantage of the water regulation system and CLOUD chamber facility, we explored the Br- CIMS sensitivity to different species in a wide range of humidity levels. For strongly bonded species, e.g., iodine (I2), Br-CIMS sensitivity to these species increases as the humidity level increases, whereas Br-CIMS sensitivity to weakly bonded species, e.g., glyoxal (CHOCHO), exhibits the opposite trend. A decent understanding of Br-CIMS response to different species under various humidity conditions allows us to provide valuable measurements in CLOUD 16, especially helping us understand the role of CHOCHO in nucleation events."
15 April 2024
Laboratory and mechanistic studies of complex VOC oxidation systems
Dr. Qing Ye,
NCAR Atmospheric Chemistry Observations & Modeling Lab
"Improving our knowledge of the atmospheric oxidation chemistry of volatile organic compounds (VOCs) is crucial to our understanding of how air pollutants form and evolve. One major challenge in the understanding of VOC oxidation arises from the extreme complexity in the oxidation processes which convert the precursor to new oxidized products and exponentially increase the number and diversity of species in the reaction mixture. To study the complex oxidation processes, laboratory chamber experiments and mechanistic simulations are two commonly used tools that have different advantages and limitations. In this seminar, I will compare a chamber dataset on alpha-pinene oxidation with a mechanistic dataset generated by a hyper-explicit mechanism generator, GECKO-A. The measurement dataset was collected by a suite of advanced analytical instruments, with the goal of achieving a near-complete description of the reactive carbon. The measurement-mechanism comparisons on a species-to-species level and an ensemble property level will be discussed. I will then show how targeted adjustments to the mechanisms in GECKO-A affect its overall agreement with chamber observations."
5 April 2024
New observations and models of ozone photochemistry in wildfire and urban plumes
Michael Robinson,
Brown Group,
ANYL Dissertation Defense
"Atmospheric reactions of nitrogen oxides (NOx = NO + NO2) and volatile organic compounds significantly impact the composition of the troposphere in the form of ozone (O3) and secondary organic aerosol. Despite decades of research and precursor regulation, O3 pollution in the United States continues to be a problem in urban areas. Increasing wildfire occurrence and intensity in the western United States raises the question of wildfires' impact on this air quality issue. Critical questions regarding this topic include: 1) which photochemical regime does O3 production occur in western wildfires, 2) how do O3 photochemical regimes compare across four major cities in North America, 3) does changing O3 photochemical regimes in North America necessitate new approaches to quantifying chemical regimes. In this defense, I address these questions with 1) aircraft observations and modeling of western wildfires to describe and quantify the photochemical regime and 2) an analysis of isoprene peroxy radical fate in four urban areas from aircraft and ground site observations."
2 April 2024
Oxidation of Monoterpenes in the Atmosphere and Indoor Environments
Olivia Jenks,
de Gouw group,
ANYL Dissertation Defense
"Monoterpenes are emitted into the atmosphere from vegetation and indoor products like personal care items and building materials. Once in the atmosphere, monoterpenes undergo oxidation by ozone (O3), hydroxyl radicals (OH), and nitrate radicals (NO3), forming secondary organic aerosol (SOA). These aerosols play crucial roles in the climate system, in limiting visibility, and impact human health. Aerosols reflect sunlight, contributing to a cooling of climate, and influence cloud properties. However, their small particle size enables them to penetrate deep into the lungs of humans, posing risks to cardiovascular and respiratory health. My first project involved characterizing the gas-phase products of monoterpene oxidation, focusing on Δ-3-carene, α-pinene, β-pinene, ocimene, and limonene. Innovative techniques were developed to identify and quantify these products, including the use of tubing delay experiments with the Vocus mass spectrometer. Field studies in Los Angeles corroborated findings from the lab, highlighting the importance of both daytime and nighttime chemistry. The composition of the monoterpene oxidation products observed in the field were compared to that of the lab studies, contributing to a comprehensive understanding of atmospheric VOC oxidation processes. My second project investigated the impact of germicidal ultraviolet (GUV) irradiation on indoor VOC oxidation. GUV has been widely used for air disinfection, particularly in public settings like hospitals and schools. However, its effect on indoor air quality remains poorly understood. By irradiating air that contains common indoor volatile organic compounds (VOCs), like limonene, in a controlled chamber, the impact of GUV light on the monoterpene ozonolysis process was studied. This work contributes to a better understanding of the implications of GUV disinfection on indoor air quality and human health and will inform strategies for improving indoor air quality and reducing health risks associated with VOC exposure in indoor environments."
1 April 2024
Investigating SOA Formation from Volatile Methyl Siloxanes
Hanalei Lewine,
ANYL 3rd year, Browne group
"Volatile methyl siloxanes (VMS) are anthropogenic organosilicon molecules that are used in a variety of applications including in personal care products. Decamethylcyclopentasiloxane (D5) has been detected at high mixing ratios indoors and outdoors, with 90% of the D5 in cosmetics being released to the atmosphere. VMS have recently been identified as precursors to aerosol in urban areas, however lab studies of D5 oxidation have been unable to fully characterize D5’s SOA yield. The dominant oxidation product, the siloxanol, has been observed in lab and ambient aerosol, however absorptive partitioning alone cannot explain the amounts measured. Previous SOA experiments did not control for seed and wall effects, and did not measure gas phase chemistry leading to SOA. Here, we performed chamber experiments to investigate how the presence of seed aerosols impacts D5 SOA formation, if multigenerational chemistry is required for D5 SOA, and how RO2 fate impacts D5 SOA. We measured the gas phase products of D5 oxidation using chemical ionization mass spectrometry (CIMS) and measured particle size distribution using SMPS. We find that at lower OH exposures, with only one generation of oxidation chemistry, we still make SOA. Despite challenges in data analysis, it is clear that seed aerosol is important for D5 SOA formation."
18 March 2024
Comparison of Common Vapor Pressure Estimation Methods through Modeling of the Reactions of Linear and Branched Alkenes with OH/NOx
Emmaline Longnecker,
ANYL 3rd year, Ziemann group
"Modeling atmospheric reactions that lead to the formation of secondary organic aerosol (SOA) is an important tool for understanding the current and future impacts of human activity on the environment. Vapor pressure is a key parameter in modeling these reactions, as it largely determines the gas-particle partitioning of atmospheric oxidation products. However, the vapor pressures of many atmospherically relevant molecules are still poorly constrained. To aid modeling efforts, several structure-activity relationships (SARs) based on group contribution methods have been developed for estimating compound vapor pressures. The current study evaluates how four of these SARs: SIMPOL, EVAPORATION, SPARC, and Nannoolal, impact the modeled predictions of SOA yields for reactions of C8-C14 1-alkenes and C9-C15 2-methyl-1-alkenes with OH radicals in the presence of NOx. The models include well-constrained, quantitative reaction mechanisms developed by our research group from several previous environmental chamber studies of product yields, gas-particle and gas-wall partitioning, and secondary reactions with OH radicals. Based on our previous studies, there was no need to account for particle-phase oligomer formation. Comparison of modeled and measured SOA yields provided insight into the major products responsible for SOA formation over the large range of carbon numbers, and the sources of discrepancies between model predictions and measurements. The wide range of agreement exemplifies the impact of vapor pressure in modeling atmospheric reactions and indicates the need for further development of estimation methods."
11 March 2024
Career panel with ANYL alumni:
We have asked the speakers to present for 12-15 min on their perspective on careers outside academia for PhD chemists, and in particular for graduates of our ANYL PhD program. For example, they may address the following questions: what path took you to where you are? What strengths from the ANYL program helped you in your career? What could have the program done to make that better? What do you wish someone had told you when you were a PhD student? What advice would you give to current PhD students and postdocs? A Q&A session will follow, prioritizing questions from current students and postdocs.
Ingrid Ulbrich,
Achieving Academic Success
"Ingrid Ulbrich (Ph.D., Jimenez Group, 2011) discovered in grad school that teaching was a much greater passion than research. And that she needed the Ph.D. for teaching jobs in higher ed. So she pushed through to complete her research (applying PMF to tropospheric organic aerosol; H-index = 26 as of 2020) and began helping students get better at problem solving. After teaching CU (Gen Chem to grad-level data analysis), she became the General Chemistry Lecture Coordinator at Colorado State University. While teaching first-year courses she saw the true struggles of students – not underdeveloped math skills or challenges dissecting word problems – but lacking a life vision, believing the voice that says they’re not smart enough, and inability to manage major life events while prioritizing academics. After encountering a curriculum that helps students learn 'how' to learn and become self-growers, Ingrid discovered how to empower students to overcome those struggles. Ingrid founded a nonprofit in 2021, Achieving Academic Success, to develop students as master learners who pursue their life visions on a path of continued growth and create the lives they want to live."
&
Erin McDuffie,
US EPA
"Dr. Erin McDuffie is a physical scientist at the U.S. Environmental Protection Agency. Erin received her PhD from the Analytical, Environmental, and Atmospheric Chemistry program at the University of Colorado Boulder in 2018. During her PhD, Erin worked with Dr. Steve Brown in NOAA’s Chemical Sciences Laboratory, where she focused on in situ sampling and simple chemical box modeling to better understand the sources and chemical transformations of ozone and fine particulate matter. After graduate school, Erin transitioned to using global atmospheric chemistry transport models to study air pollution impacts on human health as a post-doctoral research fellow at Dalhousie University with Dr. Randall Martin (now at Washington University in St. Louis). Erin completed a 2020-2022 AAAS Science and Technology Policy Fellowship at the U.S. EPA in Washington, D.C., and is currently an atmospheric scientist in EPA’s Climate Change Division, working on a wide range of science and policy questions related to better understanding, communicating, and mitigating future risks of climate change. "
4 March 2024
From the air to the sea: Addressing environmental challenges through fundamental chemistry
Dr. Ryan Davis,
Sandia National Laboratories,
University of New Mexico
"Environmental systems, such as the hydrosphere and atmosphere, are a complex balance of chemical and physical processes that impact nearly every facet of life. In the atmosphere, aerosol particles – tiny particles of liquid and/or solid floating in the air -- have a strong influence on air quality, climate, and human health. For example, the aerosol effect on climate is one of the largest sources of uncertainty in climate models. Human respiratory aerosol can also transmit certain diseases. Understanding aerosol particles is a unique challenge because they can exhibit chemical and physical properties that are not observed in bulk solution. In the first part of this talk, I will discuss efforts to understand and characterize the unique properties of aerosol particles using levitation-based techniques to understand aerosol phase state. With this approach we have demonstrated unique supramolecular effects on the phase state of aqueous aerosol particles composed of organic and inorganic compounds. The environmental and public health implications for droplet-based supramolecular chemistry will be discussed. In the second part of the talk, we will turn discussion to the hydrosphere, where anthropogenic activities have contaminated global water sources with environmentally-persistent chemical species. One such class of “forever chemicals” are per- and poly-fluoroalkyl substances (PFAS), which are highly resistant to natural degradation processes. PFAS are used widely in a range of consumer and industrial products and their widespread use has led to a global distribution. Certain PFAS have been linked to a myriad of environmental and human health problems, so there is a need to remove these compounds from water supplies. Here, I will discuss ongoing efforts to destroy, sequester, and detect PFAS. Along the way, I will highlight other areas of my ongoing research at Sandia National Labs, including gas- surface interactions on metal surfaces, machine-learning based climate models, and the development of analytical chemistry capabilities."
Sandia National Laboratories is a multimission laboratory managed and operated by National Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International Inc., for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE- NA0003525. This talk describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the talk do not necessarily represent the views of the U.S. Department of Energy or the United States Government.
28 February 2024
Atmospheric Aerosols: Quantification and Method Development
Melinda Schueneman,
Jimenez Group,
CU ANYL Dissertation Defense
"The composition of atmospheric aerosols is influenced by the source/atmospheric conditions (e.g. rural, polluted, and/or biomass burning) as well as gas (g)↔particle (p) partitioning in an air mass. Several analytical techniques can be used to measure aerosol chemical composition, including Extractive Electrospray Ionization Mass Spectrometers (EESI, molecular ions of components of aerosols) and Aerosol Mass Spectrometers (AMS, typically bulk aerosol composition). Here, two methods for understanding aerosols generated in laboratory experiments are introduced. The first method focuses on a procedure that allows for calibration of molecular species. The second involves the quantification of secondary organic aerosol (SOA) formation and g↔p partitioning for known Volatile Organic Compound (VOC) precursors.
In part I, a new calibration technique is designed and tested, which combines High Performance Liquid Chromatography (HPLC), aerosolization, SMPS, and Positive Matrix Factorization (PMF) to calibrate the EESI and AMS. Species in complex aerosol mixtures (like SOA) can be separated and calibrated for, in the absence of reference standards.
In part II, the SOA formation potential from the oxidation by OH of different biomass burning VOC precursors was quantified. An iterative solver within a kinetic model was developed, including the effect of vapor wall loss (VWL) in atmospheric chambers. This allowed constraining g↔w↔p partitioning for the reaction products of each VOC. I compared the volatility of SOA to that of primary OA (POA) in a simulated wildfire and found that POA appears to be less volatile, thus more likely to be retained in the particle phase as wildfire smoke ages.
The results of this work will help to better understand and model aging biomass burning emissions and provide new methods for online aerosol chemical composition measurements."
26 February 2024
Efficiency of Urban Ozone Photochemistry during the 2023 AEROMMA and CUPiDS Airborne Field Campaigns
Wyndom Chase,
CU ANYL 3rd year, Brown group
"The summer 2023 Airborne Emissions and Reactions Observed from Megacities to Marine Areas (AEROMMA) and Coastal Urban Plume Dynamics (CUPiDS) field campaigns provided extensive in-situ observations of ozone (O3) and its precursors in the largest United States urban areas (New York City, Chicago, and Los Angeles). We present the distribution of O3 in these megacities, along with the distribution of its precursor nitrogen oxides (NOx = NO + NO2) and total reactive nitrogen (NOy), as measured from the NASA DC-8 and NOAA Twin Otter aircraft platforms during AEROMMA and CUPiDS, respectively. Several metrics calculated from in-situ trace gas measurements can aid the understanding of the photochemical regimes in which O3 production takes place. The O3 production efficiency (OPE) is a measure of the amount of O3 produced per unit NOx that is emitted and oxidized and is often taken as the ratio of enhanced O3 or Ox (= O3 + NO2) to NOz (= NOz - NOx) within an urban plume transect. The spatial and temporal variability of OPE probes the evolution of O3 sensitivity within a given urban area. Comparison of this metric between urban areas, in this case New York City, Chicago, and Los Angeles, elucidates differences or similarities across major U.S. urban areas. This analysis establishes a foundation for future work that will incorporate additional O3 sensitivity metrics, as well as chemical box modeling and remote sensing observations, to gain greater insight into the current landscape of U.S. O3 photochemical pollution."
19 February 2024
Observing Wildfire Emissions and Improving Air Quality Forecasting Using Remote Sensing Data
Lindsey Anderson,
CU ANYL 3rd year, de Gouw group
"Human-caused climate change and the buildup of fuel from fire suppression practices has led to an increase in the frequency and intensity of wildfires in the western United States. Wildfire emissions of particulate matter and reactive gases impact human health, affect local and downwind ozone (O3) formation, and influence the climate. The magnitude and chemical composition of wildfire emissions in operational air quality forecasts depend on the vegetation type and the burned area. However, laboratory and field measurements have shown that the composition of wildfire emissions also depends on whether the wildfire is experiencing mainly flaming combustion or mainly smoldering combustion, which changes throughout its lifetime. Including a parametrization of how the composition of wildfire emissions changes with evolving combustion conditions could improve air quality forecasts, especially for pollutants such as O3. Here, we will show how remote sensing observations of trace gases from the Tropospheric Monitoring Instrument (TROPOMI) can be used to observe changes in the composition of wildfire emissions over time, as wildfires transitions from mainly flaming to mainly smoldering combustion. We will also discuss how the Hourly Wildfire Potential (HWP) index, which is derived from NOAA’s High Resolution Rapid Refresh (HRRR) model predictions of wind speed, temperature, humidity, and soil moisture, relates to trace gas composition from TROPOMI and can be used to parametrize how NOx emissions vary with forecasted changes in combustion conditions. Using this technique, we have found improvements in forecasted NOx and O3 during the FIREX-AQ field study. These are the first experimental simulations in which the chemical composition of wildfire emissions varies online in a high-resolution (3km x 3km) convection-allowing air quality forecast. Looking forward, we also plan to use HWP in order to parametrize the vertical distribution of emissions, because this is also dependent on the dominant combustion type."
12 February 2024
Making Air Quality Count: Low-cost Sensors. Public Health and Urban Planning
Prof. Priyanka deSouza,
Urban and Regional Planning Department,
CU-Denver
"Air pollution is responsible for ~ 7 million premature deaths every year. 90% of these deaths occurred in the Global South. How can countries in the Global South improve air quality? This talk takes a multipronged approach to answer this question. First: air pollution data is critical for the development of effective air quality management plans. Few cities in the Global South have extensive regulatory air pollution monitor networks, and when they do, the data are often not publicly available. This talk proposes novel methodologies to leverage data from low-cost sensors and satellite data to fill in the air pollution data gaps. However, air pollution data is only one piece of the puzzle. To convince policy-makers of the urgency of tackling air pollution this talk also evaluates the health impact of air pollution in the Indian and African contexts. Finally, how does data ultimately lead to action? This talk ends by describing what actions air quality measurements have led to in Kenya, and what existing barriers to effective policy actions are."
Fall 2023
4 December 2023
Ozone in wildfire smoke and its influence on regional and global ozone
Steven S. Brown
Tropospheric Chemistry and Atmospheric Remote Sensing Program Lead,
NOAA Chemical Sciences Laboratory, Boulder, CO
Adjoint Professor of Chemistry,
University of Colorado, Boulder, CO
The frequency, burned area and emissions from wildfires have been increasing in North America for the last four decades. Biomass burning is a known sources of ozone precursors, nitrogen oxides (NOx) and volatile organic compounds (VOCs). Increasing wildfire emissions have influenced trends in North American urban ozone. The pyrogenic influence on ozone occurs either through ozone production within smoke plumes that is then transported to urban regions, or through the mixing of pyrogenic VOCs with urban NOx to enhance local and regional ozone production. This presentation will use data from recent airborne and ground-based field campaigns to quantify these processes. The 2016-2017 Atmospheric Tomography mission (ATom) assessed the influence of biomass burning at hemispheric and global scales. The 2019 Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ) sampled wildfire smoke across the U.S. with multiple research aircraft. The 2022 California Fire Dynamics Experiment (CalFiDE) conducted focused in-situ and remote sensing measurements in California and Oregon. Ground-based measurements in Boulder, Colorado intercepted periods of smoke influence in the Northern Front Range urban area in 2020 and 2021. Finally, the 2023 Atmospheric Emissions and Reactivity Observed from Megacities to Marine Areas (AEROMMA) campaign on the NASA DC-8 and the Coastal Urban Plume Dynamics Study (CUPiDS) on the NOAA Twin Otter observed long range smoke transported to U.S. urban areas and the associated impacts on ozone.
and
The Physical and Electrochemical Studies of Perylene Diimide Compounds
Jim Hall,
CU 1st Year
Solar photovoltaics are an important part of the worlds climate change mitigation strategies; however, the solar cells being use commercially only perform at 10-15% efficiency. Improving these cells to reduce the energy lost to thermalization can be an important step in increasing the efficiency of these cells. Perylene diimides (PDIs), a class of organic industrial dyes, have shown great promise in their ability to harness this energy loss through a process called singlet fission. This talk looks into the transient absorption measurements of some of these PDI compounds and shows their ability to downconvert this energy to be better used by a solar cell. In addition, we will explore the cyclic voltammetry (CV) of these compounds; despite several rounds of troubleshooting and problem solving, the set up being used did not show any significant oxidation or reduction events within the voltages being scanned. This is likely due to reactions with oxygen during the CV process, despite best efforts to remove it.
27 November 2023
Indoor Air Quality in Western Canada During Wildfire Episodes
Rebecca Mesburis,
CU ANYL 1st year
The increased frequency, duration, and intensity of wildfires has raised public awareness of the impact of wildfire smoke on air quality and human health. The 2023 wildfire season in Canada broke the record for the most area burned in North America’s history at about 18.5 million hectares. The main threat to public health from wildfire smoke is particulate matter, with particulate matter that have a diameter of 2.5 micrometers or less (PM2.5) contributing to most of the total particulate mass and travelling significant distances from the source of the fire. Recently, low-cost air quality sensors have been used in air quality studies due to their ability to capture high-resolution spatiotemporal data. We used the publicly available low- cost PurpleAir sensor network to gather indoor and outdoor PM2.5 data in Kamloops, Canada from January to December 2021 with the goal to assess the level of exposure to wildfire PM2.5 relative to other sources of PM2.5. Given that we obtain most of our particulate inhalation exposure when indoors, changes to the indoor environment during wildfire episodes were emphasized. On wildfire-influenced days, wildfire PM2.5 dominated the indoor exposure sources and indoor PM2.5 was almost always less than outdoor PM2.5. On non-wildfire- influenced days, no typical relationship was established between indoor and outdoor PM2.5. The analysis was limited by the number of PurpleAir sensors and knowledge of the indoor environments studied. The findings indicate that remaining indoors during wildfire events is currently an effective but finite strategy to limit PM2.5 exposure in Kamloops.
and
Exploring Alcohol Adsorption to Gold Induced by Applied Potential
Drew Blauth,
CU ANYL 1st year
Self-assembled monolayers (SAMs) play vital roles in battery development, biosensor function, and drug delivery. Extensive research has been directed towards adsorbate and substrate pairs that spontaneously form SAMs, such as thiols and gold, leaving pairs that do not spontaneously form SAMs relatively understudied. This project investigated alcohol adsorption to gold, which has been ignored by researchers due to oxygen - gold bond being relatively weak. By applying potential to gold substrates immersed in alcohol solutions and observing alcohol adsorption to gold using Surface Enhanced Raman Spectroscopy (SERS), it was determined that alcohol adsorption to gold could be induced and interrupted repeatedly by cycling between positive and negative applied voltages. While it is unclear exactly how the alcohols interact with the gold, these results suggest that applied potential could be used to create alcohol and gold SAMs, greatly broadening the applications SAMs could be used for.
13 November 2023
Impact of Cleaning on Concentrations of Volatile and Semivolatile Organic Compounds in A Normally-Occupied Residence
Nathan Sweet,
CU ANYL 1st year
The use of cleaning products alters the chemical composition of indoor air. Utilizing data from detailed observational monitoring conducted over multiple months, we explore the influence of cleaning activities in a normally occupied single-family house. To study emissions and chemistry, we quantified more than 200 VOCs using a proton-transfer reaction time-of-flight mass spectrometer and 52 SVOCs using semivolatile thermal-desorption gas chromatography. During regular professional home cleaning, we observed concentration enhancements in ~60% of observed VOCs and ~80% of reported SVOCs. Most of these concentration enhancements were not clearly linked to either primary emission from cleaning products or secondary formation through reactive chemistry. Instead, shifts in the sorptive properties of indoor surfaces may account for these observations. Individual concentrations of four chlorinated compounds, including dichloramine, increased by up to 0.8 ppb during bleach cleaning. Most cleaning-associated compounds returned to pre-event concentrations within a few hours, with some VOCs and lower volatility SVOCs persisting more than 5 hours, longer than would be expected for removal by ventilation alone. Concentrations of ultrafine particles also increased during professional cleaning, likely from nucleation events associated with bleach cleaning. Use of carpet cleaner was associated with emission of hexanediol, which persisted at elevated concentrations for days after the initial event. We infer that surface sorption dynamics influence the composition of indoor air after cleaning a home. Cleaning events can affect indoor air quality long after the cleaning is completed.
and
Using Caulobacter crescentus as a Model to Probe the Reactivity of Silver Nanoparticles
Maddie Farber,
CU ANYL 1st year
Silver nanoparticles (AgNPs) are an increasingly common environmental pollutant with antimicrobial and antibacterial properties. The elucidation of their interaction with cellular membranes and subsequent mechanisms of toxicity are critical areas of research that need to be better understood in order to manage potential adverse environmental effects. This work investigates the interaction of AgNPs with large unilamellar vesicles (LUVs) as a model membrane system. Similar studies conducted by others in this field have largely been conducted with uncoated AgNPs, ignoring the effect of environmental conditions on the formation of an eco-corona on AgNPs. Thus, in this study, the spent medium (SM) of a relevant environmental bacterium, Caulobacter crescentus, was used to form a complex eco-corona. We hypothesized that the eco-corona would mediate the in vivo reactivity of AgNPs, specifically through distinct interactions at the cell membrane. The differential reactivity of AgNPs and SM-AgNPs is shown through an in vivo toxicity study using C. crescentus. Model membranes were analyzed using dynamic light scattering (DLS), in which AgNP and LUV size and charge are characterized, and fluorescence anisotropy, where changes to LUV membrane fluidity and dynamics are interrogated. Results of the in vivo study are presented in tandem with model membrane studies in order to correlate toxicity effects seen in vivo with potential mechanisms of reactivity at the cell membrane.
6 November 2023
Explorations into the Composition of Brake Wear Particles from Semi-Metallic and Ceramic Brake Pads
Maxwell Lee,
CU ANYL 1st year
Vehicle based air pollution has always been a major contributor to air pollution from combustion-based exhaust particulate matter and emissions. As vehicle trends shift away from combustion-based drive trains, other sources of vehicle-based pollutants will become the main source of vehicle air pollution. Particularly, particulate matter is of great concern to study because it is known that brake pad wear can be a source of these hazardous particles. While a general idea of elemental composition is known for brake pad particles, the chemical composition of organic particles emitted from braking events is largely unknown. Additionally, there is no general consensus on whether brake pads marketed as “ceramic” and “semi-metallic” differ in elemental composition. These unknowns create multiple issues when attempting to find the concentration of brake pad pollution in the air near areas of high traffic. We found that the elemental composition of “ceramic” and “semi-metallic” brake pads does differ and are linked by the presence of common elements like Iron, Magnesium, and Barium, making these elements potential ways to track brake pollution. Additionally, we discovered that organic particles from brake pads form from unique tribological processes, and that using H/C and O/C ratios may be a possible method for tracking organic brake pad pollution. These results can be used to inform possible field studies near high traffic areas to determine how concentrated brake pollution is in those areas. Ideally, these tracers can be used to find trends in brake pad pollution in the context of environmental justice and show whether there are trends between brake pad pollution concentrations and the health of low-income communities.
and
Comparative Analysis of AVIRIS and WorldView-3 SWIR for Geologic Mapping in Anza-Borrego Desert State Park
Jeffrey Price,
CU ANYL 1st year
A geologic map is both a visual depiction of the lithologies and structures occurring at the Earth’s surface and a representation of a conceptual model for the geologic history in a region. The work needed to capture such multifaceted information in an accurate geologic map is time consuming. Remote sensing can complement traditional primary field observations, geochemistry, chronometry, and subsurface geophysical data in providing useful information to assist with the geologic mapping process. Two novel sources of remote sensing data are particularly relevant for geologic mapping applications: decameter-resolution imaging spectroscopy (spectroscopic imaging) and meter-resolution multispectral shortwave infrared (SWIR) imaging. Decameter spectroscopic imagery can capture important mineral absorptions but is frequently unable to spatially resolve important geologic features. Meter-resolution multispectral SWIR images are better able to resolve fine spatial features but offer reduced spectral information. Such disparate but complementary datasets can be challenging to integrate into the geologic mapping process. Here, we conduct a comparative analysis of spatial and spectral scaling for two such datasets: one Airborne Visible/Infrared Imaging Spectrometer—Classic (AVIRIS-classic) flightline, and one WorldView-3 (WV3) scene, for a geologically complex landscape in Anza-Borrego Desert State Park, California. In this talk, I will discuss the physically based portion of our approach: the spectral mixture residual. The mixture residual uses the wavelength-explicit misfit of a linear spectral mixture model to capture low variance spectral signals. For this study area, the spectral mixture residual revealed greater spectral dimensionality in AVIRIS than WorldView (99% of variance in 39 versus 5 residual dimensions). These results illustrate the potential of recent and planned imaging spectroscopy missions to complement high-resolution multispectral imagery—along with field and lab observations—in planning, collecting, and interpreting the results from geologic field work.
30 October 2023
Drivers of Biogenic Reactive Nitrogen Oxide Emissions in Southern Indiana Deciduous Forests
Clara Lietzke,
CU ANYL 1st year
In the troposphere, reactive nitrogen oxides (NOy = NO, NO2, HONO) are harmful pollutants. Conversion of NO to NO2 propagates the radical reactions that oxidize volatile organic compounds (VOCs) and generate hydroxyl radical (OH). NO2 is directly photolyzed to produce ozone (O3), a harmful air pollutant and main component of smog. Anthropogenic emissions of nitrogen oxide and nitrogen dioxide (NOx = NO + NO2) have declined with the enactment of air pollution control policy, increasing the importance of biogenic NOy emissions. Previous work characterizing sources and sinks of NOy has been focused on soil microbial emissions, although emissions of NOx from plant matter have also been directly observed. To determine the short-term impact of leaf senescence on local NOy levels, we conducted the Fall Forest Exchange of Reactive Nitrogen (FFERN) field campaign, where we observed substantial NO emissions from American Sycamore leaf litter, particularly under specific stressors like dew and freezing events. Here, we investigate the mechanism of NOy emissions from American Sycamore and other tree species leaves in addition to their response to different stressors, such as drying, rehydration, freezing, and cutting.
and
Base-mediated depolymerization of amine-cured epoxy resins
Katherine Stevenson,
CU ANYL 1st year
Carbon fiber-reinforced epoxy composites are used in multiple industries, including for aerospace, automotive, and wind energy applications, due to their excellent strength-to-weight ratios and tunable material properties. Fortunately, recycling strategies for carbon fiber-based composites are emerging, with the primary focus on recovery of fibers due to the cost and energy intensity in their production. In addition to fiber recovery, there is an opportunity to recycle the epoxy components, such that ideal recycling strategies would yield both post-consumer fibers and epoxy monomers for reuse. Here, we examine potassium tert-butoxide-mediated cleavage of C–O and C–N bonds in amine-cured epoxy resins. We accomplish this via developing model compounds that reflect both C–O and C–N linkages in amine-cured epoxy composites, before expanding to both model linear thermoplastics and thermosets. We obtain excellent yields of both phenol (up to 97% molar yield) and amine products (up to 99 mol%) from aromatic and/or aliphatic amine-based model compounds. This system enables up to quantitative yield of bisphenol-A and up to 58% molar yield of aniline from model thermoplastic epoxy amines and 71% molar yield of BPA from a reaction with a thermoset substrate. These data correspond to 15% mass recovery of BPA from a commercial epoxy thermoset.
23 October 2023
Impact of Sulfur on Planetary Haze: Implications for Habitability
Eleanor Browne
CU ANYL Faculty
Atmospheric composition plays a critical role in creating and maintaining a habitable environment. For instance, the formation of aerosol particles (“hazes”) may affect the surface ultraviolet radiation flux and temperature and may serve as a source of complex organic molecules including nutrients. Although the composition of aerosols in planetary atmospheres is diverse, organic aerosols are of particular interest for our understanding of habitable worlds. Organic aerosol may serve as a potential biosignature for certain anoxic atmospheric compositions, and it may have played an important role in the climate and biogeochemistry of early Earth. Recently, we have explored how the addition of trace H2S (0-10 ppmv) affects organic haze analogs produced from ultraviolet photochemistry of reducing (N2/0.1% CH4) and weakly reducing (N2/0.1% CH4/0-2% CO2) gas mixtures. In these laboratory experiments, we analyze the aerosol products in real time using a suite of analytical instrumentation developed for studying modern Earth’s atmospheric chemistry. Specifically, we measure aerosol chemical composition using a quadrupole aerosol mass spectrometer, aerosol size and number with a scanning mobility particle sizer, and aerosol optical properties and water uptake using photoacoustic and cavity ringdown spectroscopies. We find that the inclusion of trace amounts of H2S in the precursor mixture significantly enhances the formation of organic aerosol mass, increases particle effective density, alters aerosol optical properties, and leads to the formation of organosulfur aerosol. Notably, we find no evidence of S8 aerosol – a finding that challenges predictions that H2SO4 and S8 were the primary sulfur reservoirs in Earth's Archean atmosphere. We further explore how the changes in aerosol optical properties could influence the radiative forcing of a planet using a modified gray radiative scheme and we discuss the implications of our water uptake measurements on cloud formation and radiative forcing. Overall, our results suggest that coupled carbon-sulfur atmospheric chemistry significantly impacts organic haze with implications for planetary climate, habitability, biosignature detection, and prebiotic chemistry.
and
The pollution impacts of disinfection-only air cleaners, and applying frequency combs for aerosol analysis
Jose-Luis Jimenez
ANYL Faculty
Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly summarize ongoing projects such as the upcoming NASA ASIA-AQ campaign and the ASCENT long-term monitoring site in Denver. However, I will mostly focus on upcoming projects of direct interest to first year PhD students. Airborne disease transmission was successfully labeled as a superstition by American epidemiologist Charles Chapin in 1910, with limited progress until the COVID-19 pandemic. The pandemic has ushered in a paradigm shift in which most respiratory viruses are understood to have important or dominant airborne transmission. For this reason, many types of air cleaners focused only on disinfection have been developed and sold during the pandemic. Over the last year we have investigated the formation of pollutants from many of these air cleaners, including germicidal ultraviolet light (GUV). Multiple types of air cleaners form O3 and PM (SOA) indoors. I will summarize recent experimental and modeling results, showing that multiple types of air cleaners may kill more people from pollution than they save from disinfection. A likely PhD position in our group will expand recent and ongoing GUV experimental work to multiple types of air cleaners, and will quantify the SOA formation potential of indoor air.
Dual frequency combs are new light sources with unique properties for analytical spectroscopy. The mid-IR contains important chemical information about organic functional groups, but analysis of aerosols suspended in air in this region with techniques such as FTIR was considered infeasible, due to interferences from narrow absorption lines of abundant gases such as CO2. As part of the collaborative IARPA SAURON project led by CU-Boulder Engineering, a PhD student in our group will collaborate with the SAURON team to use new and emerging DFC mid-IR sources to explore the analysis of aerosols in an environmental chamber (and eventually simple field experiments), exploring the ability to subtract interfering gases and measure the spectrum of aerosols of different composition, concentration, and mixing state.
10 October 2023
Particle Formation and Growth in New Chemical Regimes
Eleanor Browne
CU ANYL Faculty
Understanding the mechanisms through which aerosol particles form and grow is critical for constraining a planet’s energy budget, however, our knowledge of the key processes governing particle formation and growth in diverse environments remains weak. In the coming decades, our knowledge of particle formation and growth will continue to be challenged as changing climate and anthropogenic emissions alter the chemical regimes of modern-day atmospheric chemistry on Earth and measurements from, for instance, the James Webb Space Telescope and NASA’s Dragonfly mission offer increasingly chemically resolved insights into planetary atmospheres much different from our own.
In this talk I will highlight three of our projects on particle formation and growth in new and/or understudied chemical regimes. In the first part I will discuss our field measurements in an agricultural region, focusing on reactive nitrogen and carbon species that contribute to particle formation and growth. I will briefly touch on how vertical gradients in atmospheric composition and physical properties (e.g., temperature) affect particle formation and growth. Next, I will discuss our laboratory investigations of the atmospheric chemistry of volatile methyl siloxanes. These solely anthropogenic compounds have recently come under scrutiny for their potential toxicity and environmental persistence. Moreover, field measurements suggest that they are present in urban nanoparticles. Our work investigating the kinetics, mechanism, and aerosol yield of volatile methyl siloxane oxidation addresses key gaps in our understanding of their environmental chemistry. Lastly, I will describe our laboratory experiments exploring aerosol formation in anoxic planetary atmospheres. I will primarily focus on how organosulfur formation affects aerosol mass, composition, and optical properties in Archean Eon analog experiments and will discuss the implications for habitability and the understanding of the evolution of Earth’s atmosphere.
9 October 2023
Laboratory studies of the formation of atmospheric aerosol under different chemical regimes
Jesse Kroll,
MIT Dept. of Civil and Environmental Engineering
MIT Dept. of Chemical Engineering
A large fraction of fine particulate matter (aerosol) in the atmosphere is secondary in nature, formed from the atmospheric oxidation of gas-phase compounds. This oxidation chemistry can govern the amount and properties of aerosol particles, and hence their impacts on climate and health. Much of our understanding of aerosol formation derives from laboratory studies, which to be most useful for atmospheric modeling should cover the range of atmospheric oxidation conditions as well as possible. One particular challenge is matching the chemistry of organic peroxy (RO2) radicals; these key intermediates can react via a number of channels – bimolecular reactions with different radical species (NO, HO2, RO2…) as well as unimolecular (isomerization) reactions – each of which may have its own reaction product distribution and aerosol yields. This talk will describe our group’s efforts to measure aerosol formation in an environmental (“smog”) chamber, across the full range of RO2 conditions found in the atmosphere. For the oxidation of a given aerosol precursor, we vary RO2 chemistry in the chamber by changing concentrations of various reactants, and estimate RO2 fate using chemical ionization mass spectrometry (CIMS) of gas-phase products as well as mechanistic modeling of the oxidation chemistry. Chemical systems examined include the oxidation of dimethyl sulfide (CH3SCH3, emitted to the atmosphere by phytoplankton) to form sulfate aerosol, and the oxidation of isoprene and alpha-pinene (C5H8 and C10H16, emitted by plants) to form organic aerosol.
2 October 2023
Long-path atmospheric measurements using dual frequency comb spectroscopy
Kevin Cossel
NIST, Boulder, CO
Open-path measurements of atmospheric gas species over km-scale path lengths are well suited to quantify emissions from sources like oil and gas production, agricultural activities, forest fires, and industry. Our group at NIST has developed open-path dual frequency comb spectroscopy (DCS) as a tool to provide accurate measurements of multiple trace gas species simultaneously across path lengths ranging from 100 m to >10 km.
We have used these systems for a number of field measurements. In the first campaign, we deployed a prototype system in the mid-infrared spectral region to a new oil and gas well installation in order to measure emissions during the different stages of unconventional well development. In another measurement, we deployed to the Platteville Atmospheric Observatory in north-eastern Colorado or 4 months. This site is located in the Denver-Julesburg oil and gas basin and in an area with a large number of confined animal feeding operations, leading to a complex mixture of trace gas emissions. By using measurements of ethane and NH3, we can attribute the observed CH4 to the oil and gas and agricultural sectors. We also see HCHO plumes that are correlated with C2H6, indicating oil and gas related sources of HCHO (likely from combustion). Finally, we took a system to a beef cattle stocker site to measure emissions of methane and NH3 over several months.
Current opportunities in our group include field measurements working to understand urban greenhouse gas emissions and air quality links, testing mitigation of greenhouse gas emissions in agriculture, and measuring emissions from forests as well as laboratory studies looking at emissions of greenhouse gases and reactive nitrogen from cyanobacteria and combustion.
and
Expanding the Use of 19F NMR Spectroscopy in Per- and Polyfluoroalkyl Substance Analysis
Andre Schaum,
ANYL 1st year, CU Boulder
Aqueous film-forming foams (AFFFs) have been used to extinguish liquid-fuel fires since the 1960s and
are significant historical sources of per- and polyfluoroalkyl substances (PFAS) in the environment.
Though in the process of being phased out of use in the United States, large stockpiles of AFFFs still exist
nationwide, often stored in poorly labeled containers and tanks. For proper assessment and disposal, rapid
and inexpensive analytical methods are needed to quantify total fluorine, determine PFAS composition,
and identify AFFF type. Current analytical methods that provide quantitative measures of individual
PFAS in AFFFs, such as liquid chromatography – tandem mass spectrometry (LC-MS/MS), are often
complicated, time-consuming, and expensive. Methods for total fluorine analysis, though quantitative,
provide relatively little information on the chemical nature of PFAS in AFFFs. Fluorine Nuclear Magnetic
Resonance Spectroscopy (19F NMR) has previously been used to characterize PFAS in environmental
matrices, technical mixtures, and analytical standards, though its application to AFFFs has been limited.
Here, a 19F NMR method was developed for rapid qualitative and quantitative analysis of fluorine content
in AFFFs and used to identify the manufacturing method of two AFFFs of unknown origin.
18 September 2023
Chemistry of Volatile Organic Compounds in the Atmosphere
Joost de Gouw,
ANYL Faculty, CU Boulder
Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products.
In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments.
Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments to better understand their sources and fate. One particular study involves the impact of the Marshall Fire in Boulder County on indoor air in homes that were near the burnt area. Second, we are working on the emissions and chemistry of VOCs in urban air. One study that will be highlighted involved field measurements in Los Angeles in the Summer of 2022. Third, we are working on the indoor air chemistry induced by air cleaners such as germicidal UV lamps that are effective at inactivating airborne viruses. Finally, we are also using data from satellite remote sensing instruments to measure the pollutants from oil and natural gas production, in urban air and from wildfires.
and
From Coastal Megacities to Remote Oceans: Small Molecules in the Anthropocene
Rainer Volkamer,
ANYL Faculty, CU Boulder
Anthropogenic enhancements of natural processes modify atmospheric chemistry in the urban and remote marine atmosphere. For example, marine iodine emissions have tripled in recent decades due to enhanced deposition of pollution ozone over oceans, affecting particle formation and the ozone layer. Furthermore, increasing emissions from wildfires pose challenges to public health and urban air quality. The Volkamer group develops advanced instrumentation (optical and mass spectrometric) to measure small molecules and condensable vapors relevant to particle formation, public health and climate discussions. We seek to better quantify and understand the interconnections between marine ecosystem, aerosols and their control on atmospheric chemistry. We also study the emissions and oxidative chemistry of coastal Megacities using research aircraft and satellites. Examples from recent field and laboratory experiments are discussed that aim to develop a molecular level understanding to test and advance atmospheric models. Opportunities for graduate research exist in 1) field measurements using research aircraft (i.e., TI3GER-2022, CUPiDS-2023, TI3GER2-2025 projects), 2) laboratory experiments of particle formation and multiphase chemistry (incl. at CLOUD/CERN), and to 3) develop instrumentation by exploiting synergies between optical spectroscopy and mass spectrometry.
11 September 2023
Chemistry of Organic Compounds in the Atmosphere and Indoor Air
Paul Ziemann,
ANYL faculty, CU Boulder
"Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative studies of indoor air chemistry at CU. In this talk I will provide a brief overview of recent research in my lab for the purpose of informing first-year chemistry graduate students."
Summer 2023
21 July 2023
It’s Okay to be Salty: Salt Deliquescence and Efflorescence Relevant to Mars and Earth
Marium Fernanders,
Tolbert group,
ANYL PhD thesis
"The presence of salts in Martian soil gives rise to new mechanisms of liquid water formation through deliquescence and efflorescence of sediment salts. Observations by the Phoenix lander, Curiosity rover, and Mars Reconnaissance Orbiter (MRO) have detected hydrated salts in the Martian regolith, including chloride, perchlorate, and chlorate compounds. This thesis uses laboratory studies to investigate water uptake and loss by model systems to gain insight into the water cycle on Mars.
With recent discoveries of chlorine in the Martian soil, past research has focused on the deliquescence and efflorescence of perchlorate and chloride salts. However, less is known about chlorate salts. Chlorate is one of several intermediate oxidation states between perchlorate and chloride, however of the other oxychloride species chlorate is the more stable species and therefore more likely to exist on the Martian surface. Here, sodium chlorate and magnesium chlorate were studied to observe their moisture absorption and release behavior. It was found that sodium chlorate displayed temperature-dependent deliquescence and magnesium chlorate exhibited water uptake regardless of temperature. This could mean that magnesium chlorate can take up water at single digit relative humidities, making it an extremely Mars-relevant salt for study.
Understanding the behavior of pure salts is crucial, but it's also important to consider real-world conditions that influence water uptake and release in sediment salts. Additionally, this thesis investigated the behavior of magnesium nitrate hexahydrate under Martian and Atacama Desert conditions. It was found that pure magnesium nitrate is unlikely to undergo aqueous cycling on Mars because the temperature and relative humidity required for nitrate deliquescence happens at relative humidities that is to wet at the same temperature present on Mars. However, in the Atacama Desert, nitrates in the soil can uptake water throughout the year, with peak uptake occurring in the fall season, potentially forming stable or metastable brines. A potentially year-round source of water could be used for microbial life to survive under the hyper-arid conditions present on the desert surface.
Finally, complex sediment samples from Don Juan Pond's slope streaks were investigated as a model for Recurring Slope Lineae present on Mars. It was found that chlorine-bearing salts, such as sodium and calcium chloride, drive water uptake and deliquescence, while insoluble mineral components do not hinder water absorption. Efflorescence and deliquescence occurred at similar relative humidities, preventing supersaturation. Based on laboratory data and environmental conditions in the Lake Vanda region, episodic occurrence of slope streaks throughout the year, with higher prevalence in the southern summer and spring seasons, was predicted."
15 May 2023
Formation, Abundance, and Evolution of Molecular Products in α-Pinene and β-Pinene Secondary Organic Aerosol
Dr Christopher Kenseth,
Department of Atmospheric Sciences, University of Washington
"Secondary organic aerosol (SOA) contributes substantially (15–80% by mass) to the global burden of atmospheric fine particulate matter (PM2.5), which exerts large but uncertain effects on climate as well as adverse impacts on air quality and human health. The oxidation of α-pinene and β-pinene (C10H16), emitted in appreciable quantities from forested regions (~85 Tg y-1), represents a dominant source of SOA. Deciphering the molecular composition, and in turn formation and aging mechanisms, of α-pinene and β-pinene SOA is essential to reducing uncertainty in assessment of their environmental and health impacts. However, molecular characterization of α-pinene and β-pinene SOA, which generally consist of hundreds or more compounds of diverse classes, is significantly hindered by their chemical complexity. This seminar will present an overview of research aimed at constraining the formation, abundance, and evolution of molecular products in SOA derived from the ozonolysis and photooxidation of α-pinene and β-pinene using a combination of environmental chamber and flow tube experiments, liquid chromatography/electrospray ionization mass spectrometry (LC/ESI-MS), and organic synthesis."
Spring 2023
1 May 2023
Air-Sea Exchange of Reactive Gases: Chemistry at Interfaces and Partitioning in Aqueous Droplets
Randall Chiu,
Volkamer group,
ANYL PhD thesis
"Many puzzles remain regarding the interaction of halogens with oxygenated volatile organic compounds (OVOC) in the marine boundary layer (MBL). Observations of the OVOC glyoxal (CHOCHO) over the remote Pacific Ocean are difficult to reconcile with its brief (2-4 hour) atmospheric lifetime and high water solubility (Henry’s law constant ~4×105 M/atm). In those same airmasses, concentrations of reactive halogen species, specifically bromine monoxide (BrO) are below detection limits even though models predict up to 1.3 pptv. Presented here is a surface reaction that holds promise for explaining one or both puzzles. In the lab, fatty acids on a simulated sea surface microlayer (SML) undergo photochemical transformation to unsaturated aldehydes (2-alkenals), subsequent ozonolysis of which produces glyoxal. In a follow-up study, 2-alkenals were shown to react with Br to produce HBr in high yield. Depending on the concentration of fatty acids in SML, fatty acid photochemistry holds promise as a candidate for the missing BrO sink.
The second work presented are aircraft ozone eddy covariance (EC) flux over the Pacific Ocean during the 2023 Technical Innovation Into Iodine and GV Environmental Research (TI3GER) technical campaign. Ozone EC fluxes were measured with three independent ozone sensors of two different designs (NCAR Fast O3, a nitric oxide chemiluminescence instrument, and KIT Fast Airborne O3, a coumarin dry chemiluminescence instrument), which has not been done before. All three instruments were shown to be suitable for EC flux experiments. Particularly interesting case studies are presented in which ozone EC flux exhibits different spatial variability than water vapor EC flux. In addition, the availability of three independent EC flux measurements was used to constrain the uncertainty and limit of detection (LOD) methods in the literature."
24 April 2023
The Role of Organic Heterogeneous Nuclei in Contact Crystallization of Atmospherically Relevant Salts
Kyle McMillan,
ANYL 3rd year, Tolbert group
The overall radiative effect of atmospheric aerosols on climate is currently a major source of uncertainty in climate models. Essential in resolving this uncertainty is a complete understanding of the particle phase state in the atmosphere. Often overlooked in current studies on the particle phase is the role of collisions in inducing contact nucleation. Thus, more research that focuses on determining which atmospherically relevant compounds are most likely to be involved in contact nucleation under varying conditions is warranted. Of interest here was evaluating the effectiveness of organic-containing contact nuclei (CN) in inducing contact efflorescence of aqueous salt-containing droplets. To do this, contact efflorescence was probed on freely floating aqueous ammonium sulfate (AS) droplets using a long-working distance optical trap. In this setup, optically levitated droplets were contacted with both glassy (D-(+)-raffinose and citric acid) and surface-active (stearic acid and cis-pinonic acid) organic CN over a range of relative humidity (RH) values above the homogeneous efflorescence RH of AS. The purpose of these experiments was to determine if certain organic-containing CN could induce contact efflorescence of aqueous droplets at an RH significantly above the homogeneous efflorescence RH of AS. Far-field imaging was employed as a way of probing crystallization either in the contact or homogeneous modes, and was supplemented with near-field imaging, which was used to track contact events. Droplet compositions before and after crystal nucleation were characterized using Raman spectrometry to track the liquid water content of the droplet. Promising in these experiments is the effectiveness of surface-active organics—in particular, cis- pinonic acid—in inducing contact efflorescence of AS, which has major implications for the particle phase state of the troposphere. Continued experiments in this area will provide key insight into the interactions that cause contact to be effective at initiating nucleation and will ultimately lay the groundwork for analogous contact ice nucleation studies.
17 April 2023 - special seminar
Environmental Analytical Chemistry in an Art Museum
Julia Bakker-Arkema, Ph.D.,
Associate Research Scientist, Metropolitan Museum of Art
(Ziemann Group alum, 2020)
As a preventive conservation scientist at a museum, my work is centered around improving environmental conditions during the conservation, transportation, storage, and display of cultural heritage objects. I will share some current work using gas-chromatography-mass-spectrometry (GC-MS) to identify and quantify volatile organic compounds emitted from conservation materials that have the potential to damage the surfaces of art and artifacts, and hopefully I’ll give you a sense of what it means to be an analytical chemist in an art museum. During my Ph.D. research in the Ziemann lab, I investigated the oxidation of volatile organic compounds and the formation of secondary organic aerosol through environmental chamber studies. My experience and interest in environmental analytical chemistry led me down various paths both in and out of the laboratory, including science policy on ozone management in the front range, an indoor air field campaign in Texas, science communication workshops for Women in Science & Engineering, and website design and video production with the Sloan Foundation. I’ll discuss each of these pursuits, and how I applied the skills I learned to launch a career in teaching and research at the Metropolitan Museum of Art in New York City. This talk is aimed at graduate students, and all questions are welcome!
17 April 2023
Design and Characterization of the CU-Boulder Environmental Chamber Facility & Long-term and High-time-resolution Measurement for the Characterization of Aerosol Properties in the Denver Metropolitan Area
Seonsik Yun,
ANYL 3rd year, Jimenez group
"Environmental reaction chambers are essential tools for studying atmospheric chemistry and physics based on laboratory experiments. The design of chambers varies concerning the chamber sizes, temperature/humidity controls, light sources, air purification, and operating conditions to simulate atmospheric chemistry reactions and are often used to investigate secondary organic aerosol formation. Here we present a new state-of-the-art CU-Boulder environmental chamber facility and its design and characterizations.
The Atmospheric Science and Chemistry mEasurement NeTwork (ASCENT) is a new comprehensive, high-time-resolution, long-term measurement network in the U.S. to characterize aerosol chemical composition and physical properties. As one of 12 sites of the ASCENT, La Casa site is located near downtown Denver, Colorado, and will be equipped with a suite of advanced aerosol instrumentation: Aerosol Chemical Speciation Monitor (ACSM, non-refractory aerosols), Xact (trace metals), Aethalometer (black/brown carbon), and Scanning Mobility Particle Sizer (SMPS, aerosol number size distribution and concentration). Additional instrumentation, such as a Vocus Proton Transfer Reaction Time-of-Flight Mass Spectrometer (Vocus PTR-TOF, gas phase volatile organic compounds) and a radon detector (RAD7), will also be deployed to address a comprehensive data analysis on Denver urban aerosols. In this talk, I will present the preliminary results from the ASCENT instrumentation and future research opportunities."
11 April 2023
Measurements of Volatile Organic Compounds in Urban Air by Proton-Transfer Reaction Mass Spectrometry
Andrew Jensen,
de Gouw Group
CU ANYL Dissertation Defense
"Volatile organic compounds (VOCs) in urban environments are emitted to the atmosphere from many biogenic and anthropogenic sources. VOC oxidation contributes to the formation of secondary products, including ozone and secondary organic aerosol, which negatively impact air quality and human health. As emissions change, VOC measurements are necessary to reassess their role in urban air quality. Here, I present results from measurements of ambient VOCs in three urban environments made by proton-transfer reaction mass spectrometry (PTR-MS) with part-per-trillion by volume detection limits.
I first detail the quantification of VOCs measured in Boulder, CO in spring 2021 with a focus on instrument characterization. I developed a PTR Data Tool to parameterize instrument response which, in turn, informs the quality of the measurements and the quantification of VOCs which cannot be directly calibrated. These findings help streamline and improve future PTR-MS measurements.
VOCs were also measured in Changzhou, an industrial Chinese city, before, during, after the 2020 COVID-19 lockdowns which shut down non-essential activities. I quantified strong reductions in industrial and transportation emissions. These estimates help inform emission inventories and chemistry-transport models during this global perturbation of air pollution, as well as possible future scenarios with reduced emissions.
Finally, VOCs were measured in the Los Angeles basin, California during summer 2022. Ambient concentrations of primary VOCs from vehicles have declined over the preceding decade due to emission regulations while concentrations of secondary VOCs were largely unchanged, in part, due to faster oxidation and unregulated emission sources. This study expands the detected subset of urban VOCs and allows for a more detailed understanding of atmospheric sources and fates."
10 April 2023
Pollution distribution in the Denver metroplex: Chemical, sociological, and historical insights
Alexander Bradley,
ANYL 3rd year, de Gouw group
"Prior studies have shown that people of color in the United States are exposed to higher levels of pollution than non-Hispanic White people. We show that the city of Denver, Colorado displays similar race and ethnicity-based air pollution discrepancies by using a combination of high-resolution satellite data, air pollution modeling, historical demographic information, and areal apportionment techniques. TROPOMI NO2 columns and modeled PM2.5 concentrations from 2019 are higher in communities subject to redlining, a discriminatory mortgage appraisal practice from the 1930s by the federal Home Owners’ Loan Corporation (HOLC). We calculated and compared Spearman coefficients for pollutants and race at the census tract level for every city that underwent redlining to contextualize the disparities in Denver. We find inequitable siting of polluting infrastructure leading to higher populations of people of color living near point sources, including 40% higher Hispanic and Latino populations; Traffic analysis and emission inventory data show that people of color are more likely to live near busy highways. Unequal opportunities for people of color has allowed for pollution disparities to persist despite attempts by the city to rectify them . Finally, we identify core causes of the pollution disparities to provide direction for remediation."
4 April 2023
Optical Properties of Brown Carbon Found in Earth and Planetary Haze Aerosol
Kevin Jansen,
Tolbert group
CU ANYL Dissertation Defense
"Atmospheric aerosol has long been documented to be capable of both scattering and absorbing incoming solar radiation, leading to either a net cooling or warming effect on planetary atmospheres. Despite the optical properties of some forms of aerosol such as inorganic salts being well characterized, the optical properties of organic aerosol are not as well established. While most organic aerosol is only capable of scattering light in the atmosphere, a fraction of organic aerosol does absorb light. Unlike inorganic “black carbon” which absorbs light throughout the visible wavelength range, so-called organic “brown carbon” is capable of absorbing light at in the near ultraviolet tailing off in the visible. Brown carbon aerosol can account for a significant fraction of the total light absorption at lower wavelengths in various atmospheres. For example brown carbon account for up to twenty percent of Earth’s light absorption at wavelengths <400nm, implying brown carbon can have a major effect on a planet’s total radiative forcing.
Part of the uncertainty in the magnitude and direction of brown carbon forcing stems from the fact that atmospheric aerosol contains numerous organic compounds, each with their own optical properties. Depending on the aerosol source, ambient conditions, and any potential atmospheric processing, the optical and chemical properties of brown carbon can vary dramatically over time.
This thesis examines the effects of both formation conditions and atmospheric processing on two different brown carbon aerosol systems. First, the optical properties of brown carbon formed via aqueous reactions of carbonyls and ammonium found in Earth’s atmosphere were characterized under varying pH conditions. It was found that brown carbon formation is heavily dependent on the pH, with brown carbon formation being favored under basic conditions. At the same time, brown carbon was also formed under acidic, atmospheric conditions in aerosol particles that are incapable of forming in acidic bulk phase solutions, indicating a unique reaction only accessible in aerosol particles. In addition, the ozonolysis of this brown carbon in the atmosphere was simulated in a flow tube to monitor the potential loss of the aerosol’s light absorption. It was found that despite readily reacting with ozone, a recalcitrant fraction of brown carbon retains its ability to absorb light, indicating it can have a persistent warming effect as it remains in Earth’s atmosphere.
Brown carbon is not limited to the Earth's atmosphere, but has been detected in the atmospheres of other planets as well. For instance, organic brown carbon has been found on Titan, Saturn's largest moon and may have been important on the Archean Earth as well. For planetary atmospheres without obvious sources of large organic molecules, brown carbon is likely formed through photochemical reactions of small molecules including methane. Here, light absorbing aerosol was generated by exposing mixtures of CH4/CO2/H2S in N2 to deep UV light to mimic aerosol formation potentially found in planetary hazes. It was found that the light absorption of the haze aerosol decreased as the concentration of CO2 increased. This trend was found to be due to the formation of non-absorbing, sulfate salts. In addition, the aerosol was found to be more hygroscopic as the CO2 concentration increased, implying the aerosol could grow in size and scatter light more efficiently, implying the CO2 could have a significant effect on the optical properties of planetary haze aerosol and subsequently its radiative forcing.
Because brown carbon has been found in both Earth's atmosphere and in the atmospheres of other planets, these studies of the optical properties of brown carbon could assist in modeling the radiative forcing and climate trends of both Earth and various planetary systems."
3 April 2023
Chemical and Meteorological Controls on New Particle Formation in the Southern Great Plains
Bri Dobson, ANYL 3rd year,
Browne group
"New particle formation (NPF) is estimated to account for 40 – 70 % of cloud condensation nuclei and therefore plays a central role in our understanding of aerosol-cloud interactions. It is well established that the likelihood of NPF is influenced by both chemical precursors, such as sulfuric acid, ammonia, amines, and organic compounds, as well as physical atmospheric conditions. However, the complexity of the process leads to large uncertainties in the representation of NPF in models. A complete understanding of the conditions that lead to NPF requires observations of both gas-phase chemistry as well as physical and meteorological conditions. Additionally, these measurements must be made in diverse ecosystems as both chemical and physical conditions vary by region.
We investigated NPF in agricultural regions, which have been understudied compared to urban and forested regions. To do so, we deployed an atmospheric-pressure interface time-of-flight mass spectrometer (APi-TOF) and a chemical ionization time-of-flight mass spectrometer with ethanol reagent ion (EtOH-CIMS) to the US Department of Energy Atmospheric Radiation Measurement (ARM) Southern Great Plains (SGP) site in rural Oklahoma in October 2021 and Spring 2022. These mass spectrometry measurements of gas-phase compounds along with the long-term meteorological measurements from the ARM SGP site allow us to further investigate the chemical and physical conditions that contribute to NPF. In this talk, I will present a case study of two of the observed NPF events during our spring observing period. Changes in transported gases and atmospheric conditions cause differences in new particle formation and growth over the course of a few days at the same location."
20 March 2023
Ozone photochemistry and free radical budgets in the Los Angeles basin: A comparison of ground-based observations in 2021 and 2010
Michael Robinson,
ANYL 3rd year, Brown group
"Radical precursors and termination products are important factors to understanding sensitivities of ozone (O3) production to nitrogen oxides (NO + NO2 = NOx) and volatile organic compounds (VOCs). During the summer of 2021, an extensive set of photochemical measurements were conducted at a ground site on the Caltech campus in Pasadena, CA to evaluate emissions and photochemistry in the Los Angeles (LA) basin. Here we present the calibration and measurement of important radical precursors and compare them to observations from the CalNex 2010 field intensive. Major radical sources include formaldehyde (HCHO) and other aldehydes, nitrous acid (HONO), nitryl chloride (ClNO2), ozonolysis of alkenes, and O(1D) + H2O. In addition to radical precursors, radical termination products were measured, including peroxy acetyl nitrates (PAN), nitric acid (HNO3), organic nitrates and organic peroxides. The comparison of radical precursors and termination products illustrates shifts in photochemical regime resulting from NOx and VOC emissions changes over the past ten years in the LA basin, as well as other influences such as temperature, seasonality and day of week."
14 March 2023
The Effects of Trace H2S in Laboratory Experiments of Planetary Organic Haze Chemistry
Nathan William Reed,
Tolbert group / Browne group
CU ANYL Dissertation Defense
Planetary organic hazes from methane (CH4) photochemistry and atmospheric sulfur gases are each common in planetary atmospheres, including the Archean Earth and, likely, exoplanetary atmospheres. A planetary organic haze can affect a planet’s radiative forcing and interpretations of observable spectra, as well as act as a source for prebiotic chemistry and nutrients for life. However, cross reactions between inorganic sulfur and organic molecules to form organosulfur have yet to be explored in haze chemistry. The objective of my thesis is to explore the coupling between hydrogen sulfide (H2S) and organic haze chemistry. Here I present the results of laboratory experiments using aerosol mass spectrometry, differential mobility analysis, and optical measurements to explore how trace H2S influences the compositional, physical, and optical properties of aerosol from organic haze analogs produced by CH4 photochemistry. I investigated the chemistry of aerosol formed in reducing (CH4/H2S gas mixtures in N2, varying trace H2S) and weakly reducing (CO2/CH4/H2S gas mixtures in N2, varying CO2) atmospheric conditions.
When I included trace H2S in precursor mixtures, the total organic aerosol mass increased in both conditions, despite H2S not being a carbon source. Moreover, I found evidence for the formation of organic reduced sulfur (ORS) and organic oxidized sulfur (OOS) compounds in the reducing and weakly reducing conditions, respectively. At lower CO2 mixing ratios, I attributed the total sulfate signal to be entirely OOS. Thus, in contrast to previous thought, I show that ORS and OOS are potentially significant atmospheric sulfur reservoirs. Further, I found the compositional changes in the reducing conditions lead to changes in the aerosol optical properties, as the total extinction (absorption plus scattering) of light increased with increasing H2S mixing ratios. I found that trace H2S dramatically influences the organic aerosol composition, mass, and optical properties by the formation of organosulfur (ORS and OOS) compounds and enhancing organic aerosol formation. These results have implications for Archean atmospheric chemistry, prebiotic chemistry, planetary climate, and spectral interpretations for exoplanetary atmospheres.
Thus, future work should consider the potential impacts of trace H2S on organic haze chemistry.
13 March 2023
Metal Oxide Particles as Atmospheric Nuclei: Exploring the Role of Metal Speciation in Heterogeneous Efflorescence and Ice Nucleation
Zachary Schiffman, ANYL 3rd year,
Tolbert group
"ABSTRACT: Mineral dust can indirectly impact climate by nucleation of atmospheric solids, for example by heterogeneously nucleating ice in mixed-phase clouds or by impacting the phase of aerosols and clouds through contact nucleation. The effectiveness toward nucleation of individual components of mineral dust requires further study. Here, the nucleation behavior of metal oxide nanoparticle components of atmospheric mineral dust is investigated. A long working distance optical trap is used to study contact and immersion nucleation of ammonium sulfate by transition metal oxides, and an environmental chamber is used to probe depositional ice nucleation on metal oxides particles. Previous theory dictates that ice nucleation and heterogeneous nucleation of atmospheric salts can be impacted by several factors including morphology, lattice match, and surface area. Here, we observe a correlation between the cationic oxidation states of the metal oxide heterogeneous nuclei and their effectiveness in causing nucleation in both contact efflorescence mode and depositional freezing mode. In contrast with the activity of contact efflorescence, the same metal oxide particles did not cause a significant increase in efflorescence relative humidity when immersed in the droplet. These experiments suggest that metal speciation, possibly as a result of cationic charge sites, may play a role in the effectiveness of nucleation that is initiated at particle surfaces."
6 March 2023
Advancing Process-based Understanding of How Humans Are Changing the Global Sulfur Cycle
Prof. Eve-Lyn Hinckley,
Univ. of Colorado Ecology and Evolutionary Biology
"Today, the nature of how humans alter the global sulfur (S) cycle is changing. As atmospheric S deposition has declined in response to air quality regulation in the United States and Europe, there has been an increase in S fertilizer applications reported in many large-scale regional crop systems. In addition, intensification of agriculture has driven increased S inputs for other uses: as a pesticide, regulator of soil pH, and soil conditioner. Given that excess S can cause soil acidification and mobilization of heavy metals in ecosystems, it is critical to develop methods to trace the “fingerprint” of agricultural S through complex landscapes, quantify S forms and transformations in soils and surface waters, and determine the consequences of its use. In this talk, I will describe both new trend analyses and process-based studies that provide compelling evidence for how the forms, amounts, flows, and consequences of S have changed from what they were in the 1960s and 1970s when the dominant human manipulation of the S cycle was through mining and fossil fuel emissions. I will highlight studies from my research group that show exciting new methodological developments using radio- and stable isotopes of S adapted from the marine literature to trace S applications through large agricultural regions. I will also discuss the collaborative actions that researchers, land managers, and regulators may take to address the consequences of excess S in the environment. Ultimately, I will make the case for how an element that has been far less investigated than carbon, nitrogen, and phosphorus, should be a priority for study in the coming years."
27 February 2023
Investigations into the health effects of wildfire smoke
Prof. Colleen Reid,
Univ. of Colorado Geography and Institute for Behavioral Science
"Wildfires have been increasing in frequency and duration in the western U.S. and the wildfire season has been increasing in length such that many regions now claim that there is no wildfire season anymore but that wildfires have become a year-round threat. While there are many causes of the increase in wildfires in the western U.S., it is becoming clear that wildfires not only affect forests and grasslands, but also have impacts on the health of populations downwind. Indeed studies have shown that fine particulate air pollution () is decreasing in most areas of the United States, except for areas most affected by wildfires, where an increasing trend in can be attributed to wildfire smoke. Studies of the health impacts of wildfire smoke are challenging due to lack of sufficient air pollution monitoring across space, the complexity of the components of the smoke that could affect human health, the numerous health outcomes that have been found to be linked to wildfire smoke, and more. In her talk, Dr. Reid will dive into her research that investigates how to better assess population exposure to wildfire smoke, how it impacts human health, and which communities are more affected by wildfire smoke. She will also provide a glimpse into her ongoing work to understand the health tradeoffs of interventions to protect health from wildfire smoke."
20 February 2023
Assessment of indoor air quality using substance-specific guide values and TVOC
Prof. Tunga Salthammer
Department of Material Analysis and Indoor Chemistry, Fraunhofer WKI
"The primary goal of indoor hygiene studies is to create a pleasant and healthy environment for people. This requires a sufficient supply of fresh air and the setting of climatic parameters that lead to thermal comfort. However, the concentration of air pollutants must also be kept as low as possible. A complete measurement program to study indoor air quality can be both time-consuming and expensive. For this reason, parameters that can be measured easily and quickly are often used for an initial assessment of the situation. One of these parameters is TVOC (Total Volatile Organic Compounds).
The current situation makes it necessary to subject VVOC, VOC, SVOC and TVOC measurements to a critical analysis due to their widespread use in questions of indoor air quality and to the evaluation of emissions of organic compounds from building and consumer products.
A detailed description of the measurement methods is followed by a critical evaluation of the various TVOC values, their possible applications and guide values for individual compounds. The aim is to provide a deeper understanding of TVOC in order to use this parameter correctly and to be able to better assess published results. The analytical definition of VOCs also suggests total values TVVOC and TSVOC for very volatile and semi volatile organic compounds. However, due to the physical properties of the potential target chemicals, this is associated with a number of drawbacks.
Early work on TVOC was trend-setting and still influences current indoor guide values, even if many VOCs are no longer representative from today's perspective. The spectrum of indoor VOCs has shifted significantly: away from classic solvents and more towards reactive compounds and indoor chemistry. However, within the framework of surveys TVOC can make contributions to identifying statistical trends and reference values can be extracted from the data collected in this way. The online measured TVOC values are at best suitable for screening IAQ. TVOC does not take into account the toxicological properties of the individual substances for inhalation exposure. This would not even be possible, since different substances usually have different toxicological endpoints that cannot simply be summed up for a reliable risk assessment. Therefore, measurements of individual substances are usually evaluated using substance-specific guide values.
Consequently, TVOC cannot be used in connection with health-related and odor-related issues. Nevertheless, such references are repeatedly made, either intentionally or unintentionally. This happens in an analytical but also in an interpretative sense. Authorities, practitioners and architects use TVOC as an acceptance criterion for buildings, but without specifying the methodology. Overall, there is a great deal of uncertainty about TVOC. This applies in particular to the question of whether and why a TVOC value can be helpful and which statements cannot be made on the basis of the TVOC."
Reference: Salthammer, T. (2022). TVOC revisited. Environment International, 167, 107440, DOI link: https://doi.org/10.1016/j.envint.2022.107440 (published under CC BY-NC-ND 4.0).
13 February 2023
Springtime Arctic ozone depletion forces northern hemisphere climate anomalies
Marina Friedel,
IAC, ETH Zuerich
"Large-scale chemical depletion of ozone due to anthropogenic emissions of chlorofluorocarbons (CFCs) can occur, albeit infrequently, over the Arctic. Such ozone depletion events are consistently followed by surface temperature and precipitation anomalies over vast parts of the Northern Hemisphere. Using chemistry-climate models, it can be shown that the loss of stratospheric ozone is an important cause of these surface anomalies, as it leads to persistently cold temperatures in the lower stratosphere and alters stratospheric dynamics. Including chemical processes involving ozone in forecast models could therefore increase predictability on a sub-seasonal to seasonal scale. Similarly, the long-term trend in Arctic ozone caused by the decline of CFCs also has important implications for stratospheric temperature and dynamics. The imminent recovery of the ozone layer will heat the polar stratosphere, and its fingerprint is expected to be seen all the way down to the surface, as model simulations show. Therefore, a realistic representation of ozone in climate models is required to reduce uncertainty in climate projections.”
6 February 2023
Frequency comb spectroscopy: from Nobel prize to practical application
Prof. Greg Rieker & Prof. Scott Diddams,
CU Engineering
"The first frequency comb laser was demonstrated on the CU Boulder campus in 1999 in Jan Hall’s lab at JILA. In the two decades since, the technology has proliferated across a vast array of scientific fields from timekeeping and astronomy, to low-noise frequency synthesis and precision spectroscopy for fundamental physics. In recent years, it made the jump to practical applications at the forefront of climate and energy, for example as the foundation of a sensor network detecting methane leaks across 750 sq. miles of oil and gas infrastructure. Prof Diddams will discuss the evolution of frequency comb lasers, metrology and sensing techniques developed in his laboratory at NIST and now CU, which are pushing the boundaries of sensing from the UV to the THz, and from macro to micro-scale comb systems. Prof. Rieker will discuss the push toward robust, fieldable frequency comb systems and practical applications, ending with recent collaborative work between the laboratories on sensing in high-speed chemically reacting systems."
30 January 2023
Formation and dissociation of hydrocarbons under interstellar conditions
Prof. Jordy Bouwman,
CHEM & LASP, Univ. of Colorado
"Hydrocarbons of all sizes and shapes are found throughout the various stages of star and planet formation. Recently, using radio astronomical observations, a variety of cyclic and even polycyclic hydrocarbons have been detected in the very cold (10 K) Taurus molecular cloud, challenging our understanding of the chemical formation pathways under these low-temperature and low-density conditions. In photon dominated regions, on the other hand, very large Polycyclic Aromatic Hydrocarbons (PAHs) of 50 carbon atoms and larger are commonly detected as a class based on the characteristic mid-infrared emission bands that they emit after being electronically excited by ultraviolet and optical radiation. These large PAHs are exposed to a very strong radiation field that can alter their molecular structure and may even lead to dissociation. In this seminar, I will show how experimental studies using synchrotron and free electron laser radiation – in conjunction with quantum chemical computations – allow us to reveal the formation and dissociation mechanisms of interstellar (aromatic) hydrocarbons at a molecular level of detail."
23 January 2023
Secondary Organic Aerosol Mass Yields from NO3 Oxidation of α-Pinene and Δ-Carene: Effect of RO2 Radical Fate
Doug Day,
(ANYL ResSci, Jimenez group)
"Dark chamber experiments were conducted to study the SOA formed from the oxidation of α-pinene and Δ-carene under different peroxy radical (RO2) fate regimes: RO2 + NO3, RO2 + RO2, and RO2 + HO2. SOA mass yields from α-pinene oxidation were <1 to ∼25% and strongly dependent on available OA mass up to ∼100 μg m–3. The strong yield dependence of α-pinene oxidation is driven by absorptive partitioning to OA and not by available surface area for condensation. Yields from Δ-carene + NO3 were consistently higher, ranging from ∼10–50% with some dependence on OA for <25 μg m–3. Explicit kinetic modeling including vapor wall losses was conducted to enable comparisons across VOC precursors and RO2 fate regimes and to determine atmospherically relevant yields. Furthermore, SOA yields were similar for each monoterpene across the nominal RO2 + NO3, RO2 + RO2, or RO2 + HO2 regimes; thus, the volatility basis sets (VBS) constructed were independent of the chemical regime. Elemental O/C ratios of ∼0.4–0.6 and nitrate/organic mass ratios of ∼0.15 were observed in the particle phase for both monoterpenes in all regimes, using aerosol mass spectrometer (AMS) measurements. An empirical relationship for estimating particle density using AMS-derived elemental ratios, previously reported in the literature for non-nitrate containing OA, was successfully adapted to organic nitrate-rich SOA. Observations from an NO3– chemical ionization mass spectrometer (NO3–CIMS) suggest that Δ-carene more readily forms low-volatility gas-phase highly oxygenated molecules (HOMs) than α-pinene, which primarily forms volatile and semivolatile species, when reacted with NO3, regardless of RO2 regime. The similar Δ-carene SOA yields across regimes, high O/C ratios, and presence of HOMs, suggest that unimolecular and multistep processes such as alkoxy radical isomerization and decomposition may play a role in the formation of SOA from Δ-carene + NO3. The scarcity of peroxide functional groups (on average, 14% of C10 groups carried a peroxide functional group in one test experiment in the RO2 + RO2 regime) appears to rule out a major role for autoxidation and organic peroxide (ROOH, ROOR) formation. The consistently substantially lower SOA yields observed for α-pinene + NO3 suggest such pathways are less available for this precursor. The marked and robust regime-independent difference in SOA yield from two different precursor monoterpenes suggests that in order to accurately model SOA production in forested regions the chemical mechanism must feature some distinction among different monoterpenes."
and
A study of iodine chemistry in the Indian and Southern Ocean marine boundary layer
Swaleha Inamdar,
(ANYL Professional Research Assistant, Volkamer Group)
"Reactive atmospheric iodine significantly affects the tropospheric chemistry by ozone (O3) depletion and participation in various heterogeneous reaction cycles mainly involving O3, causing changes to the atmospheric oxidation capacity. There is growing experimental evidence that halogen chemistry plays a key role as part of tropospheric photochemistry. Much of the proposed halogen chemistry in literature is propagated through the reactions of a series of halogen atom and radicals. Although, some measurements of iodine oxide (IO) are reported around the globe, the remote open ocean remains undersampled. Recently, first reported observations of IO in the Indian and Southern Ocean marine boundary layer (MBL) suggest that atmospheric IO may not be well correlated with the inorganic fluxes. Further, biogenic fluxes play a significant role in active iodine chemistry in this region. This contradicts with the studies reporting that inorganic iodine emissions are responsible for 75% of the reactive iodine in the tropical Atlantic MBL. Thus, to improve our understanding of iodine chemistry in this region, field observations in the remote open ocean of the Southern Ocean are carried out in this study via several ship-based expeditions in this region. To identify the geographical emissions of reactive iodine precursors, this study compiles observations from the Indian and Southern Ocean MBL for a comprehensive region-specific parameterization for estimating the inorganic iodine fluxes via sea surface iodide concentrations. This study addresses whether the existing parameterization tools for inorganic iodine fluxes are sufficient to explain the detected IO in the remote open ocean MBL."
Fall 2022
5 December 22
Vapors and Aerosols: NIST’s forensic approach to arson and cannabis intoxication
Jennifer Berry,
National Institute of Standards and Technology
"The National Institute of Standards and Technology (NIST) is world-renowned for making standards and precise measurements with advanced measurement techniques. The Fluid Characterization Group, part of the Boulder-based Materials Measurement Laboratory, is rooted in precise thermodynamic measurements of fuels and has successfully developed technologies and methods for forensic vapor and aerosol detection. The group’s Dynamic Vapor Microextraction (DVME) was originally developed for vapor pressure measurements of low volatility, unstable molecules but has been expanded towards a variety of forensic applications, particularly for the detection of arson from fire debris. The instrumental development for this new application needs to be thoroughly planned and designed to encompass a large number of factors and their interactions, breaking from a one-factor-at-a-time method. Conducting a sensitivity analysis permits the exploration of multiple variables at the same time while limiting the number of experiments needed. When coupling a sensitivity analysis with DVME in a fire debris study, we found that both the metric and the experimental plan played a crucial role in determining the importance of different instrumental and sample factors. Beyond fire debris, the group has also been developing and testing breath collection techniques to detect cannabinoids in breath after cannabis use. Aerosols and condensed vapors will be collected with two different devices to investigate changes in breath compounds and concentrations after cannabis use. While cannabinoids have been detected in breath aerosols, additional explorations into exhaled breath condensate as a part of a 128- participant study will use a cannabinoid targeted and metabolomics untargeted approach to explore if this type of noninvasive breath collection is a valid method as well. With the growth of forensic research in NIST Boulder, numerous opportunities are available for collaborations, internships, and postdoctoral fellowships at NIST Boulder."
28 November 22
Resolving The Complex Chemistry of Combustion
Prof. Nicole Labbe,
CU Mechanical Engineering
"High temperature gas reacting systems are among the most complex chemical kinetics systems to model. For example, a prototypical gas phase reacting system is combustion, where fuels can undergo tens of thousands of unique competing reactions via thousands of unique chemical species. Further complicating the chemistry, these systems can be highly pressure dependent, and even modest pressure fluctuations can drastically change both reaction rate and species distributions. With such large combinatorial possibilities for reactions and product species, theory alone is often unable to fully resolve the chemical mechanism due to computational limitations. Furthermore, the breadth of reactions and species present limits the ability of experiments to fully resolve the chemistry of may gas phase reacting systems. However, through careful combination of both theoretical and experimental techniques, at least the most critical reactions can often be revealed for accurate chemical model development. In this work, we demonstrate how a combined approach using both semi-automated theoretical kinetics and gas phase speciation experiments was used to resolve several kinetics disputes in the literature due to unresolved kinetic mechanisms. First, we demonstrate how theoretical kinetics was the key to clearing up a discrepancy as to the source of methyl ketene in the pyrolysis of a biodiesel component, ethyl propanoate. Next, we show that a new tunable lab-scale VUV light source was able to experimentally prove a theoretically predicted keto-enol tautomerization for acetone for the first time. Finally, we explore how a combined experimental and theoretical approach resolved how the location of the double bond in methylcyclohexene dramatically changes the sooting propensity between each of the three possible isomers."
14 November 2022
ANYL Alumni Career Panel: Melissa Trainer (NASA), and Dan Bon (CDPHE)
Dr. Melissa Trainer is a planetary scientist at NASA's Goddard Space Flight Center (GSFC) with expertise in the composition of planetary atmospheres and the production of organic molecules and aerosols via in situ synthesis pathways. Dr. Trainer currently serves as a Deputy Principal Investigator (PI) for the Dragonfly mission to Saturn's moon Titan, part of the NASA Planetary Science New Frontiers Program. She is also the lead for the Dragonfly Mass Spectrometer (DraMS), which enables the investigation of Titan's surface composition and characterization of potential prebiotic chemistry. She has spent more than two decades characterizing the properties of Titan and early Earth aerosol analogs, with publications on the chemical, optical, and isotopic properties of organic hazes. Dr. Trainer is a member of the Mars Science Laboratory Sample Analysis at Mars (SAM) instrument team, for which she led the campaign to conduct the first in situ multi-year study of the seasonal variations of the composition of the Mars atmosphere through surface mass spectrometry measurements. She received her undergraduate degree in Chemistry from Franklin and Marshall College in 2000, and her PhD in Analytical Chemistry from the University of Colorado-Boulder in 2006. She has been a scientist at NASA since 2009.
&
Daniel Bon, PhD. Supervisor, Air Toxics Measurement and Special Projects Unit, Technical Services Program, Air Pollution Control Division, Colorado Department of Public Health and Environment. Daniel Bon completed his PhD in Atmospheric Chemistry in 2011 and the University of Colorado, Boulder and the NOAA ESRL Chemical Sciences Division, where his research focused on PTR-MS field measurements of volatile organic compounds in the atmosphere. Since 2011, Dr. Bon has worked for the Colorado Department of Public Health and Environment, Air Pollution Control Division. From 2016-2022, Dr. Bon built and operated the Colorado Air Monitoring Mobile Laboratory. In 2021, he became the Unit Supervisor for the newly created Air Toxics Measurement and Special Projects Unit created to respond to HB21-1189 and HB22-1244 Air Toxics bills passed by the Colorado State Legislature. His growing team includes 7 scientists and 3 mobile measurement labs. Students could learn about what we do by reading the summaries of the new Colorado Air Toxics bills our group is working to implement and a recent press release: hb21-1189, hb22-1244, press release
We have asked the speakers to present for 12-15 min on their perspective on careers outside academia for PhD chemists, and in particular for graduates of our ANYL PhD program. For example, they may address the following questions: what path took you to where you are? What strengths from the ANYL program helped you in your career? What could have the program done to make that better? What do you wish someone had told you when you were a PhD student? What advice would you give to current PhD students and postdocs? A Q&A session will follow, prioritizing questions from current students and postdocs.
7 November 2022
The Ice Nucleating Properties of Southern Great Plains Mineral Dusts
Guy Symonds,
ANYL 1st year, CU Boulder
"Mixed phase clouds, a collection of supercooled liquid and frozen water droplets, are potentially important regulators of the Earth’s climate. In the troposphere, water droplets are primarily frozen through the immersion-mode freezing mechanism, where a rare particle within the droplet acts as a template and catalyzes the production of the ice phase. Commonly, these ice nucleating particles in the atmosphere are some type of suspended mineral dust. The research being presented examines the ice nucleating behavior of previously unprobed dust samples in the Southern Great Plains area in Oklahoma, United States of America. This location was subject to a field campaign due to its isolation from major industrial activity on the North American continent and so it serves as a “Continental background site”. By examining the ice nucleating activity of these dusts, the contribution to ambient aerosol in the Southern Great Plains area can be determined, as well as their relative contribution to total aerosol ice nucleating activity. Furthermore, these dusts were subjected to aging processes designed to simulate environmental weathering processes to further investigate the variability of their ice nucleating activity and how their freezing properties correlate with chemical changes caused by the aging."
and
Formation of a Polariton Through the Creation of Micro Cavities with an Active Layer of PFO
Nicole Silver,
ANYL 1st year, CU Boulder
"The presence of an exciton polariton, a quasiparticle formed through the strong coupling between a photon and an electron hole pair, creates important properties that have the potential to significantly increase the absorption efficiency of organic semiconductor materials in a solar cell. In my undergraduate research at Cornell University in Andrew Musser’s Light Matters Research Group, I focused on finding evidence of a polariton in the organic semiconductor material Perfluorooctanesulphonate (C8F17O3S), also known as PFO. This presentation will outline the intricate process of forming micro cavities, which involves the development of a spin coating technique and identification of several parameters specific to the polymer PFO. These are determined through absorption spectroscopy, profilometry, evaporation in vacuum, angle dependent reflectivity, and simulations. The process of creating a micro cavity and using an angle dependent reflectivity goniometer to discover two polariton branches in a PFO micro cavity will be discussed along with the identification of polariton branches in two other materials, TM82 and TM83."
31 October 2022
Chemistry of Organic Compounds in the Atmosphere and Indoor Air
Paul Ziemann,
ANYL faculty, CU Boulder
"Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative studies of indoor air chemistry at CU. In this talk I will provide a brief overview of recent research in my lab for the purpose of informing first-year chemistry graduate students."
and
Sustainable Magnesium Production via Molten Salt Electrolysis and G-METS Distillation
Madison Rutherford,
ANYL 1st year, CU Boulder
"Current methods of magnesium production are prohibitively expensive and resource intensive with low energy efficiency and high environmental impact. Molten salt electrolysis (MSE) using a reactive liquid cathode, e.g., tin, combined with vapor compression distillation in a gravity-driven multiple effect thermal-system (G-METS VCD) can significantly reduce the energy requirement and environmental impact of both magnesium primary production and recycling. This presentation presents a techno-economic model of cost, energy consumption, and emissions associated with magnesium primary production via MSE and G-METS VCD. The model includes a mass balance with 17 elements, electrolysis process energy balance with carbon or solid oxide membrane anodes, and detailed operating and capital cost estimates. Based on the properties of magnesium and expected operating conditions, the cost of magnesium production using this process could be comparable to or lower than that of aluminum production. Initial electrolysis experiments show high current efficiencies with both carbon and SOM anodes, and future work on G-METS VCD is outlined."
24 October 2022
Quantifying particulate halogens in the atmosphere: Airborne measurements and instrumentation
Dongwook Kim,
ANYL 3rd year, Jimenez group
"Halogens in the atmosphere play important roles in ozone chemistry. Halogen chemistry studies have been mainly focused on gas-phase chlorine, iodine, and bromine chemistry. Particulate halogens can be a reservoir for reactive halogens and can directly interact with ozone. However, they have not been well-constrained in chemical transport models due to a lack of measurements on a global scale. Recently, we have quantified submicron particulate halogens, as well as speciation of oxidation states, from several airborne datasets measured by the University of Colorado High-Resolution Aerodyne Aerosol Mass Spectrometer (CU-HR-AMS). We have identified sources that were previously not well recognized. We present particulate halogen measurements (i.e., I, Br, ClO4-) over urban, remote, and wildfire conditions as well as instrumental development for stratospheric aerosol sampling.
(1) On aircraft platforms, AMS uses an aerodynamic lens that requires constant upstream pressure to work consistently. Airborn interfaces that provide that (pressure-controlled inlets –PCI-) have historically performed less well at high altitudes. We show the recent development of a new PCI inlet design coupled with a PM2.5 aerodynamic lens towards the goal of sampling PM1 aerosols up to ~15 km altitude.
(2) The first quantitative detection of iodine (both particle and gas phase) in the stratosphere has been reported (Koenig et al., 2020). It suggested that particulate iodine is a major fraction in the stratosphere. We present particulate iodine measurement up to the lower stratosphere over the Pacific Ocean during NSF TI3GER and its implications for stratospheric ozone.
(3) Reactive halogens (i.e., chlorine and bromine) can affect urban air quality by providing radical sources. We show that particulate bromine is emitted by anthropogenic sources and compare it with other ground measurements. Also, we identify particulate iodine sources and discuss the potential impact on air quality.
(4) We quantify the emission of particulate iodine from Western US wildfires during FIREX-AQ. We found that particulate fore could be the major form of iodine emission from US wildfires.
(5) Exposure to perchlorate affects the human endocrine system. While the atmospheric sources of perchlorate are highly uncertain, snow and ice core records suggest that chlorofluorocarbons (CFCs) might be important precursors for the photochemical production of perchlorate in the stratosphere. We show the first global in-situ perchlorate measurements from the ATom campaign over the remote oceans and comparison to the GEOS-Chem model with updated perchlorate-related mechanisms. Also, we show anthropogenic emissions of perchlorate that may affect the tropospheric perchlorate burden. "
26 September 2022
Particle formation and growth
Eleanor Browne,
ANYL faculty, CU Boulder
"The Browne group uses a combination of laboratory experiments and field measurements to understand the sources and transformations of trace gases in the atmosphere with the ultimate goal of understanding the impacts of this chemistry on air quality, climate, and nutrient delivery to ecosystems. Here, I will discuss some of our recent work investigating particle formation and growth in an agricultural setting."
19 September 2022
Chemistry of Volatile Organic Compounds in the Atmosphere
Joost de Gouw,
ANYL faculty, CU Boulder
"Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products.
In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments.
Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments to better understand their sources and fate. One particular study involves the impact of the Marshall Fire in Boulder County on indoor air in homes that were near the burnt area. Second, we are working on the emissions and chemistry of VOCs in urban air. One study that will be highlighted involved field measurements in Los Angeles in the Summer of 2022. Third, we are working on a chamber study to better understand the formation of secondary organic aerosol from biogenic VOCs. Finally, we are also using data from satellite remote sensing instruments to measure the pollutants from oil and natural gas production, and from wildfires."
and
Understanding aerosols for pollution, climate change, and disease transmission
Jose Jimenez,
ANYL faculty, CU Boulder
"Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly present results from different projects over the last year, as well as some future directions for our group. (1) I will introduce our aircraft research program, including results from recent projects, and how they set up scientific questions for the upcoming NASA ASIA-AQ project on multiple megacities (if politics allows: Tokyo, Seoul, Manila, Bangkok, Kolkata, Dhaka, Hanoi, Singapore, and Kuala-Lumpur). (2) I will present preliminary results from the CalNexT-2022 field study in the Los Angeles area, where we returned (along with Prof. de Gouw’s group and other colleagues) to the Caltech campus 12 years after CalNex-2010. Analysis so far suggests that ½ of the SOA potential in ambient air is due to species less volatile than sesquiterpenes and aromatics (c* < 1e4 ug m-3). (3) I will discuss recent results on COVID airborne transmission, as well as the indoor chemistry due to germicidal ultraviolet (GUV) air disinfection and other commercial “air cleaners”.
For those interested in COVID-19 aerosol transmission, you can find a summary of the evidence supporting airborne transmission of COVID-19 at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00869-2/, a review for airborne transmission of all respiratory diseases at https://doi.org/10.1126/science.abd9149, an overview of how airborne transmission really works at https://doi.org/10.1111/ina.13025, and an overview of the historical reasons for the confusion on this topic at https://doi.org/10.1111/ina.13070."
12 September 2022
From Coastal Megacities to the tropical UTLS: Iodine and Ion induced Particle Formation
Rainer Volkamer,
ANYL faculty, CU Boulder
"The tropical upper troposphere and lower stratosphere (UTLS) is a unique yet understudied atmospheric environment. The combination of higher flux of ion inducing cosmic radiation and lower temperatures makes ion-induced nucleation a more significant source of particles in the UTLS than in the lower troposphere. The Volkamer group develops advanced instrumentation (optical and mass spectrometric) to measure small molecules, condensable vapors and ambient ions that are relevant to particle formation, public health and climate discussions. We seek to better quantify and understand the interconnections between marine ecosystem, aerosols and their control on atmospheric chemistry. We also study the changing oxidative chemistry in coastal Megacities using research aircraft and satellites. Examples from recent field and laboratory experiments are discussed that aim to develop a molecular level understanding of the fundamental processes that affect a) new particle formation from iodine, and b) apply high-resolution mass spectrometry on high-flying aircraft to sample ambient ions in the UTLS. Opportunities for graduate research exist to 1) conduct and analyze field measurements using research aircraft (i.e., TI3GER-2022, CUPiDS-2023 projects), 2) laboratory experiments of particle formation (incl. at CLOUD/CERN) and multiphase chemistry, and 3) develop instrumentation to exploit synergies between optical spectroscopy and mass spectrometry."
Summer 2022
27 May 2022
Calibration of Iodide-CIMS for oxygenated hydrocarbons in the gas-phase: Application to measure Henry’s Law of 1,2-ISOPOOH
Joey D'Alesio,
Volkamer group
"Two gas-phase quantification methods for Chemical Ionization Mass Spectrometry using Iodide reagent ions (Iodide-CIMS) were developed in this work: the integration method and the flow rate method. Both methods utilize a Hamilton syringe pump to relate the detected signal to the gas-phase concentration, also known as a calibration coefficient, injected into a VOCUS 2R API-LToF mass spectrometer configured with an AIM source and equipped with VUV light to generate iodide reagent ions. These quantification methods can be combined with any chemical ionization mass spectrometer utilizing a large variety of target analytes. The integration method was used to determine a calibration coefficient, which was applied to partitioning experiments in order to determine the Henry’s Law coefficient for 1,2-ISOPOOH. Henry’s Law describes the ratio of the presence of a compound in the aqueous-phase and the gas-phase when partitioning is in equilibrium. Henry’s Law applies only to dilute solutions. The 1,2-ISOPOOH Henry’s Law coefficient was determined to be 2.61E+05 M/atm +/- 1.81E+04 M/atm. The reported value is bracketed by available literature values, which range from 1.00E+05 M/atm to 1.30E+06 M/atm. The flow rate method was used during initial calibration development to determine preliminary calibration coefficients for a suite of atmospherically relevant acids. This method will continue to be optimized for future laboratory and aircraft calibrations."
23 May 2022
Role of Oxidative Conditions on the Formation of Aerosol and Organic Nitrates from the Reactions of Monoterpenes with NO3 and OH radicals
Marla DeVault,
Ziemann group
"Monoterpenes are widely emitted biogenic volatile organic compounds, which react quickly in the atmosphere and the oxidized products of which can partition into secondary organic aerosol (SOA). Understanding the types of products that are formed, how they might react in the particle-phase, and the effect of oxidative conditions on these factors are key for improving atmospheric models. Specifically, we are interested in the formation of organic nitrates and their impact on SOA. In order to study this, five monoterpenes were oxidized by NO3 radicals, to simulate nighttime conditions, and OH radicals in the presence of NOx, to simulate daytime conditions. The SOA collected from these ten reactions show a prevalence of acetal and hemiacetal dimers and trimers for NO3 radical oxidation for cyclic monoterpenes, which are notably absent from the OH/NOx oxidized SOA. This talk explores what mechanistic processes lead to this and other differences in particle-phase organic nitrates derived from these two reactions."
Spring 2022
18 April 2022
New approaches to ambient ion analysis reveal chemical trends
Daniel Katz, ANYL 3rd year,
Browne group
"Atmospheric ions control the electrical properties of the atmosphere, influence chemical composition via ion-molecule and/or ion-catalyzed reactions, and affect new particle formation. Understanding the role of ions in these processes requires knowledge of ionic chemical composition. However, determining the chemical composition of these ions is analytically challenging owing to the low concentration of ambient ions in the atmosphere (~100s-1000 ions/cm3). Here, we analyze measurements of the composition of ambient cations and anions collected using an atmospheric pressure interface time-of-flight mass spectrometer (APi-TOF) during the 2016 Holistic Interactions of Shallow Clouds, Aerosols, and Land- Ecosystems (HI-SCALE) campaign. We utilize a newly developed technique, binned positive matrix factorization (binPMF), in conjunction with Resolution-enhanced Kendrick Mass Defect (REKMD) analysis. These techniques allowed for improved chemical insight into the trends in ion composition with no requirement for a priori assignments of chemical composition. This advancement is of particular importance for measurements with low signal-to-noise. Mass spectral factors were first identified by binPMF and then analyzed using REKMD plots to elucidate chemical patterns within the factors. REKMD demonstrated that otherwise unidentified compounds were related by repeating units of CH2 and O. Back trajectories and correlation with other measurements provide insight into potential sources of the various identified factors. Overall, we demonstrate that binPMF in combination with REKMD is a powerful tool to analyze challenging mass spectrometric datasets."
11 April 2022
Measurements of VOCs in Homes Impacted by Smoke from the Marshall Fire
William Dresser, ANYL 3rd year,
de Gouw group
"The Marshall Fire was one of the most destructive fires in Colorado history, and burned and damaged over 1000 structures in Boulder County. In the immediate aftermath of the fire, there was an intense interest in accessing the persistent air quality effects of the fire both due to its close proximity to major population centers as well as the unique nature of the fire fuel, mostly man-made structures as opposed to biomass. While smoke emissions from traditional wildfires have been well studied and characterized, the emissions from building materials are less well understood and can vary significantly based on the structure. We are interested in how the smoke emissions from this fire infiltrated and then interacted in indoor environments, which have large surface reservoirs, leading to potential persistent indoor air quality effects. In the weeks following the fire, multiple instruments were deployed in a smoke-impacted home immediately adjacent to one of the burn areas to monitor effects for a period of roughly a month. Our measurements focused on gas phase Volatile Organic Chemicals (VOCs) and were carried out with a Vocus Proton-Transfer-Reaction Time-of-Flight Mass Spectrometer (Vocus PTR-TOF) and Aerodyne Gas Chromatograph (GC). Both indoor and outdoor levels were measured to look at long term trends and indoor enhancements. Ventilation experiments as well as mitigation tests using Corsi-Rosenthal boxes were done, and analyzed with respect to changes in indoor VOC concentration and exposure. Smoke remediation took place during the study, and the data give insight into enhancements in VOCs before, during, and after the cleaning."
4 April 2022
Kinetics of Oligomer-Forming Reactions Involving the Major Functional Groups Present in Atmospheric Secondary Organic Aerosol Particles
Hannah Maben, ANYL Master's Thesis Defense,
Ziemann group
"Atmospheric organic aerosol particles impact climate as well as human and environmental health. Secondary organic aerosol (SOA), which is formed by the gas-to-particle partitioning of products of the oxidation of volatile organic compounds (VOCs) emitted from biogenic or anthropogenic sources, contributes a large fraction of this material. In the particle phase, these products can undergo accretion reactions to form oligomers that impact the formation, composition, and chemical-physical properties of aerosols. While these reactions are known to occur in the atmosphere, data and models describing their kinetics and equilibria are sparse. Here, reactions of compounds containing potentially reactive hydroperoxide, hydroxyl, carboxyl, aldehyde, and ketone groups were investigated in single and phase-separated organic/aqueous mixtures in the absence and presence of a sulfuric acid catalyst. Compounds containing these groups and a nonreactive UV-absorbing nitrate group were synthesized and their reactions and products were monitored and characterized using high-performance liquid chromatography with UV detection (HPLC-UV), electrospray ionization-mass spectrometry (ESI-MS) and attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy. Reactions were observed between hydroperoxides and aldehydes to form peroxyhemiacetals, and between carboxylic acids and alcohols to form esters, and their rate and equilibrium constants were determined. No reactions were observed in other mixtures, indicating that under the conditions of these experiments only a few reaction pathways form oligomers. Reactions were also conducted with probe compounds and SOA formed in an environmental chamber reaction of α-pinene with O3 SOA. Whereas in a previous study we observed a rapid hydroperoxide reaction in this SOA, among the other compounds studied here only alcohols reacted. These results provide insight into the types of accretion reactions that are likely to occur in atmospheric aerosols, and the rate and equilibrium constants can be used to better model SOA chemistry."
1 April 2022
Atmospheric Chemistry of Volatile Methyl Siloxanes – Kinetics and Oxidation Mechanism from Experimental and Theoretical Investigations
Mitchell Alton, ANYL PhD thesis defense,
Browne group
"Volatile methyl siloxanes (VMS) are solely anthropogenic chemicals that have come under recent scrutiny for their environmental persistence and tendency to bioaccumulate. Millions of tons of these chemicals are produced every year, with an estimated 30 kilotons of decamethylcyclopentasiloxane (D5) emitted into the environment every year, with additional contributions from other VMS. A large source of VMS to the environment is from use of personal care products containing these compounds. Due to environmental concerns about the impact of these compounds, the European Union placed restrictions on the quantity of VMS in wash-off personal care products in 2018 with recommendations to increase the restrictions to include some industrial processes in 2021. Although there is significant interest in these high-production chemicals, the understanding of the environmental fate of these compounds is incomplete. It is predicted that >90% of VMS released to the environment will partition into the atmosphere. Once these compounds are in the atmosphere, they can react with hydroxyl radicals (OH) or chlorine atoms (Cl) through hydrogen-abstraction reactions, though the previously reported rate constants for reactions with OH vary by more than a factor of 3 and only one measurement exists for the rate constant with Cl atoms. Additionally, previously published works reported inconsistent oxidation products of these compounds, likely due to unconstrained oxidation chemistry in their experiments. To better constrain the atmospheric chemistry and fate of these compounds, I measured the kinetics of seven VMS with OH radicals and Cl atoms in a 1 cubic meter FEP Teflon™ chamber I designed and built. Additionally, I measured the oxidation products of VMS in a variety of atmospheric conditions to better constrain the oxidation mechanism. Finally, I used quantum theoretical calculations to investigate the plausibility of the reactions proposed from our experimental results."
14 March 2022
Spectroscopic studies of oxygen and iodine using cavity enhanced extinction spectroscopy
Henning Finkenzeller, ANYL PhD thesis defense,
Volkamer group
"Atmospheric oxygen extinguishes approximately 2 W m-2 solar radiation in oxygen-oxygen collision-induced absorption (O2-O2 CIA). This heats the atmosphere, affects radiative transfer, and needs to be considered in atmospheric spectroscopy. The lack of O2-O2 CIA spectra below 335 nm wavelength is limiting remote sensing applications. Here we report measured spectra of the O2-O2 CIA cross-section between 297-500 nm using Cavity Enhanced Extinction Spectroscopy. Several heretofore unmeasured weak absorption bands (at 315, 328, 420, and 495 nm) are characterized for the first time in the gas phase under controlled laboratory conditions, i.e., at atmospheric pressure and variable temperature (293, 263, and 223 K). The spectra are optimized for use in hyperspectral remote sensing applications, provide opportunities to develop theory, and warrant application in radiative transfer calculations to re-assess O2-O2 CIA heating rates in the atmosphere.
Iodine is a reactive trace element in atmospheric chemistry that destroys ozone and efficiently nucleates particles. Iodine emissions have tripled since 1950 and are projected to keep increasing. While iodic acid (HIO3) is widespread and grows particles more efficiently than sulfuric acid, its gas-phase formation mechanism is unresolved. Here, in CLOUD experiments which generate iodine radicals at atmospherically relevant rates, we show that iodooxy hypoiodite, IOIO, is efficiently converted into HIO3 via reactions (R1) IOIO + O3 → IOIO4 and (R2) IOIO4 + H2O → HIO3 + HOI + O2. The laboratory derived mechanism is corroborated by theory and shown to explain field observations of daytime HIO3 in the remote lower free troposphere. The new mechanism provides a missing link between iodine sources and particle formation and - since aerosol iodate is readily reduced in the atmosphere, thereby recycling iodine back to the gas phase - suggests an important catalytic role of iodine in aerosol formation."
7 March 2022
Polymer Sorption of VOCs for Indoor Air Quality and Atmospheric Sampling
Melissa Morris, ANYL 3rd year,
Jimenez group
"Polymers comprise a substantial fraction of the surfaces in indoor environments, where we spend about 90% of our time. It is well-known that polymers absorb gas-phase volatile organic compounds (VOCs), which directly affect indoor and outdoor air quality, but few studies have investigated the interactions between polymers relevant to indoor materials and VOCs. To better understand the role of carpets on indoor air quality, we have extended recent studies from our groups on polymer-VOC interactions to include polymers relevant to carpets (nylon, polyester, polypropylene, polyethylene), as well as carpet itself. We use a Vocus proton transfer mass spectrometer to measure sorption of a series of 2-ketones by polymer or rolled-carpet tubes. Then, we use two sorption models adapted from Pagonis et. al. (2017) and Algrim et. al. (2020) to quantify sorptive capacities (and for highly-sorptive polymers, VOC diffusion coefficients) for these materials. The polymers were found to increase in sorptive capacity as: nylon, polyester, polypropylene and polyethylene. Polypropylene and polyethylene (often used in carpet backing) are more sorptive for the same geometry. However, nylon and polyester (the materials of carpet fibers), do comparable sorption when scaled up to account for the enormous surface area of carpet fibers. The sorptive capacities of carpet polymers suggest that carpet indoors is comparable in sorptive capacity to paint (Algrim et al. 2020) and wood (Ziola et al. 2022). Sorption experiments also showed that nylon carpet irreversibly sorbs C4-C9 acids. While testing carpet-relevant polymers for VOC sorption, we tested a few additional polymers relevant to atmospheric sampling, including TSI conductive silicone tubing. We found that conductive silicone tubing only transmits very high-volatility compounds (C* > 1e7 ug m-3, such as terpenes and more volatile species). We have demonstrated the use of a system with tubing of multiple polymer materials as a separator of gas-phase compounds into volatility classes. This technique could be useful e.g. for measuring chemical characteristics (i.e. OH reactivity or SOA potential) of complex mixtures of gas-phase compounds (such as ambient air) according to volatility class."
28 February 2022
Reduction of Iodate in aqueous organic and inorganic thin films
Margarita Reza, ANYL 3rd year,
Volkamer group
"Iodine species are known to catalytically destroy ozone, and in the case of Iodic acid (HIO3), efficiently forms particles. Iodic acid is a source of particulate iodate (IO3-); this has been detected in aged stratospheric air alongside gas-phase IO radical, suggesting the existence of recycling mechanisms reducing IO3- to form volatile iodine species. The reduction of iodate is explored through a series of photochemical coated wall flow tube (CWFT) experiments. A quartz glass tube was coated with an aqueous solution containing sodium iodate in a matrix (i.e., either ammonium bisulfate (ABS), 1,2,3,4-butanetetracarboxylic acid (BTCA), citric acid (CA), or Fe(III) citrate (Fe-Cit) and CA) at a concentration ratio iodate:matrix = 1:100. A thin aqueous film forms on the inside walls by passing a stream of humidified air through the CWFT (~80% RH). For each film, the formation of gaseous iodine (I2) was followed in three types of experiments: (1) dark reaction with H2O2 involved flowing H2O2 through the CWFT for several hours in the dark; (2) photochemical experiments involved irradiating the CWFT with visible lights and UVA lights, separately; and (3) dark-aging experiments involved flowing H2O2 through the CWFT for several hours in the dark, followed by irradiation with visible light. The evaporation of gas-phase I2 from the aqueous films was measured by cavity enhanced differential optical absorption spectroscopy (CE-DOAS) coupled to the CWFT. The cleanliness of the materials, cleaning procedures used, and the absence of uncontrolled chromophores reducing iodate, that might be intrinsic to the CWFT setup was confirmed in blank experiments. The I2 released from aged films irradiated with visible light (type 3 experiments) was found to be substantially greater than that from irradiated fresh films (type 2), or fresh films exposed to H2O2 in the dark (type 1). This increase of I2 in H2O2 aged films, independently observed in both inorganic and organic matrices, suggests that a secondary inorganic chromophore is formed from the reaction of iodate with H2O2. A photochemical pathway was discovered in which visible light is sufficient for reducing iodate to I2. This is relevant in the atmosphere because it helps to explain the co-existence of particulate IO3- and gas-phase IO radicals recently observed by aircraft measurements in the stratosphere. Multiphase re-cycling of I2 from particulate IO3- in absence of ultraviolet light further suggests that a catalytic reaction cycle is more active than previously thought, and involves three steps: (1) gas-phase HIO3 formation from I2 photolysis, (2) condensation and dissociation of HIO3 to form IO3-, and (3) IO3- reduction to re-cycle I2. This catalytic reaction cycle destroys O3 by multiphase chemistry, and highlights a possible catalytic role of HIO3 in particle formation. This photochemistry is currently missing in atmospheric models, seems relevant to predictions about the partitioning of iodine between the gas- and particle phases, invigorates particle formation from iodine oxoacids, and seems relevant to better understand iodine’s role in the recovery of the ozone layer."
21 February 2022
An Investigation of Carboxylic Acid Chemistry in Indoor and Outdoor Environments
Anna Ziola, ANYL 3rd year,
Ziemann group
"Carboxylic acids are prominent organic molecules in indoor and outdoor environments and also have chemical properties that make them useful probes for investigating the chemistry of indoor and outdoor air. Even though the average person spends nearly 90% of their lifetime indoors, we know very little about the processes that impact volatile organic compounds (VOCs) in indoor environments, where there are a variety of surfaces that VOCs can interact with. To understand the role of wood surfaces, we have used an iodide chemical ionization mass spectrometer (I-CIMS) and an attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectrometer along with a model to quantify and better understand how carboxylic acids interact with a variety of wood species and varnishes. These experiments have provided parameters for use in models and shown that the behavior of carboxylic acid VOCs indoors relies more on the identity of the varnish and less on the identity of the wood species. We have also taken advantage of the chemical properties of carboxylic acids to study the oxidation of alkenes in low NOx environments. According to data from CalNex in 2010 and LAAQS in 2020, the concentration of mid-day NO, a prominent component in VOC oxidation mechanisms, has decreased by about 75% in Los Angeles in the past decade. There have been many studies on the oxidation of VOCs in high NOx environments, but far fewer in the absence of NOx. To gather more knowledge about low NOx chemistry, we reacted 6-heptenoic acid, a C7 1-alkene with a terminal carboxylic acid group, with OH radicals in an environmental chamber while using an I-CIMS to identify and quantify gas-phase products. We also collected aerosol particles on filters, derivatized the carboxylic acid groups, and identified and quantified the products using high performance liquid chromatography (HPLC) in tandem with an electrospray ionization mass spectrometer (ESI-MS). Therefore, by taking advantage of carboxylic acid properties, we have been able to better understand their interactions with indoor surfaces and gain insights into the oxidation of alkenes under low NOx conditions."
14 February 2022
Longer term aging of organic aerosol: photobleaching and chemical transformations
Rachel O'Brien,
College of William and Mary
"Organic aerosol particles can influence the climate through either directly absorbing or scattering solar radiation or by acting as nuclei for cloud droplets. Some aerosol particles are dominantly scattering while others contain organic molecules that can absorb solar radiation in the visible region, termed brown carbon (BrC). We still have large uncertainties in the magnitude of these climate effects and a better understanding of the removal rates for the particle mass and absorption properties (i.e. color) is needed. One removal process is photolysis, where absorption of solar radiation leads to fragmentation of the organic molecules and the loss of particle mass and/or color. However, the photolysis rates and the overall extent of mass that can be removed via direct photolysis in laboratory experiments does not match what is used in models and often differs from ambient measurements. In this talk, I will combine results from work in our lab looking at photolysis of biogenic secondary organic aerosol as well as BrC from biomass burning organic aerosol to evaluate gaps in our ability to predict the observed ambient removal rates. By probing complex mixtures from recent biomass burning experiments (e.g. FIREX samples), I will demonstrate that our current measured rates in the laboratory are overestimated and that a slower photolysis rate, as well as a potential gas-phase oxidation rate, should be used to predict the role of photolysis on organic aerosol lifetime in the atmosphere."
Fall 2021
Evaluating TROPOMI CO Column Observations Using CU AirSOF During BB-FLUX
Jake Rowe, ANYL MS Defense, Volkamer group
"The Biomass Burning Fluxes of Trace Gases and Aerosols (BB-FLUX) field campaign was carried out during the summer of 2018 with the primary goal of quantifying emission fluxes of trace gases by mass balance of actual wildfires. To characterize these fluxes, the University of Colorado Airborne Solar Occultation Flux (CU AirSOF) instrument was flown below biomass burning plumes to measure vertical trace gas columns, such as carbon monoxide (CO, with the first overtone fit from 4214 - 4254 cm-1), along the direct solar beam. The TROPOspheric Monitoring Instrument (TROPOMI) provides measurements of trace-gas maps in the Ultraviolet-Visible (UV-Vis.) and shortwave-IR (SWIR) spectral regimes (e.g. CO from 4277 - 4303 cm-1). A subset of flights during BB-FLUX were dedicated to position the aircraft below smoke plumes while the satellite measures the same scene aloft.
Radiative transfer simulations were conducted to estimate the effect of aerosol multiple scattering in smoke plumes, which is not routinely accounted for in TROPOMI CO retrievals at short-wave infrared wavelengths. Aerosol multiple scattering recovers sensitivity losses in the absence of surface albedo (by up to 80%), but the loss is greatly reduced (5-10%) if surface albedo exceeds 10%. The difference in the temporal and spatial scales, and measurement geometries sampled from the aircraft and satellite are actively addressed by 1) focusing on near coincident case studies, 2) comparing spatial integrals of CO differential vertical column densities (dVCDs) across plumes, and 3) using the FLEXible PARTicle (FLEXPART) dispersion model to correct for different sampling times. CU AirSOF revealed variations in CO dVCD integrals on the order of 24% (up to 37%) over 30 mins (consecutive transect measurements) that characterize changes in emissions on the satellite sub-pixel scale. TROPOMI CO is found to be systematically higher relative to the aircraft by +26% for the operational product (+23% scientific product) without FLEXPART correction; such high-bias is reduced to +8.7% (+5.8%) upon adding the FLEXPART correction. The small but systematic high-bias in TROPOMI CO is most likely due to unaccounted aerosol multiple scattering, atmospheric variability, or a combination thereof."
8 November 2021
Bringing Real-World Chemical Complexity to the Laboratory – Secondary Organic Aerosol Formation from Plant Emissions Increases Particle Viscosity and Alters Composition
Prof. Celia Faiola, Univ. of California-Irvine
"Climate change is influencing ecosystem health and plant biogeography. This affects the composition and spatiotemporal distribution of biogenic volatile organic compounds (BVOCs) across Earth’s surface. Perturbations to the types of compounds emitted could have significant impacts on secondary organic aerosol (SOA) production, but the chemistry of many of these compounds (including complex mixtures of these compounds) have not been studied comprehensively in a controlled laboratory environment. This presentation will summarize laboratory studies investigating SOA formation from complex mixtures of real plant emissions representing different plant emission types. Unexpected effects on aerosol chemistry, composition, and properties attributed to the presence of oxygenated monoterpenes and acyclic terpenes in the BVOC mixture were observed. Oxygenated monoterpenes (i.e. camphor and eucalyptol) that dominate emissions from sage and sagebrush produce SOA with more highly oxygenated molecules than SOA formed from more traditionally-studied terpenes. Acyclic terpenes (i.e. myrcene and farnesene) that are often associated with plant stress, reduce SOA yields and promote fragmentation reactions while increasing the viscosity of the resulting SOA. Aerosol chemistry of these compounds could become increasingly important with the expansion of drought-tolerant sage scrub and elevated frequency of plant stress conditions."
1 November 2021
Evaluation of Halogen-Induced Ozone Depletions Near Salt Lake City
Wyndom Chace, ANYL 1st year, CU Boulder
"An unexpected halogen-rich plume, originating from an industrial plant on the western shore of the Great Salt Lake, was sampled on nine separate flights during the NOAA 2017 Utah Winter Fine Particulate Study (UWFPS). Tropospheric ozone depletions were also measured in the halogen plume, analogous to halogen-induced ozone degradation in other regions of the atmosphere such as the arctic troposphere and the stratosphere. This study sought to quantify the halogen flux from the industrial plant and to characterize the photochemical processes that result in tropospheric ozone depletions. The calculated Cl2 and HCl emission fluxes were consistent with the industrial plant’s reported EPA emissions inventory; the inventory does not report fluxes of Br2 and BrCl, which also had significant calculated fluxes. The observed ozone depletions were investigated using a semi-Lagrangian plume setup in a zero-dimensional atmospheric chemistry box model (F0AM). The box model was able to reproduce the magnitude of ozone depletion observed in the daytime January 26 plume (>35 ppbv below background levels) and confirmed that chlorine and bromine radicals, generated from the photolysis of halogen species emitted by the plant, were responsible for the observed ozone depletions. These results suggest that atypical levels of industrial halogen emissions may have significant impacts on local air quality, particularly relevant in the case of this industrial plant due to its close proximity to Salt Lake City."
and
An Investigation of Ozone Chemistry During KORUS-AQ Using Observations and Modeling
Lindsey Anderson, ANYL 1st year, CU Boulder
"Ground-level ozone is a toxic air pollutant that is created from reactions of nitrogen oxides (NOx = NO + NO2) and volatile organic compounds (VOCs) in the presence of sunlight. The production of ozone is a nonlinear function of its precursors, which complicates emission regulations that target ozone reduction. In this study, ozone chemistry in Seoul, South Korea was investigated using flight observations from the Korea United States Air Quality (KORUS-AQ) field study. Measurements near the city center are characteristic of a highly polluted urban region with high levels of NOx concentration, while measurements to the southwest exhibit high levels of VOC concentration from chemical production facility emissions. The ozone production regime (NOx-limited vs. VOC-limited) in the Seoul Metropolitan Area (SMA) was determined to be VOC-limited, meaning reductions in the concentration of reactive VOCs would lead to a decrease in ozone production. Possible emission reduction regulations were then modeled to understand how they would impact ozone formation in the SMA. It was determined that emission regulations targeting ozone reduction in the SMA should include reductions in both NOx and reactive VOCs because NOx reduction alone would lead to an increase in ozone formation."
25 October 2021
Comparison of Common Vapor Pressure Estimation Methods through Modeling of Alkene OH·/NOx Systems
Emmaline Longnecker, ANYL 1st year, CU Boulder
"Modeling of atmospheric reactions is an important tool in understanding the current and future impacts of human activity on the environment. Vapor pressure is a key parameter in modeling these reactions, as it largely determines the ability of a species to transition from the gas to particle phase. However, the vapor pressures of many atmospherically relevant molecules are still poorly constrained. In order to aid modeling efforts, several group contribution methods have been developed for estimating compound vapor pressures. The current study evaluates how four of these methods: SIMPOL, EVAPORATION, SPARC, and Nannoolal, impact the modeled predictions of secondary organic aerosol (SOA) yields for the reactions of C8-C14 1-alkenes with OH radicals in the presence of NOx. The models were created in the program KinSim and included detailed reaction mechanisms and branching ratios determined in several previous chamber studies by our research group, as well as gas-particle and gas-wall partitioning. SOA yields predicted using each of the four estimation methods were then compared to the measured values. The results of the models were variable, with the maximum discrepancies ranging from an underestimate of ~40% to an overestimate of ~30% compared to the experimentally determined mass yields. This variability exemplifies the impact of vapor pressure in modeling atmospheric reactions and indicates the need for further research in development of estimation methods."
and
Redox-Active Coordination Complexes for Small Molecule Activation with Environmental Applications
Hanalei Lewine, ANYL 1st year, CU Boulder
"Nitrate and nitrite are harmful pollutants resulting from the overuse of nitrogen fertilizers in agriculture. In Nature, denitrification converts these to lower-oxidation state nitrogen species, each step being catalyzed by a different metalloenzyme such as nitrate reductase. Synthetic systems that mimic these enzymes could convert NOx- to less harmful, and potentially useful compounds like nitrogen (N2) and ammonia (NH3). The highly versatile pyridinediimine (PDI) ligand has been successful in the reduction of these species due to the potential for incorporating redox-activity, proton-responsivity, and hemilability into the ligand scaffold. A new PDI iron complex featuring a hemilabile pendant phosphine shows reactivity towards nitrate and nitrate to selectively reduce to NO on a mononitrosyl iron complex (MNIC) will be presented."
27 September 2021
Chemistry of Organic Compounds in the Atmosphere and Indoor Air
Paul Ziemann, ANYL Faculty, CU Boulder
"Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative studies of indoor air chemistry at CU."
and
Chemistry of Volatile Organic Compounds in the Atmosphere
Joost de Gouw, ANYL faculty, CU Boulder
"Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products.
In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments.
Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments to better understand their sources and fate. Second, we are working on the emissions and chemistry of VOCs in urban air. Third, we are working on a chamber study to better understand the formation of secondary organic aerosol from biogenic VOCs. Finally, we are also using data from satellite remote sensing instruments to measure the pollutants from oil and natural gas production, and from wildfires."
20 September 2021
Heterogeneity in Airborne Transmission of COVID-19 by Respiratory Aerosols
Prasad Kasibhatla (Duke Univ., CIRES Visiting Fellow)
"The recognition of the importance of airborne transmission of the SARS-CoV-2 virus by respiratory aerosols has spurred several research groups to develop and apply simple mechanistic aerosol models to investigate COVID-19 outbreaks associated with specific events. These studies have highlighted the importance of layered, non-pharmaceutical intervention strategies such as masking, ventilation, and air cleaning to mitigate transmission risk. To date, however, there is a disconnect between these micro-scale mechanistic aerosol models and the macro-scale epidemiological models that have been used to study large-scale COVID-19 disease dynamics. In this talk, I will discuss my recent research to try to close this gap. I will present a Monte Carlo analysis of transmission risk and secondary infections arising in social settings by modeling aerosol transmission of COVID-19 in thousands of indoor locations in the United State. I will show that variability of viral load among index cases plays a key role in shaping the variability in transmission risk at these locations. I will further demonstrate that aerosol transmission is consistent with the observation that COVID-19 transmission is overdispersed and will provide a mechanistic explanation for this large-scale characteristic of the pandemic. Implications of this finding with regards to the effectiveness of non-pharmaceutical interventions in curbing the pandemic will be discussed. Finally, I will outline my ideas for the next steps in this research that I will be working on during my sabbatical at CIRES."
13 September 2021
Small molecules in the Anthropocene: Oceans, Wildfires, and New Particle Formation from Iodine
Rainer Volkamer, ANYL faculty, CU Boulder
"The Volkamer group develops advanced optical instrumentation (in situ and remote sensing) to measure small molecules and aerosols that are relevant to public health discussions and climate. We seek to better quantify and understand emissions of small molecules, total carbon, and aerosols from natural and managed ecosystem (e.g., wildfires, oil & natural gas, ocean surface, UTLS), and develop a molecular level understanding of the fundamental processes that affect their chemical transformations and sinks (e.g., new particle formation). The combination of optical spectroscopy and high-resolution mass spectrometry is an emerging future theme in the group. Opportunities for graduate research exist in the areas of 1) field measurements using research aircraft (i.e., TI3GER-2022, CUPiDS-2023 projects), 2) laboratory experiments of particle formation (incl. at CLOUD/CERN) and multiphase chemistry, and 3) instrumentation to study carbon closure, and exploit synergies between optical spectroscopy and mass spectrometry."
and
Atmospheric chemistry of reduced nitrogen and organosilicon
Eleanor Browne, ANYL faculty, CU Boulder
"The Browne group uses a combination of laboratory experiments and field measurements to understand the sources and transformations of trace gases in the atmosphere with the ultimate goal of understanding the impacts of this chemistry on air quality, climate, and nutrient delivery to ecosystems. Here, I will discuss some of our recent work on the atmospheric chemistry of cyclic volatile methyl siloxanes and of reduced nitrogen compounds. Finally, I will discuss research opportunities in our group."
30 August 2021
Understanding aerosols for pollution, climate change, and disease transmission
Jose-Luis Jimenez, ANYL Faculty, CU Boulder
"Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly present results from different projects over the last year, as well as some future directions for our group. I will introduce our aircraft research program, including results from the recent NASA/NOAA FIREX-AQ wildfire smoke study, where we obtained near-molecular level aerosol speciation data at 1 second time resolution. We are investigating both the inorganic and organic composition of the smoke, including important properties such as pH and volatility. I will summarize modeling work with GECKO-A exploring the evolution of OH reactivity with OH exposure for different hydrocarbons. I will discuss our indoor air research, including the apportionment of gases in the CU Art Museum combining 3 different CIMS instruments. I will also briefly describe recent developments on the importance of aerosols for disease transmission, not just for COVID-19 but for all (or most) respiratory diseases.
For those interested in COVID-19 aerosol transmission, you can find a summary of the evidence supporting airborne transmission of COVID-19 at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00869-2/fulltext, a review for airborne transmission of all respiratory diseases at https://doi.org/10.1126/science.abd9149 (starting Thu 26-Aug), and an overview of the historical reasons for the confusion on this topic at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3904176."
Spring 2021
24 May 2021
Measurements related to the fate of organic compounds in outdoor and indoor environments
ANYL PhD Defense, Benjamin Deming, Ziemann group
"A massive quantity of organic compounds is emitted into the Earth’s atmosphere every day. Depending on their structure and the ambient conditions, these species may persist anywhere between minutes to years in the atmosphere. This presentation discusses three studies broadly related to the fate of organic species in both outdoor and indoor environments. In the first project, the interactions between volatile organic compounds and various types of tubing were quantitatively investigated. It was found that, depending on the material of the tubing in question, organic species may either adsorb onto the tubing surface or absorb into the tubing itself. Besides leading to an improved understanding of tubing used in sampling lines for field campaigns or laboratory experiments, this work also provides unique insight into the interactions of volatile organic compounds and solid surfaces. Oxidation of the aforementioned organic species in the atmosphere often leads to the production of secondary organic aerosol. Depending on the composition of the aerosol and the ambient conditions, particles may undergo liquid- liquid phase separation, forming a largely aqueous layer and a predominantly organic phase. The purpose of the second study was to investigate the distribution of atmospherically common inorganic acids between these phases. In particular, the presence of significant quantities of acid in the organic layer has important implications for the mechanisms and rates of acid-catalyzed particle-phase reactions. We found that, depending broadly on factors such as the O:C ratio and water content of the organic phases, between 0.1% and 10% of the acid in the system may be found in the organic layer. These results have important implications for particle-phase chemistry, which may further process and functionalize organic carbon in the atmosphere. The final study investigates the presence of alkenes on indoor surfaces and the effects these species have on indoor air chemistry. Upon developing a microscale derivatization-spectrophotometric method for measuring non-conjugated double-bonds, we applied it to a number of small, but informative, studies investigating open questions about indoor air and surfaces. The results show that the reaction of gas-phase ozone with surface-bound alkenes is prevalent and drives significant chemistry indoors."
Monday, 26 April 2021
An extraterrestrial start and a volcanic end to the Cretaceous-Paleogene mass extinction?
Prof. Boswell Wing,
CU Boulder, Geological Sciences
"The Cretaceous–Paleogene (KPg) mass extinction is one of the “big five” mass extinctions that occurred during the last 550 million years. Although geological records of the KPg transition preserve ample evidence of an impact, there is significant controversy about whether this impact was the sole mass extinction trigger, or whether volcanism from the Deccan traps contributed significantly to the sudden onset of the biotic crisis. In this presentation I will discuss how precise measurements of the ratio of 34S-32S in KPg sediments at high-spatial resolution can trace atmospheric sulfur injections from these two events in the immediate vicinity of the KPg extinction horizon."
Monday, 19 April 2021
Submicron Particle Composition and Acidity in Fire Plumes during FIREX-AQ aircraft study (1/2 seminar)
Hongyu Guo,
Postdoctoral Fellow, Jimenez Group
"During the Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ) aircraft study, the chemical composition of fire-emitted submicron particles was quantified with a High- Resolution Aerosol Mass Spectrometer (AMS). The western wildfire-emitted particles show similar composition across the plumes and are overwhelmingly dominated by organic aerosol (OA). The eastern agricultural fires show larger variability in particle composition with a higher inorganic fraction, in particular Cl and K. Fast (1 or 5 Hz) measurements of K in fire plumes, which show excellent correlation with SAGA filter measurements, allow a quantitative closure of the particle anion/cation balance. Although lab experiments suggest variable AMS relative ionization efficiency (RIE) of K for mixed K inorganic salts, field observations indicate a uniform RIE for fresh fire-emitted particles dominated by OA. SO4 in some fresh biomass burning plumes (both wild and agricultural fires) had major contributions from organosulfur species (as quantified by two separate methods), vs. typically a few percent in regional background air. This is consistent with limited previous observations (DC3, FLAME-3). The AMS inorganic SO4 agrees better with SAGA-MC SO4, as expected from the ion chromatography detection of the latter instrument. The organosulfur appears to be dominantly a primary emission and was removed on a similar timescale as fresh primary biomass burning OA (BBOA). Ultrahigh-resolution analysis of FIREX-AQ filter samples is used to aid in the identification of the organosulfur species. Lastly, we use thermodynamic models to estimate aerosol pH, an important lever on many particulate physical and chemical processes, based on AMS-quantified K, inorganic-only SO4, pNO3 and collocated gas measurements (NH3 and HNO3). The fresh western biomass burning particles had near-neutral pH (on average ~6), which was buffered by high levels of NH3 and contrasts with far lower pH observed for continental (~1-4) and remote oceanic (~0) submicron particles. Regional background particles during FIREX-AQ show moderate acidity (pH~3), which indicates drastically different rates for H+ -influenced processes inside and outside of the plumes."
and
Emissions, Partitioning, and Aging of Biomass Burning Organic Aerosol at FIREX-AQ (1/2 seminar)
Demetrios Pagonis,
Postdoctoral Fellow, Jimenez Group
"The chemical composition of biomass burning organic aerosol (OA) evolves rapidly as it is transported downwind of a fire. Changes in composition are driven by dilution, evaporation, and secondary OA (SOA) production. Here we present airborne OA measurements from an extractive electrospray mass spectrometer (EESI) and an Aerodyne high-resolution Aerosol Mass Spectrometer (AMS) during FIREX-AQ. By combining the bulk OA composition measured by AMS with speciated OA composition measured by EESI, we quantify the relative contributions of SOA production and primary OA (POA) evaporation to the evolution of OA composition downwind of wildland fires. Evaporation rates of bulk OA and levoglucosan (a component of POA) are quantified through a combination of thermodenuder measurements and sampling smoke plumes at varying ambient temperatures. Rates of SOA production are quantified through measurements of individual SOA components by EESI, and through positive matrix factorization of AMS spectra. We observe SOA production rates exceeding 100 μg sm -3 h -1 (standard conditions 273 K, 1013 mbar) and simultaneously measure evaporation of POA components, with minimal change in dilution-corrected OA concentration; this provides direct evidence for balance in SOA production and POA evaporation rates in near-field aging of biomass burning OA."
Monday, 12 April 2021
OS and pH estimation from AMS measurements and OH oxidation of phenolic compounds in wildfire smoke
Melinda Schueneman,
ANYL 3rd year student, Jimenez Group
"This talk will focus on two unrelated research projects: the first will highlight a paper recently published in AMT, and the second will introduce and show preliminary results for a biomass burning project. Shortened abstracts are included here for reference.
Part I: Sulfate can be present in aerosols as inorganic (mainly ammonium sulfate, AS) or organosulfate (OS). Although OS is thought to be a smaller fraction of total sulfate in most cases, recent literature argues that this may not be the case in more polluted environments. Two new methods have been proposed to quantify OS separately from AS with AMS data. We use observations collected during several airborne field campaigns covering a wide range of sources and air mass ages and targeted laboratory experiments to investigate the proposed OS methods. Four chemical regimes are defined. In polluted areas with high ammonium nitrate concentrations and in remote areas with high aerosol acidity, the decomposition and fragmentation of sulfate in the AMS is influenced by multiple complex effects, and estimation of OS does not seem possible with current methods. In regions with lower acidity (pH > 0) and ammonium nitrate (fraction of total mass < 0.3), the proposed OS methods might be more reliable, although application of these methods often produced nonsensical results. Under highly acidic conditions (when calculated pH < 0 and ammonium balance < 0.65), sulfate fragment ratios show a clear relationship with acidity. The measured ammonium balance is a promising indicator of rapid estimation of aerosol pH < 0, including when gas-phase NH3 and HNO3 are not available. These results allow an improved understanding of important intensive properties of ambient aerosols.
Part II: The intensity and frequency of fires has been sharply increasing with an expanding population, increased land clearing for agriculture, and climate change. Fire plumes introduce large amounts of diverse chemical species into the atmosphere. The abundant emissions of VOCs, particles, and NOx suggest that substantial aerosol formation should occur downwind of fires. However, typically no enhancement of total OA is observed in most field studies. To explore the relationship between POA and SOA in aging smoke, we use measurements from the Extractive Electrospray Soft Ionization Time-of-Flight Mass Spectrometer (EESI) taken during FIREX-AQ, along with EESI and Vocus measurements from chamber experiments. A suite of laboratory chamber experiments, targeting known and suspected BB SOA precursors, are being conducted to identify key tracer species in this system, for both the particle and gas phases. Key chemical species from the OH initiated oxidation of phenol, catechol, and styrene were identified from chamber studies and their formation and evolution were modeled with KinSim. Some identified products were calibrated for and identified in a FIREX-AQ research flight, and one plume was also modelled in KinSim. We present preliminary results from the analysis of these observations."
Monday, 5 April 2021
Aerosol optical closure and long-term MAX-DOAS observations
Christopher Lee,
ANYL 3rd year student, Volkamer group
"Aerosols remain a major source of uncertainty in the global radiative budget. Studies that combine vertically-resolved measurements of aerosol size distributions and refractive index (inferred from aerosol composition measurements) are needed to assess our understanding of multispectral aerosol optical closure. Here we use data from the NASA airborne HSRL-2 instrument, which retrieves aerosol extinction profiles at 355, 532, and 1064 nm from backscatter measurements. The dataset we use is from the Two-Column Aerosol Project (TCAP) in July 2012, which deployed two research aircraft above the DoE ARM mobile facility at Cape Cod, MA. This dataset is reanalyzed here to investigate the effects of aerosol water on dry aerosol size and composition, and our ability to constrain Mie calculations to obtain multispectral optical closure.
High-altitude ground-based remote sensing is well-suited for long-term profile measurements of trace gases and aerosols, and provides unique sensitivity to the free troposphere. An example of this is the University of Colorado Multi-AXis Differential Optical Absorption Spectroscopy (CU MAX-DOAS) instrument, which measures vertical profiles of trace gases (BrO, IO, HCHO, CHOCHO, NO2, O3, SO2, H2O, etc.) and aerosols. Since February 2017, two CU MAX-DOAS instruments have operated continuously at Mauna Loa Observatory, Hawaii (155.578 W, 19.539 N, 3397 msl) and Maido Observatory, Reunion Island (55.384 E, 21.080 S, 2160 msl), respectively. In March 2021, a third CU MAX-DOAS instrument was deployed at Storm Peak Laboratory (106.744 W, 40.455 N, 3209 msl) in Steamboat Springs, Colorado. Leveraging the data from these three measurement sites, the vertically-resolved spatiotemporal variation of trace gases and aerosols can be characterized in both hemispheres."
Monday, 29 March 2021
Measurements of Volatile Organic Compounds during the COVID-19 Lockdown in Changzhou, China
Andrew Jensen,
ANYL 3rd year student, de Gouw group
"The COVID-19 outbreak in January 2020 prompted strict lockdowns, reduced human activity, and reduced emissions of associated pollutants. These reduced emissions have been estimated via remote and in-situ methods, but detailed measurements of volatile organic compounds (VOCs) are lacking. We measured VOCs in Changzhou, a Chinese city on the Yangtze River, during the local COVID-19 lockdowns from 8 January through 27 March, including periods of pre-lockdown, strict measures (level 1), and more relaxed measures accompanied by the return to work (level 2). VOCs were measured using a new, compact model of the Vocus proton-transfer-reaction time-of-flight mass spectrometer (Vocus Elf PTR-TOF-MS). We used positive matrix factorization to attribute VOCs to sources and employ an ensemble approach to determine uncertainties in the measured emission reductions. These uncertainties were further informed by satellite remote sensing and in-situ monitoring measurements of criteria pollutants, where we had measurements from previous years. Four factors of interest were resolved: textile industrial emissions (62±10%; average reduction during level 1 relative to pre-lockdown), pharmaceutical industrial emissions (40±20%), fresh traffic emissions (69±10%), and aged traffic emissions (73±14%). The quantified changes in the factors due to the lockdowns serve to constrain emission inventories and inform models, particularly for sectors where activity data are sparse, as the effects of lockdowns on air quality are explored."
Monday, 8 March 2021
Aqueous Aerosols, Aerosol Chemistry, and the Chemical Disciplines
Prof. Murray Johnston,
University of Delaware
"Airborne particles strongly influence climate and human health. Understanding these impacts requires knowledge of chemical processes associated particle formation and growth, and draws upon fundamental principles in the chemical disciplines (analytical, physical, etc.). While these disciplines provide insight into aerosol chemistry, the reverse is also true: studying aerosols as opposed to bulk phases provides insight into disciplinary topics. This presentation will focus on the unique environment of aqueous aerosol particles, which can show very different reactivity from a bulk aqueous solution as well as from their dry particle counterparts. These differences have important implications for how particles grow in the atmosphere, and they help us better understand chemical measurements that rely on the formation of aerosol droplets."
Monday, 1 March 2021
Managing Chemical Complexity in Highly Parameterized Air Quality Models
Kelley Barsanti,
University of California, Riverside
"Tropospheric chemistry and air quality are strongly influenced by source emissions. In the western US, much attention has been on the air quality impacts of wildland fires; and more recently, changes in air quality associated with COVID-19 shelter-in-place restrictions. Fires emit high levels of trace gases, including semi-volatile and volatile organic compounds (S/VOCs); and primary (directly emitted) particulate matter (PM). During plume evolution, S/VOCs react to form ozone (O3) and secondary PM, thereby degrading air quality downwind. The amount of pollutants formed depends on fuel and fire characteristics, and plume dynamics and chemistry. Unfortunately, model predictions of O3 and PM from wildland fires are characterized by significant uncertainties. While there are a number of factors that lead to poor model predictions, research in our group has focused on three particular limitations: 1) incomplete identification and quantification of gaseous compounds emitted from fires that may serve as pollutant precursors; 2) incomplete understanding of the transformations of the precursors that lead to pollutant formation in smoke plumes; and 3) over-simplified representation of emissions and processes in current smoke and air quality models. Somewhat analogously, urban anthropogenic sources also emit large quantities of S/VOCs that react to form O3 and secondary PM. Changes in anthropogenic activity as result of COVID-19 restrictions have provided an opportunity to test recent hypotheses about the changing mix of VOCs emitted by urban anthropogenic sources and the relative importance of emerging anthropogenic sources for secondary pollutant formation; as well as to test our abilities to represent the chemistry of these sources in predictive models. In this talk, I will present an overview of our efforts in these areas: applying advanced analytical techniques to characterize the S/VOCs in smoke as a function of fuel species and component, and the S/VOCs over the LA Basin during COVID-19 shelter-in-place restrictions; using chemically-detailed box models to develop air quality model parameterizations; and developing a comprehensive emissions inventory that is broadly useful in air quality model applications."
Monday, 22 February 2021
An Investigation of the Gas-Phase Products of NO3 Radical Oxidation of Δ-3-Carene
Olivia Jenks,
ANYL 3rd year, de Gouw Group
"Organic aerosol can have an impact on the climate, visibility, and human health. Oxidation of biogenic volatile organic compounds forms secondary organic aerosol (SOA) by lowering the volatility of the molecule and partitioning to the particle phase. The NO3-initiated oxidation of Δ-3-carene is being studied because it connects the interaction of biogenic and anthropogenic emissions to form aerosol, and expands on previous work with ɑ- and β-pinene. In this work, I explore the first generation gas-phase products of the NO3-initiated oxidation of Δ-3-carene in a 8 m3 Teflon FEP chamber using a Vocus proton-transfer-reaction time-of-flight mass spectrometer (Vocus PTR-ToF) and a high-resolution time-of-flight chemical-ionization mass spectrometer (HR ToF-CIMS) using iodide adducts. The mass spectra of reaction products show the presence of many of the expected products, including hydroxynitrates, dicarbonyls, hydroxycarbonyl nitrates, hydroxy dicarbonyls, and dicarbonyl hydroxynitrates, but also show significant fragmentation of the parent ions through loss of water and/or nitric acid. Parent and fragment ions are grouped together taking advantage of gas-wall interactions in Teflon tubing, which provide some simple “poor-person’s chromatography,” while transmitting all the species. Understanding the mechanism of the oxidation of Δ-3-carene by NO3 radicals allows for a better understanding of the fate of organic nitrate in the atmosphere, which will improve interpretation of field data and global chemical models."
Monday, 15 February 2021
Towards improved understanding of nitrogen dioxide emissions from forest fires
Debora Griffin,
Environment Canada,
"Smoke from wildfires are a significant source of air pollution, which can adversely impact ecosystems and the air quality in downwind populated areas. With increasing severity of wildfires over the years, these are a significant threat to air quality in densely populated areas. Emissions from wildfires are most commonly estimated by a bottom-up approach, using proxies such fuel type, burn area, and emission factors. Emissions are also commonly derived with a top-down approach, using satellite observed Fire Radiative Power. Furthermore, wildfire emissions can also be estimated directly from satellite-borne measurements.
I will present recent advancements and improvements of direct emission estimates of forest fire NOx emissions by using TROPOMI (Tropospheric Monitoring Instrument) high-resolution satellite datasets, including NO2 vertical column densities (VCDs) and information on plume height and aerosol scattering. The effect of smoke aerosols on the sensitivity of TROPOMI to NO2 (via air mass factors) is estimated with recalculated VCDs, and validated with aircraft observations. Different top-down emission estimation methods are tested on synthetic data to determine the accuracy, and the sensitivity to parameters, such as wind fields, satellite sampling, instrument noise, NO2:NOx conversion ratio, species atmosphere lifetime and plume spread. Lastly, the top-down, bottom-up and direct emission estimates of fire emissions are quantitatively compared."
Monday, 1 February 2021
Understanding the Earth from a thermodynamic systems perspective
Axel Kleidon,
Max Planck Institute
"The Earth is a vastly complex system that converts the energy contained in sunlight into various forms, from the kinetic energy of motion to chemical energy of life and the electric energy that powers human societies. These conversions follow the laws of thermodynamics, which set the directions and fundamental limits, yet these also result in interactions and feedbacks that emphasise the need for an Earth system perspective. In this talk, I provide the background for this thermodynamic description of the Earth system and use examples to show how thermodynamic limits in combination with a formulation of the dominant interactions can be used to describe the emergent behaviour. I show how this approach can be used to provide simple, yet physically-based estimates of climate and climate change, as well as how it sets limits on different forms of renewable energy. I close with an outlook on potential future applications, highlighting the generality of the approach as energy, its conversions into other forms, and interactions are at the very core of literally every Earth system process."
Monday, 25 January 2021
Peroxy radicals are getting curiouser and curiouser
Prof Neil Donahue,
Carnegie Mellon University
"Organic peroxy radicals sit at the center of tropospheric chemistry. Essentially all oxidation processes produce peroxy radicals immediately after an oxidant attacks a stable organic molecule. They have gas-phase lifetimes ranging from 1-1000 seconds. They also have choices; peroxy radical branching defines tropospheric chemistry. In the textbook case, they can react either with hydroperoxy radicals or with nitric oxide, either terminating as hydroperoxides or propagating to dark places that only Paul Ziemann understands while producing nitrogen dioxide and ultimately ozone. However, we have recently found that organoperoxy radicals with additional oxygenated functional groups can undergo progressive “autoxidation” (oxidation in the presence of molecular oxygen only) via internal hydrogen atom transfer and subsequent oxygen addition. These oxygenated organoperoxy radicals also appear to react with each other very rapidly (though this is uncertain) and to produce covalently bound organoperoxides (though this should be spin forbidden). Some of these products can have exceptionally low vapor pressures, and when formed in the gas phase can thus drive new-particle formation. It is possible that this is a major new-particle formation process in the pristine atmosphere, and that it was the dominant particle formation process in the pre-industrial atmosphere. In this talk I will explore recent experimental findings from the CLOUD experiment at CERN as well as modeling frameworks we have been developing to represent this chemistry and to translate chemical behavior observed in chambers to real-world conditions."
Fall 2020
Monday, 30 November 2020
Prototype Readout System Software for The STAR Interlock Safety System at Brookhaven National Laboratory
Joseph D'alesio, ANYL First Year
"RHIC, located at BNL, collides nuclei at relativistic speeds to artificially recreate the initial conditions of the universe. The STAR Collaboration studies these collisions using a detector, the Solenoidal Tracker At RHIC. The Interlock Safety System is responsible for monitoring and displaying parameters in the STAR control room. These parameters include the temperature and pressure of the TPC gas mixture, the Oxygen Deficiency Hazard status, the Uninterruptable Power Supply status and the water cooling system status. If these parameters fall outside an accepted range, alarms will sound to notify the control room. The readout system and software allow for the shift operator to adjust detector variables while the experiment is running and thus prevent circumstances in which fires and explosions are likely. This project focuses on upgrades to the Interlock monitoring system. The current Interlock Readout monitor uses a VME to communicate with various STAR systems while the upgraded monitor uses a Programmable Logic Controller interfaced to a PC. Device support for the upgraded monitor has been written and compiled using a prototype input/output controller program to communicate to the new readout PLC. Functionally, the existing and upgraded system will have the same capabilities. However, the new readout system will be easier to maintain and more easily updated to include, for example, additional safety signal outputs. In turn, this will result in a critical readout system prepared for future operations."
Monday, 23 November 2020
First satellite mapping of nitrous acid (HONO) in wildfire plumes
Dr. Nicolas Theys,
Royal Belgian Institute for Space Aeronomy (BIRA-IASB)
"Nitrous acid (HONO) is a short-lived reactive gas and plays a key role in the atmosphere through its influence on the OH budget, and contributes to secondary aerosols and ozone formation. Laboratory experiments and aircraft field campaigns have revealed that biomass burning is a source of HONO. However, the global importance of pyrogenic HONO is poorly constrained. Here we present the first global measurements of HONO using the TROPOspheric Monitoring Instrument (TROPOMI) onboard Sentinel-5 Precursor. Using Differential Optical Absorption Spectroscopy (DOAS) applied in the UV, we analyzed one year of TROPOMI data and found that HONO is unambiguously detected in wildfire plumes from main biomass burning regions, consistent with volume mixing ratios of several ppbvs of HONO.
During July-September 2018, the University of Colorado participated to a field campaign (Biomass Burning Fluxes of Trace Gases and Aerosols (BB-Flux)), performed research flights near fire plumes in the US and successfully detected HONO for several of them using the CU-DOAS aircraft instrument. For some cases, flights were planned around the TROPOMI overpass. We exploit this unique opportunity to validate our satellite HONO retrievals. We discuss the comparison results and reasons for discrepancies. In particular, viewing observations are very different and can lead to air mass sampling differences in the presence of elevated aerosols. To overcome this problem, we compared the ratio HONO/NO2 (RHN), which is also a proxy for HONO production. We found that satellite and aircraft RHNs are in excellent agreement, within their mutual error estimates. From the global RHNs, we demonstrate that previous assessments underestimate pyrogenic HONO emissions by a factor of 2–4 across all ecosystem types.
Finally, we performed model simulations to assess the impact of HONO on atmospheric oxidants, such as OH and tropospheric ozone. We estimate that HONO emissions are responsible for two-thirds of the OH production in fresh wildfire plumes worldwide and act to accelerate oxidative plume chemistry and ozone production. Our findings suggest that pyrogenic HONO emissions have a substantial impact on atmospheric composition, which enhances regional ozone levels by up to 7 ppbv."
N. Theys, R. Volkamer, J.-F. Müller, K. Zarzana, N. Kille, L. Clarisse, I. De Smedt, C. Lerot, H. Finkenzeller, F. Hendrick, T. Koenig, C.F. Lee, C. Knote, H. Yu, M. Van Roozendae.
Monday, 16 November 2020
Role of the Metal Support Interface in H2 Activation on Supported Gold Nanoparticles
Alexander Bradley, ANYL 1st year student
"Global Hydrogen production exceeds 50 million tons per year, largely for the production of ammonia. Given its importance, a fundamental understanding of hydrogen activation is vital when designing new catalysts. Hydrogen activation on gold catalysts is poorly understood and understudied, and the weak adsorption effects allow only a few types of experimental measurements. We have developed a kinetic model for analyzing hydrogen binding parameters on gold, and a method for extracting a proposed mechanism.
Hydrogen is thought to activate homolytically on late transition metals, but recent evidence suggests that it activates heterolytically at the metal-support interface of supported gold catalysts. The thermodynamic properties of this reaction were calculated by Van’t Hoff analysis. Enthalpy and entropy terms were found to be much lower than popular supported metals (Ni, Rh, etc). Kinetic Isotope Effect (KIE) experiments were carried out to determine the nature of the hydrogen activation, and whether the mechanism includes a proton coupled electron transfer (PCET) step."
and
Theoretical Examination of Isoprene’s Behavior at the Air-Water Interface
Kyle McMillan, ANYL 1st year student
"Isoprene is the most widely emitted biogenic hydrocarbon in the atmosphere (approximately 500 Tg
emitted annually), making it a significant player in atmospheric chemistry. So far, most research
concerning isoprene’s role in the atmosphere has examined its gas-phase chemistry with little
consideration given to its potentially significant chemistry within clouds. The purpose of this study was to
elucidate isoprene’s behavior at the air-water interface of a water droplet. To do this, high level molecular
dynamics (MD) calculations were utilized to simulate isoprene’s interaction with a droplet of 10-nm
diameter. The data generated by these calculations were then used to describe both isoprene’s
conformational dynamics and chemical group orientations at the air-water interface, looking closely at the
torsion angles of both its s-trans and s-gauche conformers as well as the distances between each of its
atoms and the water molecules comprising the water droplet. Though the results of this study were not
wholly conclusive, due primarily to incomplete datasets generated by the MD calculations, there were
some indications of potentially favorable interactions of isoprene with the air-water interface, especially
for its s-gauche conformer. Should the air-water interface selectively alter the chemical properties of
either of isoprene’s two major conformers (for example, by augmenting the reactivity of either toward a
major atmospheric oxidant such as OH) this would have clear implications for isoprene’s chemistry in the
troposphere, which has been shown to vary significantly with conformational state."
Monday, 9 November 2020
Sources of Formaldehyde in Bountiful, Utah
Ryan Thalman,
Snow College
"Starting in 2013, the mean concentration of HCHO measured in Bountiful, UT exceeded the non-cancer risk threshold and the 1 in 1 million cancer risk threshold. In addition, the measured concentrations were more than double those found at surrounding locations in Utah. A Positive Matrix Factorization (PMF) analysis using PMF-EPA v5 was done using historical data (2004-2017) to better understand the sources of formaldehyde in the region. The historical data set is composed of samples that were collected every sixth day on a 24-hour basis. Beginning in February 2019 an eight-week air sampling campaign measuring formaldehyde on a two-hour averaged basis was initiated. Formaldehyde was measured using a Broadband Cavity Enhanced Absorption Spectrometer (BBCEAS). In addition, the concentrations of NO, NO2, and O3 were measured. Two-hour averaged measurements of benzene, toluene, ethylbenzene, and xylenes (BTEX) measurable by gas chromatography-flame ionization detection were also done. Corresponding back wind trajectory calculations for selected time periods were calculated to aid in the understanding of the effects of BTEX emission sources and formaldehyde formation.
I will also discuss career options related to teaching and research at Junior and Community Colleges."
Monday, 2 November 2020
Iron polypyridyl complexes for electrocatalytic proton reduction
Zachary Schiffman, ANYL 1st year student
"In recent decades the rate of human consumption has accelerated dangerously. This is especially true of energy, the usage of which is unsustainable for long-term continuation at the current rate. We should then turn to renewable energy using materials that can be used infinitely, as opposed to fossil fuels which are notably finite and are contributing to the continuously rising levels of greenhouse gas pollution.
Solar energy would be a source of clean energy. An idea for a method of harnessing and storing solar energy is inspired by Earth’s plant life. In the process of photosynthesis, plants take in sunlight and store the solar energy in the form of chemical bonds. Just as plants reduce carbon dioxide to produce sugars as fuel, we look to use the energy of the sun to reduce protons from water into hydrogen gas, which burns cleanly with no CO2 emission. Metal-based complexes have been developed which can act as catalysts for this hydrogen evolution reaction (HER). Many of these complexes are synthesized in lengthy, low-yielding processes. In terms of developing practical technology that can be used on a large scale, it is best to synthesize these complexes in a straightforward, easy synthesis using inexpensive, commercially available materials.
We produced a tridentate bismethylpyridyl amine bound to an iron (III) center. This complex is synthesized from commercially available materials in high yield, and was shown to be an active electrocatalyst for proton reduction. Cyclic voltammetric techniques were used to verify and benchmark the complex’s activity and efficiency as an electrocatalyst. It was also shown to be active as a pre-catalyst in a three-component system for photocatalytic hydrogen generation. To this end, we seek to simplify our catalyst precursors to produce a readily synthesized complex that can be obtained at low cost and in high yields. This may take us one step closer to developing a wide-spread, accessible system for artificial photosynthesis."
and
Synthesis of Phosphate Diesters for use as Ligands on Bismuth Catalysts
Bri Dobson, ANYL 1st year
"New catalysts can simplify synthetic schemes or open up new possibilities in synthetic chemistry. In particular, bismuth phosphates have shown promise as an expanding family of green catalysts. Some bismuth compounds have Lewis acid catalyst properties but need to be made more effective in order to be used industrially. Phosphate diesters with varying ester groups may be able to tune the activity of these catalysts to fit specific reactions. With the final goal of creating a tunable bismuth phosphate diester catalyst, this project attempted to find a simple, but effective method for synthesizing a variety of phosphate diesters, which could then be used as ligands on homogenous bismuth phosphate diester Lewis acid catalysts for a variety of different synthetic uses. Two schemes for phosphate diester synthesis were attempted. One of the two was successful in the synthesis of pentamethylene phosphate. Further testing with different reagents is needed to establish the applicability of this scheme to the synthesis of other phosphate diesters, but the preliminary results are promising."
Friday, 30 October 2020
Speciation and Transformation of Reduced Nitrogen in the Atmosphere: A Laboratory and Field Investigation
Aroob Abdelhamid, Browne Group
"Reduced nitrogen compounds negatively affect air quality, climate, and human health. These compounds have been shown to enhance new particle formation, promote brown carbon formation, and increase the toxicity of aerosol. Little is known about the impact reduced nitrogen has on either the gas phase or aerosol because measurements detailing their speciation and interactions with aerosol is lacking. Due to increases in food production, the concentrations of reduced nitrogen compounds are expected to increase in the future, and thus it is important to understand their speciation and transformations in the atmosphere. In the first and second studies, I present data from the Holistic Interaction of Shallow Clouds, Aerosols, and Land Ecosystem (HISCALE) field campaign conducted in August- September, 2016 in the agricultural region of Lamont, OK. The purpose of the field campaign was to study the true atmospheric speciation of ambient reduced nitrogen. For the first study, I measured positive ambient ions, which have been shown to promote new particle formation, and are largely composed of organic nitrogen (ON) compounds. Ions help inform our understanding of high proton affinity trace gases because neutral compounds that are below the detection limit of normal instruments are likely to get ionized and seen as ions. I observed water, small organic nitrogen compounds, alkylpyridines, and compounds in the m/z 250 – 400 range. The ions in the high mass region had not been identified prior to my work. Once I identified the ON in the high mass region, I determined that these high mass ions have a diurnal cycle and were correlated with neutral ammonia measurements. Importantly, these compounds were only present in the latter half of the field campaign despite ammonia being present throughout the entirety of the field campaign, indicating the complexity of these compounds’ sources.
I then discuss ambient neutral compounds seen in HISCALE. These compounds were measured with an ethanol Chemical Ionization Mass Spectrometry (CIMS), an instrument sensitive to reduced nitrogen compounds. Ammonia, methylamine, and trimethylamine showed morning signal spikes that I suggest are from evaporating dew. I measured amides which showed a nighttime spike that is unexplained with current emissions of amides and indicates an unknown local amide source. Finally, I observed imines, which can be partially explained through a combination of aerosol uptake and amine processing, but the diurnal cycle cannot be fully explained.
I finally present work that focuses on understanding the ON formed upon reactive uptake of ammonia into biogenic secondary organic aerosol done in a collaboration with the University of Eastern Finland. I show that increasing the ammonia concentration in aerosol promoted greater production of a variety of ON, especially ON with larger carbon numbers. Ammonium in the particle created low volatility ON, while increasing ammonia concentrations lead to the formation of mid-volatility ON. Finally, I observed that ON volatility is up to 3 orders of magnitude lower than the volatility of biogenic SOA compounds lacking nitrogen incorporation. My work helps constrain the impact of ammonia uptake on aerosol."
Projects:
- Abdelhamid, A.; Stark, H.; Worsnop, D. R.; Nowak, J. B.; Browne, E. C. Measurement of Organic Nitrogen Positive Ions as Part of the HISCALE Field Campaign. in prep
- Abdelhamid, A.; Buchholz, A.; Pullinen, I.; Schobesberger, S.; Virtanen, A.; Browne, E. Nitrogen Incorporation in Biogenic Aerosol and its Effect on Aerosol Volatility. in prep
- Abdelhamid, A.; Stark, H.; Nowak, J. B.; Kimmel, J. R.; Smith, J.; Jayne, J. T.; Worsnop, D. R.; Browne, E. C. Nitrogen Chemistry in a Rural Environment: Neutral Measurements during the HISCALE Field Campaign. in prep
Monday, 26 October 2020
Variability and diel dependence of O3 – NOx – VOC chemistry in western wildfire plumes: Results from the NOAA Twin Otter during FIREX-AQ
Michael Robinson, ANYL 1st year
"The variability in photochemical ozone production from western wildfire plumes is important to the accurate prediction of their influence on North American air quality. A set of photochemical measurements including ozone, nitrogen oxides, photolysis rates and a suite of volatile organic compounds (VOCs), were made from the NOAA Twin Otter research aircraft as a part of the Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ) experiment. This research aircraft sampled nine unique fire complexes in five western states. Typical research flight days included flights during the afternoon, evening and night. In general, observed ozone production in the sampled plumes is rapid, reaching maximum ozone within 30 minutes downwind. A 0-D box modeling tool was developed to further probe the chemistry driving ozone production in these plumes. This tool was used to calculate afternoon and evening ozone isopleths which allows for a comparison of each individual fire complex in a common framework. The analysis shows the sensitivity of ozone production to initial NOx and VOC emissions. However ozone isopleths are not capable of describing the temporal transition of NOx and VOC sensitive chemical regimes. A radical budget approach is used for probing the temporal evolution of the chemistry. Afternoon photochemical plumes display a rapid transition from VOC sensitive chemistry to NOx sensitive chemistry, mainly driven by radical production from photolysis of HCHO and HONO emitted directly from the fire. Evening photochemical plumes exhibit a slower transition from NOx sensitive chemistry to VOC sensitive chemistry, with a larger portion radical production from alkene ozonolysis."
Michael A. Robinson, Zachary C.J. Decker, Kelley C. Barasanti, Matthew M. Coggon, Frank M. Flocke, Carley D. Fredrickson, Georgios I. Gkatzelis, Christopher D. Holmes, Denise D. Montzka, Brett B. Palm, R. Bradley Pierce, Rebecca H. Schwantes, Geoffrey S. Tyndall, Joel A. Thornton, Paul Van Rooy, Andrew J. Weinheimer, and Steven S. Brown.
and
Model Inter-comparisons of Inorganic Nitrate Formation During KORUS-AQ Campaign
Seonsik Yun, ANYL 1st year
"Nitrogen oxides play an important role in global tropospheric chemistry. The lifetime of nitrogen oxides is controlled by organic and inorganic nitrate formation. GEOS-Chem, a global 3-D chemical transport model, has historically had an over-estimation problem of inorganic nitrates relative to surface observation networks. This study investigated this problem by using model inter-comparisons with six different chemical transport model simulations during the KORUS-AQ field campaign period. N2O5 hydrolysis reaction, a primary nocturnal inorganic nitrate formation, was investigated to compare differences in calculations of inorganic nitrate formation between the model simulations. Differences in heterogeneous loss of N2O5 between the models were analyzed with the uptake coefficient, aerosol surface area density, and N2O5 concentration. Results show that high N2O5 concentrations at nighttime in GEOS-Chem could be attributed to the high O3 concentrations at nighttime compared to the Ensemble model, which can contribute to the over-estimation of inorganic nitrates in GEOS-Chem."
Monday, 12 October 2020
Chemistry of Organic Compounds in the Atmosphere and Indoor Air
Paul Ziemann,
ANYL Faculty, CU Boulder
"Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative studies of indoor air chemistry at CU."
Monday, 21 September 2020
Organic nitrogen chemistry: Recent results and future projects
Eleanor Browne,
ANYL faculty, CU Boulder
"Organic nitrogen is a ubiquitous atmospheric component that affects biogeochemistry, air quality, and climate. Assessing the impact of organic nitrogen on these processes remains challenging because traditional measurement techniques have lacked the sensitivity and chemical resolution to characterize the speciation and chemistry of organic nitrogen. Here, I will discuss measurements made with protonated ethanol cluster chemical ionization time-of-flight mass spectrometry during the Holistic Interactions of Shallow Clouds, Aerosols, and Land-Ecosystems (HI-SCALE) campaign at the Southern Great Plains research station in Lamont, Oklahoma. As the site is located in an agricultural region, reduced nitrogen compounds are prevalent. I will present measurements of novel compounds including imines and urea and will discuss the sources and sinks of compounds at this site. Finally, I will discuss research opportunities in our group."
and
Small molecules in the Anthropocene: Wildfires, Oceans and Iodine
Rainer Volkamer,
ANYL faculty, CU Boulder
"The Volkamer group develops advanced optical instrumentation (in situ and remote sensing) to measure small molecules and aerosols that are relevant to public health and climate. We seek to better quantify and understand emissions of small molecules, total carbon, and aerosols from natural and managed ecosystem (e.g., wildfires, oil & natural gas, ocean surface, UTLS), and develop a molecular level understanding of the fundamental processes that affect their chemical transformations and sinks (e.g., new particle formation). Opportunities for graduate research exist in the areas of 1) field measurements using research aircraft (i.e., BB-FLUX, TI3GER projects), 2) laboratory experiments of nanoparticle formation and growth (incl. at CLOUD/CERN), and 3) instrumentation to study carbon closure, and retrieve aerosol optical properties."
Monday, 14 September 2020
Chemistry of Volatile Organic Compounds in the Atmosphere
Joost de Gouw, ANYL faculty, CU Boulder
"Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products.
In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments.
Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments. From the results we learn about the sources of VOCs from people, chemical products and building materials, the chemical transformations of these VOCs and other loss process such as surface uptake and ventilation to the atmosphere. Second, we are working on the emissions and chemistry of VOCs released from volatile chemical product (VCP) use to the atmosphere, which was recently discovered to be the dominant source of VOCs in urban air. In this research we make measurements of VOCs in urban air, separate the different emission sources and describe the chemical transformations of VOCs after emission. Finally, we are working on a chamber study to better understand the formation of secondary organic aerosol from biogenic VOCs."
and
Laboratory Studies of Contact Efflorescence in a Long Working Distance Optical Trap
Maggie Tolbert, ANYL faculty, CU Boulder
"Because homogeneous salt efflorescence typically requires low relative humidity (RH), atmospheric salt particles are often assumed to be aqueous throughout much of their atmospheric lifetime. Here we use a long working distance optical trap to examine heterogeneous efflorescence that occurs when a supersaturated salt droplet comes into contact with a solid particle. We compare our findings for pure salt droplets with those obtained for salt/organic droplets where the organic may be found either in a core shell structure or as an amorphous/glassy solid. We find that in many cases, single collisions with a range of nuclei promote efflorescence at relatively high RH, causing salt particles to be solid for more of their atmospheric lifetime. Similar experiments are proposed in the future to examine the role of contact in ice cloud nucleation."
Monday, 31 August 2020
Understanding aerosols for pollution, climate change, and disease transmission
Jose-Luis Jimenez,
ANYL Faculty
"Our group’s research focuses on understanding the sources, properties, transformations, and sinks of aerosols (and of the gases that interact with them), which have major effects on human health and climate. In this talk I will briefly present results from different projects over the last year, especially those most relevant to first year graduate student opportunities. I will introduce our aircraft research program, including results from the recent NASA/NOAA FIREX-AQ wildfire smoke study, where we obtained near-molecular level aerosol speciation data at 1 second time resolution. I will discuss our indoor air research, and hopefully convince you that you should never use black conductive tubing except for non-volatile aerosols. I will also describe the importance of aerosols for disease transmission, a topic in which I have been working with many of the world leaders since March 2020. I will summarize the reasons why I think COVID-19 transmission is driven by aerosols, with a smaller fraction of surface transmission, and with a minor contribution of ballistic “WHO” droplets from coughing and sneezing. I will present some ideas about how to protect ourselves better from COVID-19 in the coming months and from other respiratory diseases in the future.
For people interested in COVID-19 aerosol transmission, you can find some selected resources linked here: https://twitter.com/jljcolorado/status/1298246684539940866 and a draft summary of the evidence here: https://docs.google.com/presentation/d/11rY9tQtkFaV_M4N-hf5qp1_Xtuw8JYb4Qvv5e7BEmcc/edit# "
Summer 2020
Friday, 10 July 2020
Laboratory Studies of Secondary Organic Aerosol Formation and Heterogeneous/Multiphase Chemistry
ANYL PhD Defense, Julia Bakker-Arkema,
Ziemann Group
"The oxidation of volatile organic compounds (VOCs) in the atmosphere to form secondary organic aerosol (SOA) is known to influence climate by directly scattering and absorbing incoming radiation, and by participating in cloud formation and processing. Furthermore, SOA decreases visibility, negatively impacts human health, and provides surfaces for heterogeneous and multiphase chemistry to occur, reactions that can alter the composition and properties of the aerosol. However, mechanisms of SOA formation and the chemistry that can occur within and on the surface of the SOA remain poorly understood. This thesis presents three laboratory studies of VOC oxidation and SOA formation, and heterogeneous/multiphase chemistry, which can provide insights and improve our understanding of the complex chemistry of the atmosphere. The first study investigates the oxidation of linear 1-alkenes by OH radicals as a model system for the oxidation of alkenes in the atmosphere. Due to discrepancies in past measurements, the molar yields and branching ratios for the formation of β-hydroxynitrates, important alkene oxidation products in the atmosphere, were measured in a series of environmental chamber experiments. The measured β-hydroxynitrate branching ratios indicate that the yields of organic nitrates are lower for alkenes than for alkanes. This work also presents an extensive inventory of the inherent sources of uncertainty when performing quantitative measurements of reaction products in environmental chamber studies, which can be applied to other quantitative studies of VOC oxidation and SOA formation. The second study presents a full quantitation of the major gas- and particle-phase products for the same model alkene system, 1-alkenes + OH radicals in the presence of NOx, using a suite of sampling methods and analytical techniques. This work achieves mass closure for the products of the OH addition pathway, and when combined with past measurements, demonstrates that the important oxidation pathways are captured in the reaction mechanism. The measured product yields are used to calculate branching ratios for key reaction pathways, including for the isomerization and decomposition of β-hydroxyalkoxy radicals, important reaction intermediates. The final study determines kinetics and equilibria for the condensed phase reactions of hydroperoxides and aldehydes to form peroxyhemiacetals, a model system for the analogous heterogeneous/multiphase reactions in atmospheric SOA. The synthesis of a hydroperoxide probe molecule allows for the measurements of rate constants and equilibrium constants even in complex matrices, such as SOA formed from the ozonolysis of α-pinene. The results demonstrate that timescales of peroxyhemiacetal can be short in SOA, and thus may be important in the atmosphere."
Thursday, 4 June 2020
Development of Solar Occultation Flux Spectroscopy for Ground and Airborne Applications
ANYL PhD Defense, Natalie Kille,
Volkamer Group
Air quality is a major issue that directly affects human health, ecosystems, and climate. Area sources such as natural gas production, agriculture, and wildfires emit primary pollutants that undergo transport and chemical transformation in the atmosphere that leads to the formation of secondary pollutants. Signatures of air quality impacts are being observed on local, regional, and global scales. However, there is a need for better measurement techniques to quantify trace gas emissions from area sources. Better understanding of emissions directly relates to lowering the uncertainty in model predictions of the impacts of pollution on human health, ecosystem, and climate.
I present the development and scientific results of the University of Colorado Solar Occultation Flux instrument (CU SOF) from ground and airborne applications to address the need for better emission quantification of trace gases from area sources. Specifically, the CU SOF instrument consists of a digital mobile solar tracker coupled to a Fourier transform spectrometer that can simultaneously be coupled to a UV-Visible spectrometer. The direct-sun observations made by CU SOF provide high photon flux, are independent of boundary layer height, and measure the vertical column density of trace gases directly in the open atmosphere without any inlets. These characteristics allow the CU SOF instrument to measure trace gas emission fluxes from area sources using the mass balance method.
Scientific results from the CU SOF instrument are described from four field campaigns. During the FRAPPE campaign, ammonia fluxes from concentrated animal feeding operations in Colorado are quantified, and found to be a factor of 2-10 higher than expected. Furthermore, feedlot soils are found to be a significant source of NOx, which is a missing NOx source in national inventories, and relevant in semi-polluted and remote locations where ozone formation is NOx sensitive. Next, in collaboration with the Karlsruhe Institute of Technology, CU SOF and three COCCON type instruments were used to source apportion methane emissions in the Colorado Front Range. A tracer method is employed finding that natural gas sources dominate over biogenic sources. Finally, the CU SOF instrument was deployed from a research aircraft during the Pre-BB-FLUX and BB-FLUX campaigns. Emission fluxes from nine trace gases relevant to wildfires are measured. Carbon monoxide emissions spanning over four orders of magnitude are observed for a variety of wildfire sizes. CU SOF measurements are compared with pyrogenic carbon monoxide emission predictions from seven different satellite-based emission inventories, and are shown to reduce the uncertainty in emissions from a factor 35 to about a factor of 2. These improvements in understanding of emissions directly scale the better predictions of air quality impacts in the form of ozone and particulate matter.
Spring 2020
Monday, 27 April 2020
Fires, fuels, and fluxes: Using airborne remote sensing to understand fire emissions
Kyle Zarzana, postdoc,
Volkamer Group (1/2 seminar)
Biomass burning emits a complex mixture of gases and particles that can vary rapidly over short spatial and temporal scales. Secondary chemistry driven in part by gas-phase radicals leads to downwind formation of ozone and particles which affect climate and adversely impact public health. Quantifying the emissions of radical precursors such as NO2, HONO, and carbonyls such as HCHO is an important first step to constraining their role in smoke chemistry, but this can be difficult due to plume inhomogeneities. Column measurements along the direct solar beam integrate over the vertical variability, and when made from an airborne platform can be used to determine mass fluxes on the scale of a wildfire. In this talk I will be presenting measurements from the Biomass Burning Flux Measurements of Trace Gases and Aerosols (BB-FLUX) campaign that was conducted in the northwest United States during the summer of 2018. The University of Colorado Solar Occultation Flux (SOF) and the Zenith Sky Differential Optical Absorption Spectroscopy (ZS-DOAS) instruments were deployed on the University of Wyoming King Air research aircraft, and these instruments measured column densities of numerous gases, including but not limited to CO, NH3, C2H6, HCN, PAN, HONO, NO2, and HCHO. Using a combination of data from these two instruments I will present estimates of radical fluxes from wildfires. Additionally, I will briefly touch on several other aspects of our BB-FLUX work, such as determining carbon fluxes from fires.
and
Constraining wildfire emission inventories using airborne flux measurements
Johana Romero-Alvarez, postdoc,
Volkamer Group (1/2 seminar)
Wildfire emit significant amounts of trace gases and particulate matter that impact atmospheric processes and human health, and accurate emissions are required to quantify determine the impacts of fires. Numerous inventories have been developed to estimate emissions using either measurements of fire radiative energy or of the burned area. These inventories often vary by several orders of magnitude, even for inert species such as CO, and until recently opportunities to valid these models have been limited. The University of Colorado Solar Occultation Flux (CU SOF) instrument can determine highly time resolved fluxes for many species from wildfires, and during the summer of 2018 was deployed as part of the Biomass Burning Flux Measurements of Trace Gases and Aerosols (BB-FLUX) campaign in the northwest United States. Over 100 fluxes measurements of a variety of species including but not limited to CO, NH3, small alkanes, and HCN, were performed on 18 different fires spanning different fuel types, fuel loadings, and fire intensities. Additionally, highly detailed measurements of the fire areas were made post campaign by the National Ecological Observatory Network and the US Forest Service. This rich dataset provides a unique opportunity to evaluate and constrain emission inventories. Comparisons between the measured fluxes and the FRP-based emissions inventory used in HRRR-Smoke will be presented as well as experimentally derived conversion factors of FRP to pyrogenic CO emissions.
Monday, 20 April 2020
Atmospheric Aerosol Analysis by Online Extractive Electrospray Ionization Mass Spectrometry
Dr. Jay Slowik,
Paul Scherrer Institute
Mass spectrometry is a powerful tool for the analysis of aerosol composition. However, tradeoffs typically exist between the loss of chemical information due to thermal decomposition and/or ionization-induced fragmentation on the one hand, and lower time resolution and/or separated collection/analysis stages on the other. We address these issues through the development of an extractive electrospray ionization time-of-flight mass spectrometer (EESI-TOF), which provides online, highly time-resolved measurements of aerosol composition without significant decomposition or fragmentation. Further, the EESI-TOF provides a versatile sampling/ionization framework, as by simply changing the composition of the primary spray and mass spectrometer polarity, the instrument can be configured to optimize detection of different organic fractions or water-soluble metals, while the sampling inlet can be configured to allow separate detection of the gas and particle phase. Two applications of the EESI-TOF are presented. First, we demonstrate rapid intra-particle decomposition reactions in secondary organic aerosol generated from the dark ozonolysis of α-pinene, as well as further reaction on the exposure of the aerosol to visible light. Second, we explore the sources and processes governing SOA composition in complex urban environments.
Monday, 6 April 2020
Tweezing Out the Interfacial Properties that Control the Chemistry of Atmospheric Aerosol Particles
Ryan Sullivan, Associate Professor
Department of Chemistry, Department of Mechanical Engineering, Carnegie Mellon University
Atmospheric aerosol particles have important yet poorly understood effects on air quality, health, atmospheric chemistry, cloud microphysics, and climate change. All these impacts of aerosols are governed by their composition and chemical mixing state – how components are distributed amongst individual particles – and by their morphology or internal structure. Together these two properties determine what lies at each particle’s interface, and this in turn controls how the particle interacts with reactant gases, condensable vapors, radiation, water vapor, and clouds. We advanced the aerosol optical tweezers (AOT) technique to determine the morphology and chemical properties of phase-separated individual droplets that contain complex secondary organic aerosol (SOA). We can now perform AOT experiments on realistic mimics of mixed atmospheric particles to understand their interfacial properties and how these evolve. Our finding of the prevalence of a phase-separated core–shell morphology and the existence of a stable emulsified state of SOA have important new implications for the reactivity and impacts of atmospheric aerosol. Our recent high-accuracy measurements of droplet acidity have enabled novel explorations of the interplay between gas reactive uptake and the resulting changes in pH that can then drive phase separations and alter morphology, which in turn can impede further reactivity.
Biomass burning is a major global source of atmospheric pollutants and much research has focused on the carbonaceous emissions from wildfires. Biomass-burning aerosol is complex and highly heterogeneous, often containing appreciable amounts of inorganic solutes including chloride salts with unknown reactivity and implications for oxidant budgets and atmospheric chemistry. The natural production of a key nighttime reservoir of nitrogen oxides, N2O5(g), in biomass-burning smoke was identified through chamber experiments on authentic biomass-burning aerosol. Our discovery that N2O5(g) often reacts with the chloride salts in smoke aerosol from tall grasses to produce ClNO2(g) instead of HNO3(g) has important implications for the lifetime of nitrogen oxides and the impacts of biomass burning on atmospheric oxidants and photochemical smog production. The surprisingly low reaction probability of N2O5(g) was determined to be due to organic carbon coatings that protect the salt phases from the gaseous reactants but can be removed as the aerosol ages further. Salt deliquescence at high relative humidity can greatly increase the reaction probability as the hydrolysis of N2O5 is driven by chemistry in aqueous phases.
Tuesday, 10 March 2020
ANYL Dissertation Defense: Measurements of the Emissions and Reactions of Anthropogenic and Biomass Burning VOCs
Zachary Finewax,
CU Boulder, de Gouw Group / Ziemann Group
"Organic carbon is ubiquitous in the atmosphere, present in the gas phase (volatile organic compounds, VOCs) or as a suspension of liquid or solid particles in air (organic aerosol, OA). Oxidation of VOCs leads to the production of OA, impacting climate, health and visibility. Emissions of VOCs occur from a variety of sources, both outdoors and indoors, requiring measurement to determine the sources, and laboratory studies to investigate the ability of a given emission to produce OA.
First, laboratory studies investigated the oxidation of catechol, an important biomass burning emission, under both daytime and nighttime conditions. The organic aerosol yield, yield of its primary product, 4-nitrocatechol, and mechanism provide evidence that catechol is an important precursor to biomass burning SOA. Vapor pressure and absorption spectra of 4- nitrocatechol elucidate its fate in the atmosphere, and its role in potentially suppressing ozone formation in biomass burning plumes. Second, laboratory studies were developed to study the oxidation of resorcinol, an isomer of catechol with much lower volatility, under daytime and nighttime conditions. The differences in organic aerosol yields, and products highlights the difference in chemistry between the two isomers. Third, the development of a vaporizer for gas- wall partitioning and particle generation is described. The procedure was designed to measure gas-wall timescales and the extent of gas-wall partitioning using instrumentation commonly available in an atmospheric chemistry laboratory. Quantitative particle generation is achieved, allowing for the calibration of particle detection instruments. Lastly, this thesis presents results from a field study at a university athletic center. Emissions of VOCs from individuals exercising are quantified, sources of VOCs are characterized and chemistry of bleach with amino acids is observed from products in the gas-phase.
This thesis advances our understanding of the chemistry governing OA production in biomass burning plumes, improves the ability to study low volatility compounds in the laboratory, and provides new insight on the impacts of exercise on indoor air quality.
List of Publications
1. Finewax, Z.; Pagonis, D.; Claflin, M.; Handschy, A.; Brown, W.; Jenks, O.; Day, D. A.;
Lerner, B.; Jimenez, J. -L.; Ziemann, P. J.; de Gouw, J. A. Quantification and source
characterization of volatile organic compounds from exercising and application of
chlorine-based cleaning products in a university athletic center. In preparation for
submission to Indoor Air.
2. Claflin, M.; Pagonis, D.; Finewax, Z.; Handschy, A.; Day, D. A.; Brown, W.; Jayne, J.
T.; Worsnop, D. R.; de Gouw, J. A.; Lerner, B. Isomer-resolved measurements of indoor
air using an in situ gas chromatograph with automatic detector switching between Vocus
PTR-TOF-MS and EI-TOF-MS. In preparation for submission to Environmental Science
& Technology.
3. Finewax, Z.; Jimenez, J. -L.; Ziemann, P. J. Development and Application of a Low-
Cost Vaporizer for Rapid, Quantitative Addition of Organic Gases and Particles to an
Environmental Chamber. In preparation for submission to Aerosol Science and
Technology.
4. Finewax, Z.; de Gouw, J. A.; Ziemann, P. J. 2019, Products and secondary organic
aerosol yields from the OH and NO 3 radical-initiated oxidation of resorcinol. Earth and
Space Chemistry, 3: 1248 – 1259.
5. Finewax, Z.; de Gouw, J. A.; Ziemann, P. J. 2018, Identification, quantification, and
volatility of 4-nitrocatechol formed from reactions of catechol with OH and NO 3 radicals.
Environmental Science and Technology. 52: 1981 - 1989.
ANYL Dissertation Defense: Development and Application of Chemical Ionization Mass Spectrometry to for Measuring Terrestrial and Exoplanetary Organic Nitrogen
Jennifer Berry,
CU Boulder, Browne Lab
"Nitrogen is one of the key elements that is essential to life, but it is mostly trapped in unreactive molecular nitrogen. The form of nitrogen that is biologically, photochemically, and radiatively active is called reactive nitrogen, of which organic nitrogen accounts for around 30%. Organic nitrogen has remained relatively unstudied until the development of high resolution mass spectrometric techniques. In this dissertation, I discuss the application of chemical ionization mass spectrometry (CIMS) and atmospheric pressure interface time-of- flight mass spectrometry (APi-ToF) in new experimental conditions, from pharmaceuticals to exoplanetary haze formation, to study organic nitrogen. I also introduce new modifications of the CIMS that makes it more applicable to high importance organic nitrogen studies that require even higher time resolution. Through developing CIMS and APi-ToF for new applications, the role of organic nitrogen in terrestrial and exoplanetary environments may become more clear.
I first introduce the novel mass spectrometric imaging technique that combines laser ablation with aerosol mass spectrometry and CIMS (LA-AMS-CIMS). Both mass spectrometric methods provide the fast response, rapid data acquisition, low detection limits, and high- resolution peak separation desirable for imaging complex samples. Additionally, the two techniques provide complementary information with AMS providing near universal detection of all aerosol constituents and CIMS with a heated inlet providing molecular-level detail of both gases and aerosols.
Then I introduce the study of organic nitrogen in ambiently charged ions and neutral gases during photochemical haze production in planetary atmospheres. Ions are known to play an important role in haze formation chemistry; however, the role of ions in laboratory simulations of haze formation is poorly characterized. By using the APi-ToF, I find that the chemical composition of the cations during haze formation is complex. High molecular weight ions up to m/z 400 are observed, with organic nitrogen ions, including ions with multiple nitrogen atoms, accounting for the majority of the identified ion peaks. With the mechanism of nitrogen incorporation being still unclear, I use CIMS to make in-situ measurements of gas- phase products up to m/z 400 and find organic nitrogen species dominate the mass spectra with smaller contributions from unsaturated hydrocarbons. Through the use of structural group relationships, I investigate the partitioning of haze formation products between the gas- and aerosol-phase. I also leverage the sensitivity and fast time response of the CIMS measurements to investigate how the gas-phase chemistry evolved over the course of the experiment, identifying a general mechanism for nitrogen incorporation with far ultraviolet light.
Finally, I introduce the ongoing modification of CIMS with the crossflow ion molecular reactor (IMR). Through the development of the crossflow IMR, measurements of ON compounds with a faster time response may be possible. I discuss a few experiments optimizing the clustering distribution and total ion signal with the crossflow IMR. With further modifications and calibrations, the crossflow IMR may be exceptionally important to studying ON in the field."
Publication list:
Berry, J., Day, D., Elseberg, T., Palm, B., Hu, W., Abdelhamid, A., Schroder, J., Karst, W., Jimenez, J., Browne, E. Laser Ablation-Aerosol Mass Spectrometry-Chemical Ionization Mass Spectrometry for
Ambient Surface Imaging, Anal. Chem, 2018, doi: 10.1021/acs.analchem.7b05255.
Berry, J., Ugelow, M., Tolbert, M., Browne, E. Chemical Composition of Positive Ions During Laboratory Simulations of Titan’s Haze Formation, ACS Earth Space Chem., 2019, doi:10.1021/acsearthspacechem.8b00139q.
Berry, J., Ugelow, M., Tolbert, M., Browne, E. The Influence of Gas-phase Chemistry on Organic Haze
Formation, Astrophys. J. Lett., 885(1), L6 (7pp), 2019, doi: 10.1080/02786826.2019.1599321.
Ugelow, M., Berry, J., Tolbert, M., Browne, E. The Impact of Molecular Oxygen on Ion Chemistry in a
Hazy Archean Earth Atmosphere, [in press by Astrobiology].
Monday, 9 March 2020
Development of a “Lab-on-a-chip” platform
Victoria Arau,
CU Boulder ANYL 1st year student, Browne group
"Current detection methods for environmental contaminants (which include but are not limited to HPLC, GC-MS, LC-MS, and LC-MS/MS) are generally time-consuming, expensive, and lack portability, limiting field deployment. Herein we evaluate a microfluidic electrochemical cell with viability for an in situ field deployment. The microfluidic channel consists of an interdigitated microelectrode and granulated activated carbon electrodes for optimized sensitivity. Electrochemical response is monitored using cyclic voltammetry with a known redox standard, with consistency in this response between macroelectrode systems and this microelectrode system. The linearity of the peak-current versus square root of scan rate demonstrates chemical reversibility, suggesting operability of this device. Results here suggest feasibility for further development of this technology as a sensor with real-time in situ capabilities. "
and
Very Remote Field Studies of Frost Formation on Mars Using the Curiosity Rover
Raina Gough,
CU ANYL Research Scientist, Tolbert Group
"For the last 3 years, I have been a Participating Scientist on the Mars Science Laboratory (aka: Curiosity) mission. This rover has traversed the Martian surface since 2012, totaling 4 full Mars years of environmental and geological observations. In this talk, I will summarize some of the most interesting results from the mission and talk about my role in planning the rover's science operations. I will also discuss a series of frost-detection experiments performed on Mars that I helped to lead. Although there is little water vapor on Mars, it is expected that water exchanges seasonally and diurnally with the surface. The rover’s weather station had predicted that frost should indeed form on the soil in the early morning in the winter. However, no condensed phase water had been observed along the rover’s traverse, until recently. Last November, we successfully used the rover’s laser-induced breakdown spectroscopy (LIBS) instrument to detect enhanced hydrogen on the soil just before dawn. We believe that this indicates the presence of diurnally exchanged water, potentially frost. I will discuss these results, and also some of the unexpected hurdles to performing science on Mars."
Monday, 2 March 2020
Multiphase Atmospheric Chemistry: From the molecular to the regional and global scales
Prof. Faye McNeill,
Department of Chemical Engineering, Columbia University, NY
"Multiphase chemistry in the atmosphere is a major source of organic and inorganic atmospheric particulate matter, and also has a profound influence on gas-phase composition and precipitation chemistry. Despite considerable progress, mechanistic understanding of some key aqueous atmospheric processes is still lacking, and their representation is incomplete in most regional and global models. I will present an overview of aqueous chemical processes in the atmosphere, highlighting recent developments and critical uncertainties. I will also discuss my group’s efforts in characterizing these processes in the laboratory and improving their representation in atmospheric chemistry models."
Monday, 24 February 2020
Physics and chemistry of new particle formation in the atmosphere
Dr. Urs Baltensperger,
Laboratory of Atmospheric Chemistry, Paul Scherrer Institute, Villigen, Switzerland
"Globally, a significant source of cloud condensation nuclei for cloud formation is thought to originate from new particle formation (aerosol nucleation). Despite extensive research, many questions remain about the dominant nucleation mechanisms. Specifically, a quantitative understanding of the dependence of the nucleation rate on the concentration of the nucleating substances such as gaseous sulfuric acid, ammonia, water vapor and others has not been reached. This is of relevance for climate as the atmospheric concentrations of sulfuric acid, ammonia and other nucleating agents are strongly influenced by anthropogenic emissions. Ions, produced e.g. by galactic cosmic rays, are also known to influence nucleation rates, however their importance is still debated. By providing extremely well controlled and essentially contaminant free conditions in the CLOUD chamber, we were able to show that indeed sulfuric acid is often an important component for such new particle formation, however, for the typical temperatures encountered in the planetary boundary layer the concentrations of sulfuric acid are not high enough to explain the atmospheric observations [1]. Under these conditions, ammonia [1], amines [2] or oxidized organic molecules [3] are needed for nucleation with sulfuric acid to occur. The relevant organic molecules are highly oxygenated molecules (HOMs). HOMs produced by the oxidation of biogenic precursors are able to trigger new particle formation on their own, even in the absence of sulfuric acid [4,5]. We confirmed that this mechanism does occur in today’s lower free troposphere [6]. Moreover, the latest CLOUD results from new particle formation in urban environments, a topic of extensive current research, will be presented."
References: [1] Kirkby, J. et al., Nature, 476, 42-433, 2011 [2] Almeida, J. et al., Nature, 502, 359-363, 2013. [3] Riccobono F. et al., Science, 344, 717-721, 2014. [4] Kirkby, J. et al., Nature, 533, 521-526, 2016. [5] Tröstl, J. et al., Nature, 533, 527-531, 2016. [6] Bianchi, F. et al., Science, 352, 1109-1112, 2016.
Monday, 17 February 2020
The Role of H2S in Photochemical Methane Haze Experiments
Nathan Reed,
ANYL 3rd year student, Browne / Tolbert Groups), CU Boulder
"Although sulfur gases and organic haze from methane photochemistry are thought to be present in numerous planetary atmospheres, including the atmosphere of the Archean Earth (2.5-3.5 bya), direct interactions between their chemistries have historically been neglected. This assumption may limit the understanding of atmospheric sulfur and organic haze chemistry, and it would therefore be beneficial to examine photochemical haze production with sulfur gases present. Organic haze laboratory studies including H2S, an atmospheric sulfur gas with both biogenic and abiogenic (e.g. volcanic) sources, have until now been non-existent. To better understand the role of H2S in organic haze chemistry, I have conducted UV photochemistry experiments of CH4 gas mixtures in N2 with the inclusion of trace amounts of H2S. I investigated the product aerosol composition, number, and size distribution as a function of H2S mixing ratio. It was found that increasing the H2S mixing ratio increased the organic aerosol effective density, number, and mass loading. Further, organic sulfur compounds were found to form in the aerosol phase. I will discuss the importance of these findings in the context of organic haze and sulfur chemistry, possible mechanisms, and future steps for the project."
Monday, 10 February 2020
Revisiting dry deposition of trace gases and particles in the atmosphere
Delphine K. Farmer
Associate Professor, Department of Chemistry, Colorado State University
"Dry deposition is a key process that removes trace gases and particles from the atmosphere, and thus one factor that controls the atmospheric lifetime of pollutants and short-lived climate forcers. In fact, dry deposition is the single largest component of uncertainty in our understanding of aerosol effects on climate. Despite its importance, dry deposition of trace gases and particles is poorly constrained by observations due to the instrumental challenge in measuring surface-atmosphere exchange. Instruments must be adequately fast, sensitive and selective to measure low concentrations on the rapid (<1 s) timescale of turbulence. We have developed several measurement techniques that use the eddy covariance approach to flux measurements over terrestrial surfaces incorporating both spectroscopy and mass spectrometry. This talk will be divided into two parts – the first considering the mechanisms that control forest-atmosphere exchange of acidic organic molecules, and the second revisiting our understanding of size-resolved particle deposition in the atmosphere. We contrast these observations with previous measurements in the literature, and with commonly used resistance models, highlighting several model-measurement discrepancies. To further investigate the mechanisms of particle deposition, we use black carbon deposition as an inert tracer for particle wet and dry deposition. We show that wet deposition dominates in an agricultural environment in Oklahoma, and provide observational constraints on black carbon lifetime in this region. Our suite of observations inform a revised model parameterization, which we incorporate in a global chemical transport model to investigate how dry deposition can impact particle loading and the radiative balance of the atmosphere. Together, these new measurements highlight the importance of observational constraints in developing, validating, and revising models of fundamental chemical and physical processes in the atmosphere – and in reducing uncertainties in our understanding of climate."
Monday, 3 February 2020
Experimental Investigation of the Particle-Phase Products and Reaction Mechanism of Δ-3-Carene with NO3 Radicals
Marla DeVault,
ANYL 3rd year, Ziemann Group
"Oxidation products of monoterpenes have been shown to contribute to secondary organic aerosol (SOA) formation in the atmosphere. However, the nighttime oxidative processes, which are dominated by nitrate radical (NO3) addition to alkenes, are not well understood. In order to fill this gap, I am working to quantify the yields of particle-phase products of the nitrate radical-initiated oxidation of Δ-3-carene, a monoterpene that is primarily emitted by coniferous plants. Using functional groups analysis methods, liquid chromatography with UV-Vis detection, and electrospray and electron ionization mass spectrometry to characterize SOA formed in environmental chamber reactions, I have identified a series of acetal dimers. The mechanism derived from these measured particle-phase products will help to inform chemical transport models on the contribution of nighttime oxidation of monoterpenes to SOA. In addition, these acetal dimers are analogous to those that have been identified and quantified in the β-pinene + NO3 radical system, and appear to form from reactions of a variety of monoterpenes."
Fall 2019
Monday, 2 December 2019
Soot: So Ubiquitous, So Destructive, So Important, Yet So Elusive: Exploring the Mysteries of Soot Formation
Prof. Hope Michelsen,
Mech. Eng., CU Boulder
"There are substantial gaps in our understanding of the mechanisms controlling soot inception, particle growth, and chemical evolution during combustion. The first steps in soot formation involve the transition of gas-phase hydrocarbon precursors to physically or covalently bound complexes. These complexes are known as “incipient particles”, and the search for their formation and growth mechanisms is a subject of active research [1-3]. These incipient particles undergo further particle growth, generating liquid-like hydrocarbon particles, which eventually reach sizes in the range of 10-50 nm, known as “primary particles” [1-5]. As these particles grow, they also lose hydrogen, solidify, and agglomerate into loosely bound clusters. Under high-temperature conditions, they become graphitic, covalently bound aggregates with a dendritic structure. Soot aggregate sizes, primary-particle sizes, and volume fractions grow as particles age in the flame [4,5]. At high temperatures in the presence of oxygen, the aggregates fragment [6,7], and the primary-particle sizes and volume fractions decrease through oxidation [4,8]. There is a poor understanding of the mechanisms by which particles undergo these transitions and the parameters that influence them.
This talk will describe our current understanding of soot formation and the scientific evidence that supports this understanding. This talk will also cover the gaps in our understanding of soot chemistry, some reasons for these gaps, and what we may need to do in order to bridge these gaps and develop more insight into soot formation and evolution."
[1] H.A. Michelsen, Proc. Combust. Inst. 36, 717 (2017). [2] H. Wang, Proc. Combust. Inst. 33, 41 (2011). [3] K.O. Johansson, M.P. Head-Gordon, P. E. Schrader, K. R. Wilson, H. A. Michelsen, Science 361, 997 (2018). [4] R.A. Dobbins, C.M. Megaridis, Langmuir 3, 254 (1987). [5] R. Puri, T. F. Richardson, R.J. Santoro, R.A. Dobbins, Combust. Flame 92, 320 (1993). [6] K.G. Neoh, J.B. Howard, A.F. Sarofim, Proc. Combust. Inst. 20, 951 (1985). [7] C.A. Echavarria, I.C. Jaramillo, A.F. Sarofim, J.S. Lighty, Proc. Combust. Inst. 33, 659 (2011). [8] K.O. Johansson, F. El Gabaly, P.E. Schrader, M.F. Campbell, H.A. Michelsen, Aerosol Sci. Technol. 51 (12), 1333 (2017).
Monday, 18 November 2019
Rapid Wastewater Analysis Platform Based on Surface-enhanced Raman Scattering
Ya-Chu Chan,
ANYL 1st year, CU Boulder
"Wastewater discharged from electroplating factories contains cyanide and chromium (VI), which could pose a threat to the environment and humans if in high concentration. The standard methods used by the Environmental Protection Agency to detect both species require tedious sample pre-treatments and sophisticated instruments. Owing to these pitfalls, an alternate method that can achieve rapid and on-site detection is important to timely deal with illegal discharge. Surface-enhanced Raman Scattering (SERS) is a vibrational spectroscopic technique that provides “fingerprints” of different entities. It has the potential to apply to wastewater detection because of its high sensitivity, accuracy, and portability. However, several problems have been hindering it from becoming a quantitative analysis method. In this project, a protocol to achieve quantitative SERS is developed. Then, how the SERS signal reflects cyanide and chromium (VI) concentration is studied, and the effect of other ions in wastewater is discussed. These results build the foundation to apply SERS to real wastewater samples. To make it more relevant to atmospheric studies, this talk will end with some comments on recent applications of SERS to aerosol pH measurement."
and
An evaluation of the sources of tropospheric ozone in the UK
Johana Romero,
ANYL Postdoc, Volkamer Group
"Ground-level ozone (O3) is a pollutant of concern for policy-makers because of its detrimental effect on human health, agriculture and ecosystems (Fuhrer, 2009; WHO, 2016)(Fuhrer, 2009; WHO, 2016). Near the surface, O3 has an atmospheric lifetime typically of hours. However, in the free troposphere, its lifetime could range from days up to several weeks (Stevenson et al., 2006) allowing O3 to be transported from its point of production downwind over long distances easily crossing countries and even continents (Monks et al., 2015). This trans-boundary nature of O3 and its precursors complicates compliance of air quality standards within the territories (HTAP, 2007). To develop effective emissions policies, it is, therefore, necessary to understand the extent to which domestic emissions and trans-boundary inflow contribute to the O3 distributions across the UK.
An online O3 tagging method based on the procedures described in (Emmons et al., 2012) and (Butler et al., 2018) was implemented into the Weather Research and Forecasting model coupled with Chemistry (WRF-Chem) to explore the contributions of foreign anthropogenic NOx emissions to the surface O3 in 12 receptor regions in the UK in summertime 2015. Simulations represent well the observed O3 from 20 EMEP ground sites within the UK and Western Europe. Results confirm the importance of short-range transport of O3 from continental Europe to the UK as well as from no-controllable O3 sources, such as the hemispheric ozone, which account for 71% of the total modelled O3 from May to August. The contribution of O3 from European NOx emissions is principally due to the transport of O3 rather than NOx reservoir.
It is shown that emission controls would be required in different source regions for compliance of ozone standards such as maximum daily 8 hours average MDA8 O3 of 50 and 60 ppbv. As an instance, emissions controls in France are important for the south and southeast of the UK, while domestic emissions controls are more relevant for the Midlands and the north of the UK. By contrast, attainment of lower exposure thresholds, e.g., accumulated ozone over 40 ppb AOT40 metric would primarily require the regulation of the hemispheric ozone levels.
The O3 tagged simulation further show that O3 from Germany, the Benelux, France and ship emissions in the North Sea are responsible for the build-up of O3 in the southeast UK during a summertime 2015 pollution episode. Furthermore, process analysis diagnostics demonstrates that vertical mixing in the morning can bring O3 and precursors from the residual layer to the ground, which contributes to the build- up of ozone during pollution episodes."
References
Butler, T., Lupascu, A., Coates, J., & Zhu, S. (2018). TOAST 1.0: Tropospheric ozone attribution of sources with tagging for CESM 1.2.2. Geoscientific Model Development, 11(7), 2825–2840. Retrieved from https://doi.org/10.5194/gmd-11- 2825-2018
Emmons, L. K., Hess, P. G., Lamarque, J. F., & Pfister, G. G. (2012). Tagged ozone mechanism for MOZART-4, CAM-chem and other chemical transport models. Geoscientific Model Development, 5(6), 1531–1542. Retrieved from https://doi.org/10.5194/gmd-5-1531-2012
Fuhrer, J. (2009). Ozone risk for crops and pastures in present and future climates. Naturwissenschaften, 96(2), 173–194. Retrieved from https://doi.org/10.1007/s00114-008-0468-7 HTAP. (2007). Hemispheric Transport of Air Pollution 2007. Retrieved from 10.18356/2c908168-en
Monks, P. S., Archibald, a. T., Colette, a., Cooper, O., Coyle, M., Derwent, R., ... Williams, M. L. (2015). Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmospheric Chemistry and Physics, 15(15), 8889–8973. Retrieved from https://doi.org/10.5194/acp-15-8889-2015
Stevenson, D. S., Dentener, F. J., Schultz, M. G., Ellingsen, K., van Noije, T. P. C., Wild, O., ... Szopa, S. (2006). Multimodel ensemble simulations of present-day and near-future tropospheric ozone. Geophysical Research Letters, 111(8). Retrieved from https://doi.org/10.1029/2005JD006338
World Health Organization. (2016). Ambient Air Pollution: A global assessment of exposure and burden of disease. World Health Organization. Retrieved from 9789241511353
Monday, 11 November 2019
Ozone Production in Los Angeles: Investigating Spatial Differences in the Impacts of NOx Emission Control
Margarita Reza,
ANYL 1st year, CU Boulder
"Los Angeles has historically experienced the highest ozone in the U.S. Strategies to reduce high ozone have included reduction of emissions of both nitrogen oxides (NOx) and volatile organic compounds (VOC), the two main precursors to ozone. Observations of ozone on weekdays and weekends, as well as from many intensive atmospheric experiments in LA, have indicated that for decades ozone production chemistry in LA has remained sensitive to VOC rather than NOx. However, very recent literature has suggested that LA ozone chemistry may be transitioning to a chemical regime that is sensitive to NOx, which would mean that LA air quality is positioned to benefit from NOx emission control on diesel truck traffic that are currently in the implementation phase. This study investigates this possible chemical- regime transition by first looking at the temporal trends in NOx concentrations at nine monitoring stations throughout LA. Spatial variability is found in the observed rate of decrease of NOx, suggesting that ozone has been affected differently throughout the LA basin. To investigate this potential spatial variability in the impacts of NOx changes, and, potentially, the change in ozone chemistry sensitivity to NOx, data gathered from the Geostationary Trace Gas and Aerosol Sensor Optimization (GeoTASO) airborne instrument on board the NASA UC12-B King Air on June 26 th and 27 th , 2017 was used. An analytical model was used to simulate ozone production in LA to identify the sensitivity of ozone production to NOx. Understanding the dependence of ozone production on NOx is critical for developing effective control strategies in this area."
and
Nanopore Sensing for Single-Molecule Glycomics
Melissa Morris,
ANYL 1st year, CU Boulder
"Single-molecule sensing represents the ultimate in chemical sensitivity, but is tremendously challenging to achieve. Because imaging at the nanoscale is difficult and expensive, developing techniques to measure single molecules requires ingenuity, and often relies on the interpretation of electrical signals. So how do nanopores help us measure at the single-molecule level? A nanopore is simply a nano-sized hole in a membrane or material. In the early stages of nanopore science, biological nanopores were isolated from nature, and now the field has turned to solid state nanopores. The Dwyer research group uses an electric field to create solid state nanopores in silicon nitride (SiNx) membranes, using a technique adopted from Vincent Tabard-Cossa.[1] Once the SiNx membrane has a hole, we mount it in a holder, so that it sits between two wells of electrolyte. We then monitor the electrical current to detect single molecules as they pass through the nanopore. In this study, we explored how solid state nanopores can be used to detect and characterize sugar molecules. Sugars have complex branching structures, and significant molecule to molecule variability. However, sugars are easily absorbed by the body, and their potential to be used as a new drug delivery system depends on their characterization."
[1] Kwok, H., Briggs, K., Tabard-Cossa, V. Nanopore Fabrication by Controlled Dielectric Breakdown. PLOS ONE. 2014, 9, e92880.
Monday, 4 November 2019
Impact of relative humidity on reactive uptake of glyoxal (CHOCHO) onto aerosol over the Korean peninsula during the KORUS-AQ 2016
Dongwook Kim,
ANYL 1st year, CU Boulder
"Glyoxal (CHOCHO) plays an important role in secondary organic aerosol (SOA) formation through aqueous phase chemistry. However, the heterogeneous processes and resulting glyoxal contribution to total SOA in various ambient conditions are largely unknown. In this study, the impact of humidity and irradiation on uptake of gaseous glyoxal onto particulate matter in has been investigated based on the glyoxal measurement acquired from airborne Cavity Enhanced Absorption Spectrometer (CEAS), during KORUS-AQ field campaign, with the help of 0-dimensional box model where the parameters were constrained by the observations from DC-8. The reactive uptake coefficient of glyoxal (γ) was derived by reconciling the gap between the observation of glyoxal and the expected abundance of glyoxal estimated from the steady-state box model without the uptake process. In the presence of > 0.1 ppb of glyoxal, the OA growth rate from glyoxal uptake was estimated to be 0.48 μg/m3/hour and γ varied from 0-20x10-3 having median and mean values of 5.89x10-3 and 8.10x10-3, respectively. The typical lifetime of glyoxal during the campaign was estimated to be ~37 minutes. γ decreased at 25-35% RH range which can be explained partially by the salting-in effect. On the other hand, γ increased at 35-75% RH range which remains uncertain. Also, γ showed an increasing trend with increasing irradiation indicating that the irreversible uptake process is dominant at the highly irradiated conditions."
and
The Synthesis of Two Novel p-38α Inhibitors
Hannah Maben,
ANYL 1st year, CU Boulder
"Current p-38α inhibitors are in clinical trials to treat diseases such as cancer, Rheumatoid arthritis, and Crohn’s disease. The synthesis of two potential p-38α inhibitors, bis-1,3-(4,6-difluoro-4-{4-[quinoxalin-2- ylmethoxy]methyl}-1H-1,2,3-triazol-1-yl]pyridin-2-yl) 5-methyl-6-phenylpiperazine-2,3-dicarbonitrile (I) and 2,6-di(5,6-Dimethyl-6,7-dihydro-5H-[1,2,5]oxadiazolo[3,4-b]pyrazin-4-yl)-3,5-difluoro-4-(4-phenyl- [1,2,3]triazol-1-yl)-pyridine (II) were conducted. The syntheses involved several nucleophilic aromatic substitution (S N Ar) reactions, a Sharpless “click” reaction, and a mild reduction via sodium borohydride. All products and intermediates were sent for elemental and biological testing, and two intermediates were determined to have biological activity against colon cancer."
Monday, 28 October 2019 Gas Phase Oxidation of Campholenic Aldehyde and Lactones as an Intermediate to SOA formation
William Dresser,
ANYL 1st year, CU Boulder
"This study investigated the oxidation of campholenic aldehyde(CA) and a series of lactone compounds and their derivatives using flow tube chemical ionization mass spectrometry (FT- CIMS) to try and understand the reaction mechanisms and identify potential intermediates between the gas and secondary organic aerosol (SOA) phase. Oxidation was induced with both hydroxide and chlorine radicals and proton transfer reaction (PTR) detection as well as Iodide detection were used in the spectra analysis. Oxidation pathways were determined for both studies at low pressures and product percentages were found for the major species. In the case of CA, the expected epoxide intermediate was identified at 5-20% abundance, which was the major intermediate of interest. The lactone pathways were used to predict the reactivity of a previously identified intermediate, hydroxymethyl-methyl-α-lactone (HMML), between isoprene and SOA."
and
Determination of the Active Agents in Commercial Pygeum Products Sold for the Treatment of Benign Prostatic Hyperplasia
Daniel Katz,
ANYL 1st year, CU Boulder
"Benign Prostatic Hyperplasia (BPH) or enlarged prostate is a common condition in older men and may be a precursor to prostate cancer. Dietary supplements made from the powdered bark of Prunus africana are marketed as pygeum to treat BPH, but they are only loosely regulated by the U.S. FDA. Commercial pygeum products were tested for N-butylbenzenesulfonamide (NBBSA), ferulic acid, atraric acid, atranorin, and β-sitosterol (BSST), components of pygeum that are thought to be effective in treating BPH. Two parallel liquid-solid extractions were conducted for each product. A direct extraction used acetone:hexane to extract NBBSA, atraric acid, and atranorin. The other extraction followed saponification that released ferulic acid and BSST from their natural esters and used dichloromethane solvent. After evaporation and reconstitution, the extracts were analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS). The amount of ferulic acid, atraric acid, atranorin, and BSST varied widely among the products, but no NBBSA was detected in any product. The levels of each active compound found in the products can be used to evaluate their possible effectiveness for the treatment of BPH."
Monday, 14 October 2019
Update on OFR185 Characterization of the Aerodyne Potential Aerosol Mass Chamber
Jake Rowe,
ANYL 1st year, CU Boulder
"One disadvantage of conventional Oxidation Flow Reactors (OFRs) is the difficulty in achieving controlled, shorter (<1 day) oxidative aging processes that are also relevant to atmospheric SOA formation processes. To investigate this issue, this study characterizes two alternative mercury lamp configurations designed to attenuate 185 and/or 254 nm irradiance relative to standard low-pressure UVC mercury lamps used in the Aerodyne Potential Aerosol Mass (PAM) OFR. In the first configuration, the irradiance at 185 and 254 nm was attenuated by applying segments of Viton heat shrink tubing along standard UVC lamps. In another configuration, the 185 nm lamp output was attenuated relative to the 254 nm output by splicing segments of lamp glass that transmit either 185 and 254 nm or only 254 nm radiation. OH exposures attained by photolysis of O2/O3/H2O with these lamps were calculated from the reactive loss of CO and SO2 input to the reactor and measured under steady-state conditions as a function of photon flux and [H2O]. At a midrange relative humidity ~ 50% and τ ~ 2 min, the attainable lower-range photochemical age decreased from ~4-5 to ~0.3-0.4 days of equivalent atmospheric exposure. Thus, the combined usage of attenuated and standard UVC mercury lamps -- as demonstrated here with the PAM OFR -- significantly extends the range of attainable photochemical aging timescales in OFRs with no additional modification of OFR conditions."
and
Identification and Characterization of a Red Blood Cell Stabilizer
Anna Ziola,
ANYL 1st year, CU Boulder
"When creating controls for hematology instruments, the volume of red blood cells (RBCs) must be held constant. Each component in control samples must retain its respective volume throughout the shelf life since the size of the cells is one method of blood component characterization in hematology instruments. The plant extract mixture currently used to stabilize the volume of RBCs is losing its effectiveness, requiring hospitals to purchase and replace these controls more often to ensure accurate patient hematology reports. To identify the active ingredient responsible for RBC stabilization in the plant extract, we used high performance liquid chromatography, mass spectrometry, SDS-PAGE gel electrophoresis, the Sysmex XN-20 and Sysmex XE-5000 to identify and characterize the active ingredient originally responsible for stabilizing the volume of RBCs."
Monday, 30 September 2019
Why should environmental chemists care about theory?
Sandeep Sharma,
PChem faculty, CU Boulder
"I will begin by discussing why learning about theory might be of interest to students doing experimental research in analytic chemistry. I will then proceed to give a birds-eye view of my research program. How questions are formulated, how one goes about trying to answer them and where are the challenges. I will also discuss how this line of research might help answer some of the questions environmental chemists ask. The presentation will end with some specific problems that Paul Ziemann and I are planning to collaborate on in the near future."
and
Chemistry of Organic Compounds in the Atmosphere and Indoor Air
Paul Ziemann,
ANYL Faculty, CU Boulder
"Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have conducted a number of collaborative studies of indoor air chemistry at CU."
Monday, 23 September 2019
Laboratory Studies of Contact Efflorescence in a Long Working Distance Optical Trap
Maggie Tolbert,
ANYL faculty, CU Boulder
"Because homogeneous salt efflorescence typically requires low relative humidity (RH), atmospheric salt particles are often assumed to be aqueous throughout much of their atmospheric lifetime. Here we use a long working distance optical trap to examine heterogeneous efflorescence that occurs when a supersaturated salt droplet comes into contact with a solid particle. We compare our findings for pure salt droplets with those obtained for salt/organic droplets where the organic may be found either in a core shell structure or as an amorphous/glassy solid. We find that in many cases, single collisions with a range of nuclei promote efflorescence at relatively high RH, causing salt particles to be solid for more of their atmospheric lifetime. Similar experiments are proposed in the future to examine the role of contact in ice cloud nucleation."
and
Small molecules in the Anthropocene: Wildfires, Oceans and Iodine
Rainer Volkamer,
ANYL faculty, CU Boulder
"The Volkamer group develops advanced optical instrumentation (in situ and remote sensing) to measure small molecules and aerosols that are relevant to public health and climate. We seek to better quantify and understand emissions of small molecules, total carbon, and aerosols from natural and managed ecosystem (e.g., wildfires, oil & natural gas, ocean surface), and develop a molecular level understanding of the fundamental processes that affect their chemical transformations and sinks (e.g., new particle formation). Opportunities for graduate research exist in the areas of 1) field measurements (i.e., BB-FLUX, TI3GER project), 2) laboratory experiments to better understand air-sea exchange, and anthropogenic enhancements of biogenic organic aerosol (incl. at CLOUD/CERN), and 3) instrumentation to develop small light weight sensors for mobile air-quality networks (e.g., RTD transit)."
Monday, 16 September 2019
Chemistry of Volatile Organic Compounds in the Atmosphere
Joost de Gouw,
ANYL faculty, CU Boulder
"Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products.
In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments.
Several different ongoing and future projects will be presented in this seminar. First, we use PTR-TOF for measurements of VOCs in indoor environments. From the results we learn about the sources of VOCs from people, chemical products and building materials, the chemical transformations of these VOCs and other loss process such as surface uptake and ventilation to the atmosphere. Second, we are working on the emissions and chemistry of VOCs released from volatile chemical product (VCP) use to the atmosphere, which was recently discovered to be the dominant source of VOCs in urban air. In this research we make measurements of VOCs in urban air, separate the different emission sources and describe the chemical transformations of VOCs after emission. Finally, we are working on a chamber study to better understand the formation of secondary organic aerosol from biogenic VOCs."
and
Organic nitrogen chemistry: Recent results and future projects
Eleanor Browne,
ANYL faculty, CU Boulder
"Organic nitrogen is a ubiquitous atmospheric component that affects biogeochemistry, air quality, and climate. Assessing the impact of organic nitrogen on these processes remains challenging because traditional measurement techniques have lacked the sensitivity and chemical resolution to characterize the speciation and chemistry of organic nitrogen. Here, I will discuss measurements made with protonated ethanol cluster chemical ionization time-of-flight mass spectrometry during the Holistic Interactions of Shallow Clouds, Aerosols, and Land-Ecosystems (HI-SCALE) campaign at the Southern Great Plains research station in Lamont, Oklahoma. As the site is located in an agricultural region, reduced nitrogen compounds are prevalent. I will present measurements of novel compounds including imines and urea and will discuss the sources and sinks of compounds at this site. Finally, I will discuss research opportunities in our group. These include further measurements at SGP in Spring 2020 and Fall 2021 as well as instrument development and chemical transport modeling."
Monday, 9 September 2019
Atmospheric nanoaerosols: An instrument for chemical analysis of freshly nucleated particles and their formation from biogenic organic precursors
Andrea Wagner,
ANYL Postdoctoral Researcher, CU Boulder, Volkamer Group
"A large fraction of atmospheric aerosol particles are formed via new particle formation from the gas phase. But the chemical analysis of freshly nucleated particles smaller than 30 nm is challenging due to their tiny mass. In this talk I present a new instrument that, in combination with a chemical ionization time-of-flight mass spectrometer, analyzes these nucleation mode particles. The measurements are size-resolved and parallel to gas-phase analysis. Additionally, mechanisms for the formation of particles from purely biogenic organic precursors in chamber measurements are presented."
and
Recent Results and Upcoming Projects Investigating Aerosol Sources, Properties, Processes, and Fate
Jose Jimenez,
ANYL faculty, CU Boulder
"This fall ½ seminar provides an opportunity to introduce our group and its upcoming opportunities to the starting graduate student class, as well as to provide a summary of key results to the ANYL division as a whole. Our group’s research focuses on understanding the sources, properties, transformations, and sinks of submicron aerosols (and of the gases that interact with them), which have major effects on human health and climate.
In this talk I will briefly present results from different projects over the last year, especially those most relevant to incoming student opportunities. I will introduce our aircraft research program, where we are currently participating in the FIREX-AQ campaign sampling biomass burning smoke with the NASA DC8 aircraft. We are deploying an aerosol mass spectrometer (AMS) and, for the first time, an extractive electrospray (EESI) MS, with excellent results. I will also summarize results from the ATOM campaign, the first to sample the global remote troposphere systematically with vertical coverage. In particular global models are found to predict organic aerosols (OA) ok, but for a combination of wrong reasons as primary OA (POA) is greatly overestimated. On the other hand, models tend to predict pH which is substantially higher than the observations, with a pH ~ 0 being typical of the remote troposphere. A meta-analysis of large urban studies shows that urban secondary OA (SOA) leads to ~320,000 excess deaths per year globally, and should be the target of future regulations. The budget of organic carbon of indoor air is presented and compared to that of outdoor studies. Finally, fundamental measurements of the organic accommodation coefficient are presented using a new chamber technique.
Potential opportunities in our group involve the CalNexT campaign studying urban SOA in Los Angeles, studies of indoor air chemistry and surface interactions, analysis of aircraft campaign data and participation in future campaigns, and chamber experiments targeting both fundamental processes and signatures of urban SOA sources. Modeling tools such as KinSim and GECKO-A can be applied to the different problems as needed."
Summer 2019
Monday, 19 August 2019
Fates of Oxygenated Organics in Outdoor and Indoor Environments, Chemical Reactions and Partitioning
Lucas Algrim,
ANYL Student, CU Boulder, Ziemann Group
"Volatile organics are emitted in indoor and outdoor environments where they are then subject to various losses such as deposition, or chemical transformation. In outdoor environments chemical reaction dominates, which will create products of higher volatility that stay in the gas phase or products of lower volatility that can partition to the particle phase to generate SOA. In indoor environments, which have lower concentrations of oxidants and greater surface area, deposition to and partitioning with surfaces are of increased relevance.
In the first section of this thesis, Chapters 2-4, we probe SOA forming potential of functionalized precursors. SOA yields were measured for OH radical-initiated reactions of the 2-6 dodecanone, 1-5 decanol, and 1-5 decyl nitrate positional isomers and also n-decane, n-dodecane and n-tetradecane in the presence of NOx. Yields decreased in the order n-tetradecane > dodecanone isomer average > n-dodecane, and the functionalized isomer yields decreased as the functional group moved toward the center of the molecule, with 6-dodecanone being an exception. Trends in the yields can be explained by the effect of carbon number and functional group presence and position on product vapor pressures, and by the isomer-specific effects of the functional group on branching ratios for the various alkoxy radical isomerizations, decompositions, and reactions with O2. Analysis of particle composition indicates within each isomer series, the SOA products are similar for each isomer. The results demonstrate that the presence of a functional group on a precursor alters gas and particle phase chemistry and provide new insights into the potential effects of molecular structure on the products of the atmospheric oxidation of volatile organic compounds.
In the second part of the thesis, Chapter 5, we parameterize the partitioning of VOCs to indoor paint films. Diffusion coefficients, Dc, of organics in a commercial paint were measured and found to correlate well with compound vapor pressures, C*. Experiments were performed by monitoring VOC partitioning in a painted flow tube. A model was constructed to replicate the VOC time traces observed during passivation and depassivation as a function of two parameters, Cw, and Dc. Modeled and measured Dc values agreed reasonably well, and modeled Cw values aligned with those calculated from the mass of paint in the flow tube. The relationship between Dc and C*, and the determination of Cw from mass of paint allow for modeling of VOC partitioning to paint films in indoor environments that indicate >50% of the VOCs with C* of 108 μg m-3 or less that contact a paint film of typical thickness, will fully permeate the paint film, regardless of emission duration."
Tuesday, 23 July 2019
Aircraft measurements of bromine and iodine from the sea surface to the lower stratosphere
Theodore Koenig,
ANYL Student, CU Boulder, Volkamer Group
"Bromine and iodine change atmospheric oxidative capacity, deplete ozone, modify NOx = (NO2 + NO) and HOx = (OH + HO2), and in turn impact air quality and human health as well as radiative forcing and climate. The radical monoxides, BrO and IO, have large structured rovibronic absorptions in the Ultraviolet-Visible (UV-Vis) spectrum which results in rapid and active photochemistry as key intermediates in the impacts listed above, and also makes them detectable to differential optical absorption spectroscopy (DOAS). I present measurements of BrO and IO using DOAS, particularly from aircraft, from which I infer total gas-phase bromine and iodine, Bry and Iy, using chemical box-models and examine the implications of these measurements. We find active multiphase chemistry relevant from the marine boundary layer MBL where gas-phase coupling to organics modulates sea-salt aerosol debromination, to the free troposphere where we find dust initiates iodine chemistry leading to miniature ozone holes west of South America, to the upper troposphere and lower stratosphere (UTLS) where multiphase chemistry and phase partitioning around the tropopause destroy ozone and change radiative forcing in the climate relevant region."
Spring 2019
Monday, 22 April 19
Methods for the quantification and identification of alkenes on indoor surfaces
Benjamin Deming ANYL 3rd year, Ziemann group
"Indoor surfaces can support organic films, which can act as sinks for semi-volatile organic compounds (SVOCs), reactors for condensed-phase reactions, sites of heterogeneous gas-condensed-phase reactions, and exposure routes for human health impacts. Although there have been some studies on indoor surface films, their size, composition, and chemistry are still uncertain. Additionally, the majority of studies investigating these films have been performed on impermeable surfaces, typically window glass. Painted surfaces, which tend to dominate by surface area, potentially differ from glass in several important ways, and are therefore an understudied aspect of indoor environments. Interior wall paint is normally composed of an organic binder and an inorganic filler, which may change the way films initially form as well as their subsequent growth. Film components may also absorb into paint and away from the surface, changing the composition of the film that remains. Oxidation indoors is largely driven by ozone and reactions with alkenes are therefore of particular interest. There are a number of sources unique to indoor environments which may contribute alkenes to surface films: cooking oils can have a high degree of unsaturation, many fragrances and cleaners contain terpene derivatives, and human beings constantly produce skin oil, which contains squalene and unsaturated fatty acids. To explore these areas of interest we characterized a method for quantitatively sampling the low-volatility, organic portion of a surface film using a surface wipe and developed a spectrophotometric method for quantifying nanomole quantities of alkenes. Samples were also derivatized, adding a readily ionizable group to non-conjugated double bonds, allowing for identification by positive-mode ESI-MS. Samples from neighboring glass and painted surfaces were collected from a variety of locations, including a classroom, graduate student offices, a bowling alley, a gym, and more. To investigate the effect of cooking on nearby surface concentrations we pan-fried three different cooking oils at high temperatures and analyzed the double bond content of the raw oil, cooked oil, and nearby surfaces. The effect of ongoing exposure to ozone on the alkene concentrations on the human envelope was explored by obtaining samples from the foreheads of volunteers at different times of the day. In a final experiment we studied the effect of using a terpene cleaner on measured surface concentrations. The results from this work should be useful to the modeling of indoor environments and help assess the importance of surfaces to indoor chemistry."
Monday, 15 April 19
Dropping Acid in the Atmosphere: Is It Just a Phase?
Prof. Andrew Ault,
Univ. of Michigan
"Atmospheric aerosols are incredibly complex chemical systems with thousands of species present in yoctoliter to attoliter volumes, which makes measuring their chemical and physical properties an analytical challenge. Despite these instrumental demands, measuring aerosol properties is essential, as air pollution leads to 10% of global deaths annually, primarily due to the effects of atmospheric particles. These aerosols are also the most uncertain aspect of radiative balance leading to climate change. The Ault Laboratory is focused on understanding the complex heterogeneous and multiphase chemistry occurring within aerosols through systematic physical chemistry studies, the development of new analytical methods and sensors, and measurements of complex systems in that atmosphere. We conduct these studies this through a combination of spectroscopy, microscopy, and mass spectrometry techniques. This seminar will focus on the acidity, phase, and morphology of mixed organic-inorganic atmospheric particles. Specifically, we will focus on the acid-catalyzed ring opening reaction of isoprene epoxydiols (IEPOX), formation of organosulfates and polyols, and subsequent changes to diffusion in viscous materials. From this we can predict future properties and amounts of secondary organic aerosol (SOA). With our novel analytical methodologies and physical chemistry studies, the Ault Laboratory is providing fundamental molecular insights into the chemistry occurring within atmospheric aerosols that have significant consequences for human health and global climate."
Monday, 8 April 19
Deliquescence and Efflorescence of Chlorate Salts under Mars-relevant Conditions
Marium Fernanders,
3rd year ANYL student, Tolbert lab
"When searching for life elsewhere in the universe, scientists have mainly focused on finding liquid water. However, in addition to liquid water, the presence of certain types of salts could also be a marker for water and perhaps life. The presence of chlorate salts, on the surface or in the sub-surface, could be an indicator of where to find life and water on present day Mars. Recent research has found that certain types of terrestrial bacteria can survive in per/chlorate-rich salt environments by using these salts as an energy source. Because of the salts’ low eutectic temperatures and ability to deliquesce (liquefy), these salts may be able to provide the ideal conditions (liquid water and energy) where life may be found in either the surface or sub-surface.
In the search for life on other planetary bodies, one of the places where scientists are looking is our closest neighbor: Mars. Mars, like Earth, exists in the “Goldilocks” zone a set area around a star where planetary bodies are not too hot or too cold for liquid water to occur. However, liquid water has not yet been detected on present day Mars. Mars with a little help from salts, could support liquid water on the surface or in the sub-surface through the process of deliquescence, where a crystal salt absorbs water vapor from the atmosphere, like a sponge, and when the conditions are just right turn into a briny droplet of water. This research proposal will study the low temperature deliquescence and efflorescence (when a briny droplet loses water through evaporation and turns back into a salt crystal) of chlorate salts and chlorate salt mixtures under Mars-relevant pressures and temperatures. By doing this, I will improve our knowledge of how chlorate salts behave under conditions where liquid water may be formed on Mars. In order to determine the conditions under which chlorates will deliquesce and eventually effloresce I will use a Raman microscope attached to an environmental cell where the pressure, temperature, and humidity can be controlled to mimic Mars-like conditions. The Raman microscope will allow me to see a salt particle turn into a liquid and back into a crystal using visual and spectroscopic identification. The Raman uses a green laser to probe a specific salt crystal or briny droplet. The light from the laser gets scattered back into a detector and I can then use the light scattering to characterize and identify the physical state, and chemical composition of crystal or droplet. The information will allow me to determine the deliquescence relative humidity (DRH), and the efflorescence relative humidity (ERH) of the chlorate salts and salt mixtures. By determining these fundamental properties as a function of temperature and pressure, we can then use NASA’s Mars rover and satellite data to determine if there are conditions on Mars where aqueous salt solutions can exist, and possibly flow, on the surface or sub-surface."
Monday, 18 Mar 19
Multiphase chemistry of volatile-organic-compound oxidation products under pre-industrial conditions
Prof Frank Keutsch,
Harvard
"Oxidation of volatile organic compounds (VOCs) is an important atmospheric process tied to the formation of secondary pollutants such as ozone and secondary aerosol. Research in the Keutsch Group has been focusing on VOC oxidation chemistry under preindustrial conditions, which primarily produces organic hydroperoxides. I will discuss recent results on the multi- phase chemistry of some of the most abundant multifunctional organic hydroperoxides, which result from isoprene oxidation. The results have implications for our understanding of gas- and aerosol aspects of the reactive carbon cycle as well as sulfate formation via cloud processing."
Monday, 11 March 2019
Chemical intuition on the oxidation mechanism of Hg(0) in the gaseous atmosphere
Prof. Theodore Dibble,
SUNY-ESF
"Mercury harms ecosystems and harms human health on account of consumption of fish near the top of the food chain. The atmosphere transports mercury around the globe from emission sources (e.g., power plants, re- emission from ecosystems). These emissions are mostly in the form of Hg(0) (atomic mercury), but deposition of Hg(II) compounds dominates transfer from the atmosphere to the Earth.
Investigations of the kinetics and mechanism of Hg(0) oxidation in the atmosphere present many challenges to experiment. Field studies are still plagued by interferences and issues of quantification. In the past several years my group has made great strides in understanding gas- phase oxidation of Hg(0). These advances have come from chemical intuition and quantum chemistry calculations. The first critical advance came in recognizing that BrHg radical was much more likely to react with atmospherically abundant radicals, •Y, such as NO2 and HOO, (see Figure 1) than with OH or Br, which had been the only two reactants included in models of BrHg• chemistry. Theory suggests very large rate constants for reactions of BrHg with Y. Experiments on BrHg• chemistry and kinetics are starting, but have yet to produce results.
The second major point was to realize that BrHgONO, like HONO, would photolyze at
tropospherically relevant wavelengths to yield BrHgO•. Subsequent studies revealed that
BrHgO• behaved a lot like OH (left half of Figure 1), although BrHgO• is better at hydrogen
abstraction than OH.
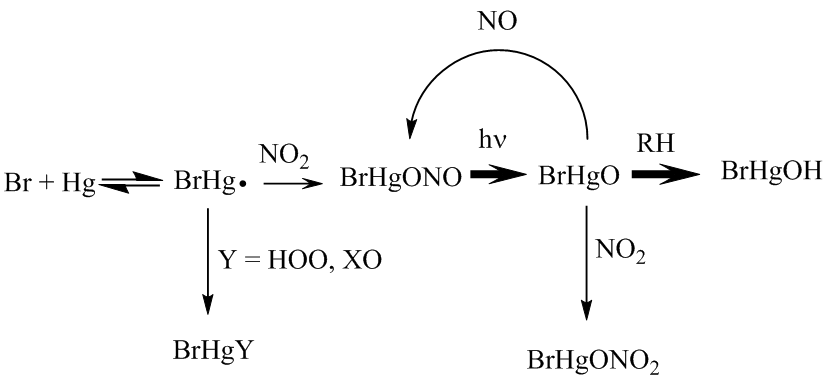
Finally, we have used theory to quantify the equilibrium constant for OH + Hg <-> HOHg and show that bond energies for HOHg-Y are essentially the same as those for BrHg-Y. With a few assumptions, this lets us build a mechanism and set of rate constants for atmospheric modeling of OH-initiated oxidation of Hg(0) to Hg(II)."
Monday, 4 March 2019
Understanding the Fate of Amines: Reactions with Oxygenates
Mitch Alton,
CU ANYL 3rd year, Browne group
"Atmospheric aliphatic amines and ammonia have been previously reported to participate in various chemical reactions including brown carbon formation, accretion reactions forming amides, imine and enamine formation, and acid-base cluster stabilization reactions that can enhance new particle formation. As anthropogenic reactive nitrogen emissions continue to increase to keep pace with world population and food demands, the interaction of these amines and ammonia with organic compounds in the atmosphere needs to be further investigated to better understand the impacts of these emissions on air quality and the environment. I will discuss a series of chamber experiments that investigated the effects of different aliphatic amines and ammonia on secondary aerosol formation from the ozonolysis of α-pinene without the use of seed aerosol. Using hierarchical clustering analysis, different fates of α-pinene ozonolysis products with amines/ammonia were identified. Various observed reactions between amines/ammonia and α-pinene ozonolysis products to form enamines, imines, amides, and acid-base clusters will be explored to show the complex chemistry that can occur during aerosol formation and growth. Finally, I will discuss how acid-base stabilization reactions are the most important contributions to particle nucleation in this system. "
Monday, 25 February 2019
Chemistry on Ice: Shedding Light on Arctic Halogen Photochemistry
Prof. Kerri Pratt,
University of Michigan
"With rapid sea ice loss and warming, there is an urgent need to understand the unique chemistry involving multiphase reactions of atmospheric aerosols and the snow-covered sea ice surface in the Arctic. Yet, the harsh environment and low analyte concentrations pose analytical challenges. The Pratt Lab utilizes novel mass spectrometry techniques to measure the complex chemistry of trace gases, aerosols, and snow in the Arctic. Using chemical ionization mass spectrometry, we are advancing understanding of Arctic halogen photochemistry through measurements of trace gas species at ppq to ppt levels, including observations of trace gases species for the first time in the ambient atmosphere. Bromine, chlorine, and iodine chemistry, and the coupling of the cycles involving these halogen species, have significant impacts on the fate of greenhouse gases and pollutants, including ozone, methane, and mercury. Sunlit and artificial light experiments conducted in the Alaskan Arctic, combined with atmospheric measurements and numerical modeling, were utilized to elucidate chemical mechanisms driving the unique multiphase processes. The new chemical insights obtained are providing crucial scientific detail needed to understand and predict changing atmospheric composition in the Arctic."
Monday, 11 February 2019
Grills and grilles: cooking and traffic as drivers of spatial variations in exposure to particulate matter
Prof. Albert Presto,
Carnegie Mellon
"Sharp spatial gradients of particulate matter (PM), organic aerosol (OA), and black carbon (BC) concentrations exist at intra-city scales (<1 km) due to intense emissions from sources like traffic and cooking activities. Typical stationary deployment of samplers is not capable of resolving these spatial gradients. By deploying an Aerodyne Aerosol Mass Spectrometer (AMS) and other high temporal resolution measurements on a mobile sampling platform, we are able to investigate the spatial variation of PM mass concentration, PM composition, and particle number concentrations within cities. Source apportionment with Positive Matrix Factorization (PMF) enables identification of contributions of traffic and other sources to the observed PM mass. Cooking and traffic sources dominate PM spatial variability.
This presentation will show results for two cities: Pittsburgh and Oakland. In locations with high local source impact in Pittsburgh, the PM1 concentration is 2 mg m-3 (40%) higher than urban background locations. Traffic emissions are the largest source contributing to population-weighted exposures to primary PM. The concentration of both cooking and traffic PM are positively correlated to their respective geographical covariates: vehicle-miles travelled (VMT) and restaurant count. VMT is a reliable predictor for traffic PM concentrations for use in air pollutant spatial models. Restaurant count is an imperfect predictor for cooking PM concentration, likely due to the highly variable emissions of individual restaurants. Cooking PM is also positively correlated to VMT, which suggests that near-road cooking emissions can be misattributed to traffic sources in the absence of PM source apportionment. In Pittsburgh, 27.7% and 8.9% of the total population are exposed to >1 mg m-3 of traffic- and cooking-related primary emissions, with some populations exposed to high concentrations from both sources. Results for Oakland show similar spatial patterns and concentration trends. While these data were collected for two cities, the source mix in many U.S. cities is similar. We therefore expect similar PM spatial patterns and increased exposures in high-source areas nationwide."
Monday, 4 February 2019
The Synthesis and Reactions of 3-Hydroxymethylphthalimides
Olivia Jenks,
CU ANYL Chem 1st Year, de Gouw group
"Phthalimides possess a wide range of physiological properties, including anti-inflammatory and immunomodulatory activities, and have been found to be useful in the preparation of specialized polymers and macrocycles. While a vast number of N-substituted phthalimides have been reported, the number of phenyl-substituted compounds is fairly limited. We have recently developed a convenient method for the synthesis of 3-hydroxymethylphthalimides, which have the potential for conversion into a number of other phenyl-substituted compounds. In this presentation, the transformation of the hydroxymethyl group to the corresponding chloride and the reactions of the benzylic halides with a variety of amines as nucleophiles will be described. With alkyl amines, nucleophilic acyl substitution at the imide ring competes with the desired alkyl substitution to yield diamides. Studies with substituted anilines show the rate of substitution varies markedly with the basicity of the nucleophile."
and
Fundamental investigation of substituent effects on threshold energy and electron density for the [1,3] thioallylic rearrangment
Mindy Schueneman,
CU ANYL Chem 1st Year, Jimenez group
"Computational chemistry provides information of reaction energetics, thermodynamics, and kinetics by integrating computer modelling and and chemical theory. Computational chemistry was used to examine the effect of electron withdrawing and donating groups on both the threshold energy and electron density for the [1,3] thioallylic rearrangement mechanism. Thioallyl compounds contain a 3 carbon allyl chain with a sulfur in the C1 position. Through the combination of DFT and QTAIM, two computational methods, it was found that the thioallylic mechanism undergoes a concerted transition state, with most of the electron density contained on the sulfur atom. In addition, the activation energy barrier was found to decrease with the addition of electron donating groups located at various parts on the allyl backbone."
Monday, 28 January 2019
Global Observations of Ammonium Balance and pH Indicate More Liquid Aerosol and Acidic Conditions than Current Models Predict
Benjamin Nault,
CU ANYL Chem Postdoc, Jimenez group
"The inorganic composition of aerosol impacts numerous chemical and physical processes and properties. However, many chemical transport models show large variability in both the concentration of the inorganic aerosols and their precursors (up to 3 orders of magnitude differences) and the composition of the inorganic aerosols. Different models would predict very different properties such as aerosol liquid water concentration, aerosol acidity (but most models do not calculate this property), heterogeneous uptake of gases, aerosols direct and indirect impact on climate, et cetera. Here, I use airborne observations from campaigns conducted around the world to investigate how the inorganic composition, and one of its key parameters, aerosol acidity, changes from the polluted regions (Mexico City, Los Angeles, Northeastern US, and Seoul) to the most remote regions (the Atmospheric Tomography campaigns 1 and 2), to provide constraints for the chemical transport models. I find that the empirical ammonium balance (ammonium balance = mol NH4 / (2×mol SO4 + mol NO3)) rapidly decreases from 0.85 in polluted regions to less than 0.2 in remote regions, contradictory to some modeling studies that suggest most of the has a balance near 1. The data imply very low NH3 in the upper troposphere, contrary to predicts of some models. Real-world aerosols are less likely to be in the solid phase and more likely to be in a metastable liquid state. Next, I explore the aerosol acidity with the E- AIM model, constrained by observations, and find that the acidity increases from the most polluted (median = 2.3) to most remote regions (median = –0.5). The chemical transport models have difficulty reproducing the aerosol acidity, showing both over and underestimation in pH. Several causes likely lead to these measurement vs model differences in aerosol acidity, including the mixing state of sea salt (internal vs. external) and total amount of NH x present in the atmosphere (NH x = NH3 (g) + NH4 + (p)), which are currently being investigated and will be briefly discussed during this talk."
and
Towards an improved representation of organic aerosol (OA) in the remote troposphere: Overall abundance, sources and physical and chemical removal
Pedro Campuzano Jost,
CU ANYL Chem Research Scientist, Jimenez group
"Organic aerosol (OA) is one of the major contributors to the PM2.5 burden in the continental Northern Hemisphere (NH); understanding its sources and aging is central to current air quality control strategies. For the remote troposphere, sparse in-situ data to date results in highly under constrained OA prediction models, with model diversity of up to three orders of magnitude. As part of the recently concluded NASA Atmospheric Tomography (ATom) aircraft mission, we have acquired four unique global datasets of submicron aerosol concentration and composition over the remote Atlantic and Pacific Oceans. Overall, OA concentrations except for the cleanest regions were comparable to sulfate, as in the Northern Hemisphere, with OA, sulfate and seasalt being the main contributors to both CCN and submicron AOD. An evaluation of state-of-the-art models (CESM, GEOS-Chem) with ATom meteorology fields shows that while overall models reproduce remote OA concentrations fairly well, they mostly fail to reproduce the large ratio of secondary to primary observed in the measurements and use unrealistic OA/OC ratios for tracking OA. Improved model parameterizations that account for these factors overestimate OA in most remote regions, suggesting that an additional, slow loss channel for OA is needed. Based on a photochemical clock analysis of the Atom data, we find an OA lifetime of about 10 days for this process, consistent with recent estimations of the OA removal rate due to OH oxidation and photolysis."
Fall 2018
Friday, 7 December 2018
TROPOMI on-board the Sentinel-5 Precursor mission: a game changer for tropospheric composition monitoring from space
Michel van Roozendael,
Royal Belgian Institute for Space Aeronomy (BIRA-IASB)
"The Sentinel-5 Precursor (S5P) is the first atmospheric composition monitoring satellite in the Copernicus Sentinel series operated by EU. Successfully launched on 13 October 2017, it carries the Tropospheric Monitoring Instrument (TROPOMI), which provides daily global observations of the nadir backscattered earthshine radiance in 3 spectral channels covering the UV, VIS, NIR and SWIR regions at the unprecedented horizontal resolution of 7x3.5 km2. BIRA-IASB has been involved in the mission preparation since early 2010 and is responsible for the algorithm baseline definition and maintenance of the tropospheric HCHO, SO2 and total ozone, as well as the operational validation as part of the S5P Mission Performance Center (MPC). After a brief introduction on the mission, instrument characteristics and retrieval methods, I focus on results obtained after one year of measurements with particular attention to species retrieved in the UV-Visible spectral range. Observations reveal the distribution of tropospheric species in unprecedented detail, allowing for a much more accurate identification of pollution sources at the level of cities, and various local emission sources both of natural and anthropogenic origin. In many cases, the sensitivity of the instrument exceed the expectations of the involved scientists."
Monday, 3 December 2018
Hydroxyl radical reactivity: Results from field campaigns in Europe and China
Hendrik Fuchs, Forschungszentrum Jülich,
Institute of Energy and Climate Research, Troposphere (IEK-8)
"OH reactivity is the total loss rate coefficient of the hydroxyl radical (OH), the main responsible agent for the oxidation of pollutants in the atmosphere. The direct measurement of this quantity is of great value as it constrains the removal rate of OH in the analysis of the atmospheric budget of OH radicals. In addition, measured OH reactivity can be compared to calculations from individual OH reactant concentration measurements in order to quantify the importance of unmeasured OH reactants. OH reactivity measurements were done on a Zeppelin airship during flights over Europe showing the vertical and horizontal distribution of OH reactants. Ground-based field campaigns in the North China Plain and the Yangtze River Delta in summer- and wintertime were performed between 2006 and 2016 giving an overview of the major OH reactants that contribute to air pollution in China."
Monday, 5 November 2018
OMI and TROPOMI: towards high resolution Air Quality and Emission monitoring
Pieternel Levelt,
KNMI and University of Technology Delft
"The Ozone Monitoring Instrument (OMI), launched on board of NASA’s EOS-Aura spacecraft on July 15, 2004, provides unique contributions to air quality monitoring from Space. The combination of urban scale resolution (13 x 24 km 2 in nadir) and daily global coverage proved to be key features for the air quality community. The OMI data is currently used operationally for improving the air quality forecasts, for inverting high-resolution emission maps, UV forecast and volcanic plume warning systems for aviation. Due to its almost 14 year continuous operation OMI provides the longest NO2 and SO2 record from space, which is essential to understand the changes to emissions globally.
In 2017 Tropospheric Monitoring Instrument (TROPOMI), was launched on board ESA’s Sentinel 5 Precursor satellite in October 2017. TROPOMI has a spatial resolution of 3,5x7 km2 in nadir; a more than 12 times improvement over OMI. The high spatial resolution serves two goals: (1) emissions sources can be detected with even better accuracy and (2) the number of cloud-free ground pixels will increase substantially. TROPOMI will continue OMI’s ozone and air quality trace gas records. Added to that TROPOMI will measure the O2 A band for better cloud detection, as well as CO and the second most important greenhouse gas CH4. TROPOMI will therefore be an important satellite mission for the EU Copernicus atmosphere service and will be followed by ESA’s sentinel 4 and 5 satellites. The first measurements of TROPOMI turn out to be above expectation.
In the coming decades air pollution in megacities will continue to be a major area of concern and the need for timely, high resolution information on emissions will increase, preferably to a level where sources can be isolated on the < 1 x 1 km2 scale. Currently we are working on new follow-on satellite instrumentation with which we envisage to improve emission monitoring to the < 1 x 1 km2 spatial resolution level (TROPOLITE).
An overview of air quality applications, emission inventories, and trend analyses will be given, based on the excellent OMI data record, followed by first measurements and results of the TROPOMI instrument. An outlook will be presented on the potentials of the TROPOMI and what new satellite instrumentation with a 1 x 1 km2 spatial resolution can bring in the air quality and climate domain."
Wednesday, 31 October 2018
Dissertation Defense: Influence of multiphase processes on the chemistry and measurement of organic compounds in indoor and outdoor environments
Demetrios Pagonis,
Ziemann Lab, CU Boulder
"Organic compounds are ubiquitous in indoor and outdoor environments, with organic
aerosols and gases impacting air quality, global climate, and human health. The life cycle of
volatile organic compounds (VOCs) in the atmosphere includes emission from indoor and
outdoor sources, oxidation in the atmosphere to form secondary organic aerosol (SOA), and
eventual deposition to a surface. Understanding each of these processes is necessary to predict
the impact of organic compounds on indoor and outdoor environments, and this thesis presents
the results of a series of studies across the life cycle of VOCs, examining the chemical and
physical processes that transform organic compounds in the atmosphere.
First, the chemistry of multifunctional hydroperoxides in SOA is studied by a series of laboratory studies utilizing a model hydroperoxyaldehyde designed to represent the highly oxidized multifunctional compounds that impact SOA growth in pristine environments. Measurements of reaction rates, equilibrium constants, and decomposition mechanisms provide insight into how chemical structure and aerosol properties affect the chemistry of multifunctional hydroperoxides in SOA. Second, emission rates, deposition velocities, reaction rates, and reaction products from a field study in a university art museum are presented. This study quantifies the significant impact of human activities on indoor VOC emissions, as well as the effect of indoor surfaces and indoor oxidants on the fate of those emissions. Lastly, this thesis presents a study aimed to improve researchers’ ability to make time-resolved measurements of gas-phase organic compounds that partition to instrument surfaces and to Teflon tubing commonly used for sampling lines. The simple chromatography model presented here accurately predicts the delay in instrument response caused by gas-surface partitioning across all the tubing lengths, diameters, flow rates, and analytes tested. Together, the studies presented in this thesis advance the understanding of, and the ability to measure, the fate of organic compounds in indoor and outdoor environments."
Monday, 29 October 2018
Non-Targeted Chemical Characterization of Waterpipe Tobacco Smoke by GC-MS and LC-MS
Andrew Jensen,
ANYL 1st year student
"As smoking waterpipes becomes more popular, it becomes necessary to understand the chemical composition of waterpipe tobacco smoke (WTS) and its impacts on human health. Current studies target known components of cigarette smoke, but no current studies acknowledge the possible differences due to the tobacco product and heating profile. The tobacco product, shisha, is made up of tobacco leaves coated in syrup which itself is about one-third glycerol. The shisha is then heated using charcoal, producing larger pyrolysis products. The resulting smoke generated from these constituents can be divided into particulate matter (PM) and vapor. Each fraction may contribute differently to smoke toxicity, making it important to understand the chemical contributions of each constituent. These constituents were studied using gas and liquid chromatography tandem mass spectrometry with a non-targeted approach.
A semi-quantitative comparison of the chromatograms allows for the identification of the sources and sinks of certain compounds in WTS. The GC-MS chromatograms of the shisha, bowl water, PM, and vapor suggest no significant portion of the WTS is trapped by the bowl-water, contrary to popular belief that the water acts as a significant filter. In the LC-MS chromatograms, charcoal alone contributes 10 chromatographic peaks while glycerol, syrup, and shisha each contribute 5, 11, and 32 peaks respectively. A similar comparison of the shisha PM and vapor chromatograms shows that the vapor phase contributes 2 unique peaks while the PM contributes 43 peaks and 11 peaks are shared between the two phases. This study has begun the process of identifying the unique chemical characteristics of the individual sources and sinks of WTS. Toxicity studies can identify WTS fractions of interest, then the unique compounds in these fractions can be isolated by the methods presented in this study."
and
Experimental and Theoretical Studies in the Gas Phase Kinetics of Atomic Halides with Methacrolein and Alkyl Bromides
Kyle Mackey,
ANYL 1st year student
"In the studies conducted by the Wine Group the rate coefficients of the reactions of bromine radicals with methacrolein and chlorine radicals with several alkyl bromides were determined using the laser flash photolysis - resonance fluorescence technique as a function of temperature and pressure for use in atmospheric modeling and calculations."
Monday, 22 October 2018
Chemistry of Organic Compounds in the Atmosphere and Indoor Air
Prof. Paul Ziemann,
ANYL Chem Faculty, CU-Boulder
"Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have recently conducted a number of studies of indoor air chemistry at CU. In this talk I will describe how we conduct the studies by using a diverse array of measurement techniques."
and
Recent Results and Upcoming Projects Investigating Aerosol Sources, Properties, Processes, and Fate
Prof. Jose L. Jimenez,
ANYL Chem Faculty, CU-Boulder
"Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, properties, and evolution remain poorly understood. In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students.
Ongoing projects include global aerosol measurements and analysis as part of the NASA ATom project, which recently sampled (almost) pole-to-pole across the vertical profile. Model comparisons suggest the importance of fast OA removal channels, and a strong overestimation of primary OA in some models. Remote aerosols are very acidic with a typical pH ~ 0, which is significantly lower than predicted by global models. We are also performing a meta-analysis of urban SOA at megacities worldwide, which shows remarkably consistent results and allows us to more accurately estimate the global number of deaths due to this source. Other topics that we are working on, and that I will touch on as time permits, are RO2 chemistry in Oxidation Flow Reactors (OFR) and how it compares with large chambers and the atmosphere; gas/particle partitioning in the laboratory for different types of seed particles; the impact of different types of tubing and instruments on the measurement of intermediate volatility and semivolatile species; the sources and budget of organic carbon in indoor air; and the development of fast SOA parameterizations for global and climate models.
Some upcoming projects include include the study of emissions and chemical evolution of smoke from real fires in the western US with the NASA DC8 (NASA FIREX-AQ) with AMS and soft-ionization EESI-TOF; an upcoming indoor campaign at a weight room at the CU Athletic Dept; and potentially the CalNexT ground-based study of urban chemistry in Los Angeles (which follows up on the highly successful CalNex-2010 study)."
Monday, 8 October 18
Small molecules in the Anthropocene: Opportunities in remote pristine and polluted air
Prof. Rainer Volkamer,
ANYL Faculty, CU Boulder
"The Volkamer group develops advanced optical instrumentation (in situ and remote sensing) to measure small molecules and aerosols that are relevant to public health and climate. We seek to develop a molecular level understanding of the fundamental physical chemistry affecting their sources, transformations and sinks using a combination of field observations, laboratory experiments and modeling. Opportunities for graduate study exist as part of funded projects to 1) better understand anthropogenic enhancements of biogenic organic aerosol and new particle formation (laboratory studies), 2) to develop innovative retrievals for CU SOF, apply them to aircraft datasets that characterize wildfires quantitatively for the first time, and test and develop atmospheric models, and 3) to develop long-term datasets of halogen oxide radicals and oxygenated VOC in the remote marine atmosphere."
and
In situ production of methyl bromide, methyl chloride, and carbon disulfide in the GISP2D ice core
Christopher Lee,
ANYL 1st year, CU Boulder
"The mixing ratios of methyl bromide, methyl chloride, carbon disulfide, and carbonyl sulfide were measured via gas chromatography-mass spectrometry (GC-MS) in eleven samples from the GISP2D ice core from Summit, Greenland. The samples range in depth from 1826 meters to 2020 meters and in age from 15000 years BP to 25000 years BP. Correlations between the trace gas mixing ratios and the concentrations of major ions (Na+, NH4+, K+, Mg2+, Ca2+, Cl-, NO3-, and SO42-) in the ice at the same depth are examined. Additionally, correlations between the trace gas mixing ratios and the concentrations of sea salt and non-sea salt components of major ions in the ice at the same depth are examined. Finally, correlations between the trace gas mixing ratios and the concentrations of major ions in the ice at the same age are examined. Due to the significant difference in age (~619 years) between the air trapped in the ice and the ice itself, the same-depth correlations provide evidence for the in situ production of methyl bromide, methyl chloride, and carbon disulfide within the examined depth range. The same-age correlations provide evidence for a contemporaneous connection between carbonyl sulfide and sodium chloride."
Monday, 1 October 18
Investigating the pH of atmospheric fine particles and implications for atmospheric chemistry
Hongyu Guo,
Postdoctoral Researcher, Jimenez lab, CU Boulder
"Particle acidity is a critical but poorly understood quantity that affects many aerosol processes and properties. In this talk, I will introduce a popular pH prediction method in recent years since pH detection technique is limited. Particle pH and water (which affects pH) are predicted using a thermodynamic model and measurements of RH, T, and inorganic gas and particle species. The method was first developed during the SOAS field campaign conducted in the SE US in summer (fine particle pH = 0.94 ± 0.59), and then extended to aircraft observations in the NE US in winter (WINTER study; pH = 0.77 ± 0.96). The results are validated by reproducing particle water and gas-particle partitioning of NH4 + and NO3 - (sensitive to pH). I will show why commonly used pH proxy, ion balance or molar ratio, doesn’t necessarily represent pH. Some impacts of low particle pH were investigated, including the effects on aerosol nitrate trends and the role of acidity in heterogeneous chemistry. Despite a ~70% sulfate reduction in the southeastern US in the last 15 years, the fine particles remained highly acidic due to buffering by semivolatile NH3. Importantly, pH is not highly sensitive to NH3, a 10-fold increase in NH3 only increases pH by one unit in various locations and seasons, which has implications for use of NH3 controls to reduce PM 2.5 concentrations."
Monday, 24 September 2018
Organic nitrogen chemistry: Recent results and future projects
Prof. Ellie Browne,
ANYL faculty, CU Boulder
"Organic nitrogen is a ubiquitous atmospheric component typically accounting for between one-quarter and one- third of reactive nitrogen deposition. The chemical complexity and reactivity of organic nitrogen, however, has made it challenging to study. Consequently, little is known about the atmospheric processing of organic nitrogen and the resulting implications for biogeochemistry, air quality, and climate. Research in my group uses mass spectrometry to identify the organic nitrogen compounds present in the atmosphere and to investigate the atmospheric processing of these compounds. In this talk, I will discuss our recent work on organic nitrogen chemistry and describe new projects on aerosol measurement and chemical transport modeling."
and
Chemistry of Volatile Organic Compounds in the Atmosphere
Prof Joost de Gouw,
ANYL faculty, CU Boulder
"Volatile organic compounds (VOCs) are released from many different natural and man-made sources to the atmosphere. VOCs are removed by different oxidants on time scales of minutes to months with oxidized VOCs, ozone and fine particles as a result. These processes affect air quality and climate and are a challenge to understand due to the large number of different VOCs that are released to the atmosphere and the analytical difficulties in measuring all of these compounds as well as their oxidation products.
In our laboratory, we make measurements of VOCs by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF) and gas chromatography mass spectrometry (GC-MS). PTR-TOF allows measurements of many different VOCs with high time resolution and without the need for pre-separation or sample treatment. GC-MS allows higher chemical detail, but at the cost of time resolution. We also combine these methods to better understand the compounds that are detected by PTR-TOF in different environments.
Several different ongoing and future projects will be presented in this seminar. First, we recently acquired a new Vocus PTR-TOF and are characterizing and preparing this instrument for measurements of indoor air in the CU Athletic Center. This will allow quantification of VOCs released from student athletes as well as during pre-game, indoor tail-gate parties. Second, we are working on the emissions and chemistry of VOCs released from volatile chemical product (VCP) use to the atmosphere, which was recently discovered to be the dominant source of VOCs in urban air. This research will involve the development of new analytical capabilities, field measurements in Boulder and in Los Angeles, and laboratory work to better understand the chemistry of VCPs. Finally, we are working on a chamber study to better understand the formation of secondary organic aerosol from biogenic VOCs."
Monday, 17 September 2018
Photochemical and Dark Ageing of Organic Aerosols
Prof. Sergey Nizkorodov
Department of Chemistry, University of California, Irvine
"Atmospheric aerosols significantly affect air quality, visibility, and global climate. Organic compounds make up a significant, and often dominant, fraction of the atmospheric particulate matter. Primary organic aerosol is emitted in the atmosphere by various sources, and secondary organic aerosol is produced directly in the atmosphere as a result of a complex sequence of reactions that start with the oxidation of volatile organic compounds and end with the condensation of the low-volatility products into particles. What makes the representation of organic aerosols in climate and air quality models challenging is their astonishingly high degree of chemical complexity. Furthermore, the chemical composition of organic aerosols continuously changes as a result of various “ageing” processes, such as photolysis, hydrolysis, oligomerization, oxidation, and other reactions involving aerosol constituents and atmospheric gases. This presentation will examine the role of condensed-phase photochemical processes in the aerosol ageing, i.e., processes initiated by absorption of solar radiation by an organic compound within a particle or cloud/fog droplet. If time permits, we will also discuss “dark” ageing processes, which occur without any involvement of solar radiation and free radicals, and result in the formation of compounds with unusual properties, such as organic compounds capable of absorbing visible radiation (so called “brown carbon”)."
Monday, 10 September 2018
Molecules to particles – Experiments and simulations relevant to new particle formation in the marine atmosphere
Henning Finkenzeller
ANYL 4th year, Volkamer lab
"In this talk, I present my projects within the CLOUD consortium. CLOUD is an atmospheric simulation chamber at CERN, Geneva, Switzerland, to study the formation and growth of new particles in urban, rural, marine, and free tropospheric environments. In particular, iodic acid (HIO3) is thought to be a key precursor for new particle formation in the marine atmosphere, but the sources of HIO3 are currently unknown. Condensation of HIO3 is thought to be the primary mechanism by which iodine forms new particles at Mace Head, Ireland, and possibly other marine environments (Sipilä et al., 2016).
I have adapted the Volkamer group iodine chemistry box model to investigate known sources of HIO3 (e.g., OIO + OH) and inform missing formation pathways. In combination with theory, I have incorporated new gas-phase reactions into the model, and compared the predictions with CLOUD observations. As pre-requisite for modeling CLOUD experiments, I have developed the photolysis module (irradiation by different lamps), temperature and humidity control, the injection of precursor gases, dilution and losses to the wall. The experimental conditions (precursor and intermediate species concentrations) can be prescribed, or taken as those observed in the chamber.
I have further developed the Model for Acid Base Chemistry and Nanoparticle Growth (MABNAG) to represent reactive uptake due to Setschenow salting-in of glyoxal (CHOCHO). Glyoxal is a volatile, ubiquitous, and putatively simple gas that forms from the oxidation of aromatic hydrocarbons, isoprene, and also non-traditional precursors, e.g., fatty acids. Upon contact with wet and sulfate containing particles glyoxal-hydrate-sulfate complexes form that have extremely low volatility. I am examining the contributions to the later stages of particle growth, and am developing a glyoxal source for future experiments at CLOUD."
Summer 2018
Thursday, 19 July 2018 (Supergroup)
Secondary Organic Aerosol Production from Local Emissions Dominates OA Budget over Seoul, South Korea, during KORUS-AQ
Ben Nault,
ANYL Postdoc, Jimenez lab
"Organic aerosol (OA) is an important fraction of submicron aerosols. However, it is still challenging to predict and attribute which organic compounds and sources lead to the observed OA, including over megacities. This can especially be true for megacities surrounded by numerous other regional sources that create an OA background. Here, we utilize in-situ gas and aerosol observations collected on-board the NASA DC-8 during the NASA/NIER KORUS-AQ (KORea United States-Air Quality) campaign to investigate the sources and hydrocarbon precursors that led to the SOA production observed over Seoul. First, we investigate the role of background and transport of OA into Seoul, using observations over the West Sea and WRF-Chem FLEXPART simulations. During KORUS-AQ, we conclude that the average OA advected into Seoul was ~1-3 µg sm–3. Then, taking this background into account, the dilution-corrected SOA concentration observed over Seoul was ~140 µg sm–3 ppmv–1 at 0.5 equivalent photochemical days. This value is comparable, though higher, than what has been observed in other megacities around the world (20–70 µg sm–3 ppmv–1 at 0.5 equivalent days). For the average OA observed over Seoul (13 µg sm–3), it was found that the local production of secondary OA (SOA) overwhelmed the transport/background of OA from foreign sources. The role of local SOA production was further supported by the following methods: (1) WRF-Chem FLEXPART source contribution calculations indicate any hydrocarbons with a lifetime less than 1 day, which are shown to dominate the observed SOA production, mainly originate from South Korea. (2) SOA correlated strongly with other secondary photochemical species, including short-lived species (formaldehyde, peroxy acetyl nitrate, sum of acyl peroxy nitrates, and dihydroxy toluene). (3) Results from an airborne oxidation flow reactor (OFR), flown for the first time, show a factor of 4.5 increase in potential SOA concentrations over Seoul versus over the West Sea, a region where background air masses that are advected into Seoul can be measured. (4) Results from a box model suggest that short-lived hydrocarbons (i.e., xylenes, trimethylbenzenes, semi- and intermediate volatility compounds) were the main SOA precursors over Seoul. Toluene contributes 9% of the modeled SOA over Seoul. Along with these results, we introduce using ΔOA/ΔCO2, which provides an insight into the amount of OA produced per fuel consumption in a megacity, and which shows less variability across the world than ΔOA/ΔCO. In summary, although long-distance transport was found to influence OA in Seoul, local emissions and rapid SOA production was found to dominate the total OA concentrations during KORUS-AQ. Seoul appears to have has larger relative emissions of SOA precursors compared to other megacities, which could be targeted for air quality improvement. This study provides important insight and constraints on the SOA production over large megacities."
and
Measurements of Positive Ambient Ions in Lamont OK as Part of the Holistic Interaction of Shallow Clouds Aerosols and Land Ecosystems (HISCALE II) Field Campaign
Aroob Abdelhamid,
ANYL Graduate Student, Browne lab
"Atmospheric ions control the electrical properties of the atmosphere, influence chemical composition via ion-molecule and/or ion-catalyzed reactions, and affect new particle formation. Understanding the role of ions in these processes requires knowledge of ionic chemical composition. Due to the low concentration of ions, chemical composition measurements have historically been challenging. Recent advances in mass spectrometry, such as the atmospheric pressure interface time-of- flight mass spectrometer (APi-TOF), are now making these measurements more feasible. Here, we present measurements of ambient cations during the HISCALE II field campaign (August- September 2016) in Lamont, OK. We discuss how the chemical composition of cations varies over the course of the campaign and what patterns from this data tell us about the role of organic nitrogen. Furthermore, preliminary results of the anion data will be discussed."
Thursday, 19 July 2018
Role of Multiphase Chemistry on the Formation of Aerosol from the Reactions of Monoterpenes with NO3 Radicals and O3
Megan Claflin,
ANYL Student, CU Boulder, Ziemann lab
"Secondary organic aerosols (SOA) have been shown to influence regional and global air quality, climate, and human health. To predict and mitigate the impacts of SOA formation, it is essential to better understand the detailed mechanisms of formation, atmospheric processing, and physical properties of SOA. In this thesis, the gas-and particle-phase reaction products and mechanisms involved in the formation of SOA from the oxidation of monoterpenes in an environmental chamber were studied in an extensive series of laboratory experiments that employed a variety of online and offline analytical methods. In addition, a limited selection of laboratory-generated samples and ambient aerosol samples collected from the Southeast US were analyzed to compare the utility two different methods of functional group analysis. The research included four major studies. 1) Identification and quantification of the products that form SOA from the reaction of β-pinene with NO3 radicals. The results highlight the importance of ring-opening, alkoxy radical decomposition, and oligomerization reactions, and were used to develop gas- and particle-phase reaction mechanisms. 2) A detailed study of the thermal desorption characteristics of hemiacetal and acetal oligomers for use in understanding mass spectra obtained from the analysis of SOA containing these components. Oligomers formed by a single hemiacetal linkage, when subjected to thermal desorption analysis, will decompose to their precursor aldehyde and alcohol monomers prior to desorption and detection. Oligomers formed by more than one of these linkages, or an acetal or ester linkage, will desorb and be detected intact. 3) Quantification of the functional group composition of SOA formed from α- pinene ozonolysis over a range of α-pinene concentrations and humidities, including autoxidation conditions. The SOA analyses, when combined with results of modeling, provide insight into the effects of RO2• radical reaction regime, humidity, and particle-phase reactions in determining SOA composition. 4) Comparison of two methods for quantifying the functional group composition of organic aerosol: the Fourier Transform Infrared spectroscopy method developed by the Russell group at the Scripps Institute of Oceanography and a derivatization- spectrophotometric method developed by the Ziemann group at the University of Colorado, Boulder. Results for laboratory-generated SOA and ambient aerosol samples show that the two methods agree quite well when results for certain functional groups are combined, and that either is adequate for measuring SOA functional group composition. This study also demonstrates that the functional group composition of SOA can help in elucidating the sources and environmental conditions under which the SOA was formed."
Thursday, 21 June 2018
Vapor-Liquid Equilibria Pertaining to the Study of Alternative Fuels and the Forensic Analysis of Chemical Evidence
Megan Harries,
ANYL Student, CU Boulder / NIST
"Measurement of the vapor-liquid equilibrium (VLE) of fluid mixtures with many components presents a challenge. Data describing such mixtures, like fuels, are important for the development of alternative energy sources and to support forensic science, but there is a lack of suitable instrumentation to provide data with reasonable uncertainty for mixtures with many components. In this thesis, three different techniques for fluid characterization are explored: the Advanced Distillation Curve (ADC), the Advanced Distillation Curve with Reflux (ADCR), and PLOT-cryoadsorption.
Two pyrolysis fuels similar to gasoline and diesel fuel made from polypropylene were studied with respect to volatility, composition, and energy content using the Advanced Distillation Curve. The diesel fuel demonstrated volatility very similar to existing petroleum-derived diesel fuels. The gasoline was less volatile than existing counterparts and did not meet specifications. Two pyrolysis crude oils made from ponderosa pine and dairy manure were assessed using the ADC coupled to an approach for characterizing fluids with multiple, immiscible liquid phases. Both oils contained high water levels and require further refinement. The organic phases of each oil contained components indicative of the feedstock used.
A modification of the ADC method, the Advanced Distillation Curve with Reflux, was introduced as an approach to measuring the VLE of fluids with many components. ADCR additionally approximates the weathering of an ignitable liquid that occurs during an arson fire and measures VLE across a range of weathered conditions. The method was demonstrated using two simple mixtures. The measurements agreed well with models, indicating that ADCR is a suitable VLE metrology. Vapor-liquid equilibrium data are crucial for interpreting the results of headspace characterization used often in forensic science. One headspace method, portable PLOT-cryoadsorption, was tested in a series of experiments in the laboratory, then deployed for the first time in a field environment that simulated a cargo container. The technology was found to be rapid and sensitive to a variety of compounds of interest to forensic science. Each of the three techniques described in this thesis contribute valuable property data for multicomponent mixtures, towards the development of high-quality predictive models."
Thursday, 10 May 2018
Measurements and Modeling of Nitrogen Oxides: Tropospheric Transformations During Summer and Winter in Polluted Regions Across the U.S.
Erin McDuffie
ANYL Student, CU Boulder
"Atmospheric reactions of inorganic nitrogen oxides critically influence the composition of the troposphere, the lowest layer of the atmosphere that supports all terrestrial life on Earth. From controlling the global budget and distribution of tropospheric oxidants, to degrading local air quality through the production of ozone (O3) and secondary particulate matter (PM), understanding the underlying chemistry of reactive nitrogen oxides is vital to both improving our predictive capabilities of global tropospheric chemistry and to developing effective mitigation strategies in regions with persistently poor air quality. Despite decades of research into their chemical mechanisms, significant uncertainties remain in the seasonally dependent lifetime and distribution of nitrogen oxides. Key remaining questions include: 1) the sensitivity of photochemical pollutant production to location-specific emission sectors, 2) factors influencing nocturnal inter-conversion processes, which involve multiphase reactions, and 3) the quantitative contribution of these heterogeneous reactions to wintertime air pollution.
In this thesis, I address these questions using observational and modeling-based analyses of data collected during three U.S. field campaigns in summer 2014 and the winters of 2015 and 2017. I first present observations from summer 2014 and results from an observationally- constrained, photochemical box model. This study was the first to quantify the contribution of oil and natural gas emissions to local O3 pollution in the Colorado Front Range, a region that has been out of compliance with national air quality standards for O3 since 2008. I next present the first wintertime aircraft determinations of aerosol uptake coefficients of dinitrogen pentoxide (N2O5) and production yields of nitryl chloride (ClNO2). These parameters were derived from a custom, iterative, inorganic nocturnal nitrogen chemistry box model, fit to aircraft observations collected over the U.S. east coast in 2015. Field-determinations of these parameters are further compared to laboratory-based parameterizations to evaluate the current representation of these processes in global models. Lastly, I present results from the first aircraft observations in Salt Lake Valley, Utah. Observations and box model simulations are combined to assess the contribution of heterogeneous reactive nitrogen chemistry to wintertime PM formation in this region, which frequently violates PM air quality standards during wintertime pollution events."
Spring 2018
Monday, 30 April 2018
Inhalation and Sublingual Delivery of Medical Cannabinoids and Vaccines
Robert Sievers,
University of Colorado, Environmental Program
"The development of cannabis products that conform to pharmaceutical level quality standards would be of great benefit to those attempting to use cannabis for medicinal purposes. Currently, the cannabis industry generates products that vary substantially in consistency of dosing, time of onset, and safety of administration route. The bioavailability of these products is not optimal due to a combination of inefficient absorption, first pass metabolism effects, and cannabinoid degradation. To combat these issues, we have developed a cannabinoid-containing dry powder that utilizes isolated, highly purified cannabinoids and excipients that are generally regarded as safe (GRAS) or have recently been determined in clinical trials of formulations to be safe for inhalation. The powder is suitable for respiratory delivery from a simple dry powder inhaler (DPI). Delivery to the lungs in this manner provides a consistent dose with a rapid onset of effects and avoids the bioavailability issues and first-pass metabolism encountered with other methods of administration. Various cannabinoids can also be combined in customized ratios targeted for the treatment of specific pathologies. A new US Patent #9,895,321 to the five authors (Sievers; Robert E., Cape; Stephen P., McAdams; David, Manion; J'aime, Pathak; Pankaj) was issued on February 20, 2018. Authors: Robert Sievers, Lia Rebits, Xuno Gildelamadrid"
and
Observations of particle organic nitrates from airborne/ground platforms: Insights into method improvement, vertical/geographical distribution, gas/particle partitioning, losses, and contribution to total particle nitrate
Doug Day,
University of Colorado, ANYL Research Scientist
"Organic nitrate formation in the atmosphere represents a sink of NOx and termination of the HOx /NOx ozone formation cycles, can act as a NOx reservoir transporting reactive nitrogen, and contributes to secondary organic aerosol formation. However, particle-phase organic nitrates (pRONO2) are rarely measured and thus poorly understood. Due to the increasing prevalence of aerosol mass spectrometer (AMS) field measurements and promise of its use in determining quantitative bulk organic nitrate functional group contribution to aerosols, a detailed evaluation of quantification methods is timely. A simple method that relies on the relative intensities of NO + and NO2 + intensities in the AMS spectrum, calibrated NOx + ratio for NH4NO3 , and inferred ratio for pRONO2 has been previously proposed as a way to apportion the total nitrate signal to NH4NO3 and pRONO2, and been used by several groups using a variety of different methods and assumptions. An extensive survey of NOx + ratios measured for various pRONO2 compounds and mixtures from multiple instruments, groups, and laboratory and field measurements shows that, in the absence of a pRONO2 standard, the pRONO2 NOx + ratio can be estimated using a ratio referenced to the calibrated NH4NO3 ratio, a so-called Ratio-of- Ratios (RoR). We systematically explore the viability, accuracy, and errors associated with quantifying pRONO2 with the AMS RoR NOx + ratio method using ground and aircraft field measurements conducted over a large range of conditions. Positive Matrix Factorization (PMF) of thermal denuder measurements was conducted to further explore the efficacy of the RoR NOx + ratio method and to construct volatility basis sets (VBS) of pRONO2 for several campaigns. A broad survey of ground and aircraft AMS measurements, applying the RoR NOx + ratio method, shows a pervasive trend of higher contribution of pRONO2 to total nitrate with lower total nitrate concentrations.
Simultaneous measurements of pRONO2 (applying the AMS RoR NOx ratio method) and of total (gas+particle)
organic nitrate (totRONO2), organic aerosols (OA), and ammonium nitrate from aircraft and several
ground campaigns were used to investigate vertical/geographical distributions, gas/particle partitioning,
losses, and contributions to total particle nitrate (pTotNO3) over North America. pRONO2 and totRONO2
concentrations show strong vertical gradients, with a steep decrease from the top of the boundary layer
(BL) up through the residual layer. However, pRONO2 was 10-30% of totRONO2 with little vertical
gradient in gas/particle partitioning from the BL to upper troposphere (UT). pRONO2 contribution to OA
shows a moderate increase with decreasing OA in the BL and free troposphere (~2-3% by mass of nitrate
group) with higher contributions at the lowest OA (5-8%), mostly observed in the UT. In the BL, RONO2
gas/particle partitioning shows a trend with temperature, with higher particle-phase fraction at lower
temperatures, as expected from partitioning theory. However, the temperature trend is much weaker
than for single compound partitioning, which may be due to a broad mixture of species. Little to no
dependence of pRONO2 /OA on RH or estimated particle water was observed in the BL, suggesting that
losses of pRONO2 due to hydrolysis are too rapid to observe in this dataset and there may be a
substantial fraction of pRONO2 species that are not prone to rapid hydrolysis."
Wednesday, 25 April 2018
Chemistry on Mars: The Search for Habitable Environments with Curiosity
Melissa Trainer,
Planetary Environments Laboratory, NASA Goddard Space Flight Center
"Following on decades of exploration of Mars, our knowledge of our neighboring planet has
advanced well beyond observations of canals to the comprehensive characterization of surface
topology and regional mineralogy. There are clear lines of evidence for past liquid water and a
complex climate history. Yet some of the fundamental questions remain: Was there ever life on
Mars? Could there have been life on Mars? The Curiosity rover carries the most advanced
analytical laboratory sent to another planet, and over the past four and half years the mission
has performed a detailed in situ investigation of Gale Crater. The Sample Analysis at Mars
(SAM) instrument suite in particular has quantified geochemical indicators that demonstrate
the environment could have supported life, and has achieved detection of the first organic
molecules on Mars. Atmospheric measurements by SAM have identified signatures of planetary
change over billions of years and monitored modern activity. This presentation will recount the
most important findings on the chemistry of Mars to date, and will discuss the implications for
our understanding of whether the red planet was ever habitable."
Tuesday, 24 April 2018
Laboratory Studies of Planetary Atmospheres and Organic Hazes
Melissa Ugelow,
ANYL Student CU Boulder
"Atmospheric organic hazes are present in many planetary and satellite atmospheres, possibly including the ancient Earth. Haze composition and how a haze influences surface and atmospheric processes will greatly depend on the atmospheric composition of the planetary body. Therefore, laboratory studies are necessary to determine these atmosphere specific haze properties. This thesis focuses on the chemical, optical and physical properties of Titan and Archean Earth organic haze analogs, along with gas phase neutral and ion measurements during haze analog formation.
<p>Titan haze analogs were formed by ultraviolet (UV) excitation and spark discharge excitation of various concentrations of methane in nitrogen in a flow through reactor. The optical properties of these hazes were measured as a function of methane concentration and were found to have increasing light absorption with increasing aromatic and nitrogen content. To monitor the gas phase during haze analog formation, a new recirculating reactor was used. The concentration of smaller chained hydrocarbons and nitriles, and the isotopic fractionation of carbon in the methane and evolved ethane, was measured as a function of reaction time. Both methane and ethane become enriched in 13C relative to the starting gas mixture.
Archean Earth haze analogs were formed by UV excitation of methane, carbon dioxide, nitrogen and increasing amounts of molecular oxygen in a flow through reactor. As precursor molecular oxygen increases, the particles become more oxidized and non-absorbing. Therefore, haze produced in an oxygen containing atmosphere could form a non-absorbing haze.
Moreover, since Titan’s haze is influenced by ion-neutral chemistry, it is possible similar chemistry occurred in the Archean Earth’s atmosphere. Archean Earth haze analog production and negative ion concentrations were found to be inversely related, with aerosol mass loading decreasing with increasing precursor molecular oxygen. Additionally, the nitrogen in the ions switches from mainly organic nitrogen to inorganic nitrogen with increasing precursor molecular oxygen, possibly indicative of the chemistry that occurred during the rise of oxygen in Earth’s atmosphere. Due to the differences in haze formation and haze properties based on precursor gases, the results of this thesis demonstrate the importance of considering the atmospheric species present during haze formation."
Monday, 23 April 2018
Mechanistic modeling of reactive soil nitrogen emissions on a continental scale
Quazi Ziaur Rasool
Rice University
"Nitrogen is an essential building block of all proteins and thus an essential nutrient for all life, including crops. Biological Nitrogen Fixation is the natural source of soil nitrogen available for biogeochemical transformations. However, anthropogenic perturbation to nitrogen cycle through the combustion of fossil fuels and consistently increasing fertilization is now larger than natural sources in the United States and globally. Recent global nitrogen budgets estimate that soil reactive nitrogen (Nr) emissions (predominantly from biochemical transformations in soil) have increased by a factor of 2-3 from pre-industrial levels. These increases are especially pronounced in agricultural regions. These emissions from biogeochemical transformations can be in reduced (NH3) or oxidized (NO, HONO, N2O) form, depending on complex biogeochemical transformations of soil nitrogen reservoirs.
Reactive nitrogen in the atmosphere is a precursor for ozone and particulate matter formation and contributes to nutrient loading by being washed out by precipitation and the deposition of atmospheric nitrogen gases and aerosols. Until recently, little progress has been made in modeling of the cascade of nitrogen from soil to the atmosphere due to the complexity of and uncertainty in its transport and transformation. The lack of understanding of these multimedia transport processes is due to the typical focus of research on specific media and the difficulty in parameterizing the anthropogenically fixed nitrogen and their input into the atmosphere, primarily through mineral fertilizer application to crops, the largest source of environmental reactive nitrogen.
This talk will focus on modeling of the exchange of gaseous nitrogen species between the soil and the
atmosphere, with an emphasis on Nitrogen oxides (NO, HONO). Contemporary air quality models like U.S.
EPA’s Community Mulitscale Air Quality (CMAQ) model, typically neglect soil emissions of HONO and N2O.
Previous soil nitrogen parametrizations in CMAQ have focused on NO emissions only and in a manner
inconsistent with how soil NH3 emissions (i.e. accounting for anthropogenically fixed nitrogen from fertilizer
application and atmospheric deposition). Thus, there is a need to more mechanistically and consistently
represent the soil N processes that lead to emissions to the atmosphere. The new mechanistic scheme
addresses the spatial-temporal variability of different reactive nitrogen emissions from soil through complex
transport and transformation of soil nitrogen pools in both agricultural and non-agricultural soils. The CMAQ
model with a new mechanistic scheme for modeling reactive nitrogen emissions from soil will be described
and evaluated against observations of atmospheric particulate matter and NOx emissions. The use of
multimedia and biome-specific measurements to constrain model parameters, and how this can improve
continental scale (Continental US) models will be presented. These findings will be presented with an
emphasis on the sensitivity of the modeling system to different air-soil exchange parameterizations and how
the representation of these emissions can be improved."
Monday, 16 April 2018
Optical Properties of Absorbing Organic Aerosol
Kevin Jansen,
CU Boulder, ANYL 3rd year
"Until relatively recently, it has been assumed that organic aerosol only scatters light, thereby having a negative radiative forcing and global cooling effect. However, aerosol formed from the aqueous-phase reaction of small di-carbonyl compounds, such as glyoxal and methylglyoxal, with ammonium salts have the potential to form light-absorbing brown carbon (BrC) aerosol. Studies of BrC formation mechanisms and optical properties have been primarily preformed using bulk-aqueous solutions, although bulk-phase studies are not perfect simulations of reactions occurring within aerosol particles. In order to characterize BrC aerosol formed in the aerosol phase, we utilize Cavity ring-down (CRD) and Photoacoustic spectroscopies (PAS) to monitor the absorption and extinction of BrC aerosol formed from reactions of glyoxal and ammonium sulfate aerosol within reaction chamber. The PAS allows for the detection of BrC absorption even under conditions in which a few μg/m3 of weakly absorbing material is made. In addition, depending on the RH of the aerosol and if the aerosol was exposed to light, we observed differing losses in absorption by the BrC aerosol, which may indicate that BrC persists longer in the atmosphere than predicted from bulk phase experiments."
Tuesday, 10 April 2018
Chemistry of Volatile Organic Compounds in a Changing Atmosphere
Joost de Gouw,
CU Boulder
"Volatile organic compounds (VOCs) in the atmosphere can react to form important pollutants such as
ozone and secondary organic aerosol and can also have direct effects on human health. In this seminar, I
will present several new insights into the sources and chemistry of VOCs in urban air, from oil and
natural gas production and in biomass burning emissions.
Emissions of VOCs from motor vehicles have strongly declined for decades and as a result, other
emission sources such as from the use of volatile chemical products (e.g. cleaners, glues, coatings,
solvents and personal care products) have become more important in urban air. I will show how
measurements in urban air can be used to determine emissions of reactive VOCs, despite the fact that
they can be removed and/or formed in between the time of emission and sampling. As many volatile
chemical products are used inside buildings, I will show how measurements of indoor air can be used to
determine emissions.
Electric power generation by wind and solar is expanding rapidly, but the use of natural gas
power plants to make up demand will likely remain in the foreseeable future. The production of natural
gas in the U.S. is currently at an all-time high. Methane emissions associated with this activity have
received much attention, because they offset the climate benefits of this lower-carbon fuel. Less
attention has been paid to the emissions of air pollutants such as VOCs and nitrogen oxides (NOx). Using
results from airborne measurements during the NOAA SONGNEX campaign, I will show that a significant
fraction of VOC emissions over the lifecycle of oil and natural gas takes place during production. Using
remote sensing measurements made from the Ozone Monitoring Instrument onboard the NASA Aura
satellite, I will show that both the drilling of new wells as well as the extraction of fossil fuels that follows
contribute to emissions of NOx.
Wildfires in the U.S. have become more frequent and extensive during a longer wildfire season.
Due to the complexity of fuel composition and burning conditions, biomass burning emissions are
among the most challenging to analyze chemically, which makes it difficult to describe the atmospheric
fate and health effects. Measurements of VOCs from biomass burning emissions were made at the Fire
Sciences Laboratory in Missoula, MT during the NOAA FIREX study. I will show how different processes
such as distillation and pyrolysis can explain the composition of VOC emissions for different fuels and
phases of a burn. I will also show the results from laboratory experiments aimed at elucidating the
chemistry of functionalized aromatic compounds that are common from biomass burning.
Finally, I will briefly discuss the development and characterization of new instrumentation for
VOC measurements that will likely lead to new discoveries in our science."
Monday, 9 April 2018
Ecosystem-atmosphere fluxes of volatile organic compounds: Which ones matter?
Dylan Millet,
University of Minnesota
"Volatile organic compounds (VOCs) play several key roles in the atmosphere: their oxidation leads to the formation of health- and climate-relevant pollutants; they affect the nitrogen cycle by interacting with atmospheric NOx; and they modulate the atmosphere’s oxidizing capacity and therefore the lifetimes of greenhouse gases and other pollutants. Terrestrial ecosystems are simultaneously the largest source and a major sink of atmospheric VOCs; however, our understanding of these fluxes and their atmospheric impacts is challenged by a number of outstanding uncertainties, including complex emission sources, bidirectional exchange with the land surface, and chemical interactions with anthropogenic pollutants. In this talk I will present results from my group’s research applying observations to better characterize these ecosystem-atmosphere interactions and their effects on atmospheric chemistry. Discussion will focus on two specific themes. I will first present results focusing specifically on formic acid, which is a major source of atmospheric acidity and an integrated marker of hydrocarbon oxidation, but which has large missing sources. I will then discuss new measurements from my group using high-resolution time-of-flight mass spectrometry to directly measure forest-atmosphere fluxes of VOCs simultaneously across the entire mass spectrum, and explore these results with the aim of better understanding i) how well our models capture this 2-way land-atmosphere carbon exchange, and ii) to what degree the fluxes for the large number of ions outside of the traditionally-measured subset matter for tropospheric composition."
Monday, 2 April 2018
The peroxy radical chemistry that drives atmospheric nano-particle growth
Joel Thornton
University of Washington
"Organic carbonaceous material is a ubiquitous and often significant fraction of atmospheric particulate mass, and can be responsible for driving the growth of atmospheric nanoparticles formed from nucleation up to cloud condensation nuclei sizes. Development of a molecular-level understanding of the processes governing nano-particle growth by organic condensation has been a long running challenge. I will present new insights into to the chemistry of biogenic hydrocarbons that contribute to this process, using both in situ observations as well as controlled simulation chamber studies. An underlying theme is the role novel instrumentation techniques have had recently in allowing direct observation and quantification of a wide suite of organic molecules and chemical processes contributing to particle growth."
Monday, 19 March 2018
Separation of NOx emissions from drilling, and oil and gas extraction in the U.S. using monthly data from the Ozone Monitoring Instrument
Joep de Bruin
Visiting graduate student, de Gouw lab, CU Boulder
"Hydraulic fracturing and horizontal drilling have increased unconventional oil and gas extraction from shale reserves in the US in the last decade, making up half of total US oil and gas production at present. This activity results in NOx emissions in the extraction regions that are measurable from space using the Ozone Monitoring Instrument (OMI) on the NASA Aura satellite. The NOx emissions are a result of two different processes: (1) the drilling and hydraulic fracturing of new wells, and (2) the extraction of oil and gas after the well is completed. To distinguish the effects of drilling and extraction on the NOx emissions, a multiple linear regression to the NO2 columns as a function of time is calculated for 9 extraction regions using the number of drilling rigs and the oil and gas production data from 2007 until 2018. In 3 regions (Permian, Bakken, Eagle Ford) a significant correlation between measured and calculated NO2 columns is found, of which the Permian region shows the highest correlation. The analysis shows that half of the total NOx concentration in this region can be attributed to emissions from oil and gas processes, and that both the drilling and extraction processes have an equal share in these emissions. In other extraction regions, NO2 columns show poor correlation with the oil and gas activity due to the proximity of urban areas (Barnett, Denver-Julesburg regions), power plants (San Juan) or variations in the drilling and extraction activity over time that are too small (Uintah, Upper Green River)."
Monday, 12 March 2018
Diffusion of organics in secondary organic aerosol
Allan Bertram
University of British Columbia
"Secondary organic aerosol (SOA) can modify Earth’s climate by scattering and absorbing solar
radiation and by modifying the properties of clouds. SOA can also negatively affect air quality
and reduce visibility in urban environments. To predict the role of SOA in climate, air quality
and visibility, a good understanding of the diffusion of organics within SOA particles is required.
Through a series of laboratory studies, we have shown that the diffusion rate of organics within
SOA particles depends strongly on several factors including the relative humidity and the
precursors used to generate SOA. More recently, we have used these results to estimate the
mixing times of organics within SOA particles in the planetary boundary layer and free
troposphere."
Monday, 5 March 2018
International air quality, health, and climate impacts of cookstoves, diesel NOx, and other anthropogenic sectors via PM2.5 and O3
Daven Henze
CU-Boulder Mech. Eng.
"Diesel cars, trucks, and buses produce ~70% of global land transportation emissions of nitrogen oxides (NOx), a key PM2.5 and ozone precursor. Globally over 3 billion people presently use solid fuel for meal preparation. What are the impacts of these activities on the environmental through atmospheric chemistry and transport? Which species dominates the local and long-range health impacts of air pollution? I will first discuss the use of models and remote sensing measurements to evaluate the domestic and international contributions to PM2.5 and O3, and their impacts on human health and climate. Source-receptor relationships are developed using adjoint sensitivity analysis, constraints from remote sensing observations, and parameterized climate model sensitivities. This talk will then delve into application of these relationships to estimate impacts of diesel NOx emissions standards and solid fuel use in major markets and source regions worldwide. We find that the per-cookstove impacts on ambient air quality and global temperature changes are pronounced in several countries not typically targeted in cookstove mitigation efforts (e.g., Ukraine and Romania). We also show that real-world diesel NOx emissions in 11 markets representing ~80% of global diesel vehicle sales are significantly higher than certification limits indicate. This excess NOx contributed an estimated ~39,000 additional ozone- and PM2.5-related premature deaths globally in 2015, with a larger portion of this owing to excessive emissions from heavy duty vehicles than from defeat devices on light duty vehicles. Lastly, we present recent evaluation of the premature deaths and preterm births associated with global O3 exposure, showing that the former is possibly several times larger than previously expected, rivaling the health impacts of PM2.5 in severity."
Monday, 26 February 2018
Chemical Composition of Positive Ions During Laboratory Simulations of Titan’s Haze Formation
Jennifer Berry
CU Boulder
"Titan, one of Saturn’s moons, is the only other planetary object in our solar system with a thick nitrogen atmosphere. Titan’s haze formation is initiated by energetic electrons and UV photons leading to a complex series of ion-neutral and radical reactions that result in high molecular weight hydrocarbons and nitrogen containing species. A long history of laboratory and modelling studies of ion-neutral reactions and haze particle composition exist, but there has been little experimental work on the role of ions on haze production. Here, we use an Aerodyne/Tofwerk Atmospheric Pressure interface Time-of-Flight Mass Spectrometer (APi-ToF-MS) to measure the ion chemical composition and relative abundance during the formation of haze analogs initiated by irradiating CH4 in N2 by a deuterium lamp. The instrument’s high resolution (R>7000) allows us to detect multiple ions at the same unit mass, leading to clear identification of hydrocarbons and nitrogen containing compounds with the same nominal mass. Families of CxHy+, CxHyNw+, CxNw+, and HyNw+ are detected with increasing saturation at higher masses during the irradiation of CH4/N2 mixtures. Nitrogen incorporation into compounds could hold significance in determining the prebiotic chemistry occurring on Titan."
Monday, 12 February 2018
Assessing the Sources of Elevated Front Range Ozone Based on
Observations from the Boulder Atmospheric Observatory
Emily Fischer
Colorado State University
"The Denver Metro/North Front Range area currently violates the National
Ambient Air Quality Standard (NAAQS) for ozone. I will discuss
observations of ozone, its precursors, and other secondary species
collected at the Boulder Atmospheric Observatory during summer 2014
and 2015. My group has used our observations to 1) untangle the
contribution of different classes of volatile organic compounds to ozone
production in our region, and 2) identify how the addition of aged wildfire
smoke can change the abundance of ozone precursors."
Monday, 21 January 2018
Atmospheric Chemistry of Volatile Organic Compounds
Joost de Gouw
CIRES & Department of Chemistry and Biochemistry
"Volatile organic compounds (VOCs) are released to the atmosphere from many different sources, both natural and man-made. In the atmosphere, VOCs are removed on time scales of minutes to months and this chemistry leads to formation of ozone and organic aerosol, two air pollutants that also affect climate. We have studied the emissions and chemistry of VOCs using a combination of field and laboratory measurements with mass spectrometry and gas chromatography. I will present some examples of the projects that I have worked on with graduate students over the years and of future research directions."
and
Every Drop Counts…Looking for Water on Mars
Maggie Tolbert
CIRES & Department of Chemistry and Biochemistry
"Mars is a cold, dry planet where pure liquid water is not stable. However, recent
observations of “recurring slope lineae” (RSL) on Mars may be evidence of current liquid
water flows. Several different hygroscopic salts are known to exist in the Martian soil,
and deliquescence of those salts could provide small amounts of liquid water temporarily.
Our research group uses Raman microscopy and an environmental cell to probe the
conditions under which such liquid brines can form and persist under low Martian
temperatures. In addition, an optical trap is used to probe phase changes of individual
levitated droplets. In this talk, I will discuss laboratory results for the brine-forming
ability of several different Mars-relevant salts and salt mixtures and implications for
water on Mars."
Fall 2017
Monday, 4 December 2017
Measurements of Positive Ambient Ions as Part of HISCALE II Field Campaign
Aroob Abdelhamid
CU Boulder, ANYL 4th year
"Atmospheric ions control the electrical properties of the atmosphere, influence chemical composition via ion-molecule and/or ion-catalyzed reactions, and affect new particle formation. Understanding the role of ions in these processes requires knowledge of ionic chemical composition. Due to the low concentration of ions, chemical composition measurements have historically been challenging. Recent advances in mass spectrometry, such as the atmospheric pressure interface time-of- flight mass spectrometer (APi-TOF), are now making these measurements more feasible. Here, we present measurements of ambient cations during the HISCALE II field campaign (August- September 2016) in Lamont, OK. We discuss how the chemical composition of cations varies over the course of the campaign including before, during, and after new particle formation events. We specifically focus on the composition of organic nitrogen ions due to the potential importance of these compounds in atmospheric nucleation. We compare our results to measurements of neutral organic nitrogen compounds in order to gain insight into how organic nitrogen is chemically transformed in the atmosphere and how this influences new particle formation"
Monday, 13 November 2017
Optimization of Surface-Initiated Atom Transfer Radical Polymerization for Application to Small Analyte Detection
Nathan Reed
CU Boulder, ANYL 1st year
"The research project I contributed to as an undergraduate student sought to investigate molecular transport as a means of signal enhancement in small analyte detection. Selective transport of analyte molecules to a nanosensor location will lower the limit of detection compared to analyte collection from free diffusion alone. Polymer brushes can be grown off a detector surface directly and can then be used to bring small molecules closer in proximity to the detector. The small molecules will selectively partition into the polymer-brush surface based on attractions caused by hydrophobic effects, hydrogen bonding, or ionic interactions. To foster such controlled and specific interactions on a device surface, uniform polymer thin films needed to be synthesized and the appropriate materials used for transport of target analytes needed to be understood. Additionally, there was a need for the method to be standardized so that non-polymer scientists and engineers could replicate it with ease. Atom transfer radical polymerization (ATRP) presented a polymerization technique able meet these needs. ATRP is a controlled polymerization technique whose parameters may be tuned to meet specific needs such as functionality and length. In addition, ATRP is an approachable technique to scientists unfamiliar with polymerization techniques once synthetic methods are unified and optimized. My undergraduate research has investigated ATRP reaction conditions applicable to a broad range of polymer types, both in solution and surface-tethered, to develop a unified and optimized synthetic approach."
and
Global simulation of brown carbon and single scattering albedo calculation using a chemical transport model
Duseong Jo
CU Boulder, Postdoc
"Recent observations suggest that a certain fraction of organic carbon (OC) aerosol effectively absorbs solar radiation, which is also known as brown carbon (BrC) aerosol. Despite much observational evidence of its presence, very few global modeling studies have been conducted because of poor understanding of global BrC emissions. I will present an explicit global simulation of BrC in a global 3-D chemical transport model (GEOS-Chem), including global BrC emission estimates from primary (biomass burning and biofuel) and secondary sources. The BrC absorption leads to a general reduction of NO2 photolysis rates, whose maximum decreases occur in Asia up to 9% (19%) on an annual (spring) mean basis. The inclusion of BrC absorption reduces the overestimation of single scattering albedo (SSA) in the model, but still the model overestimates the observed SSA by AERONET. To further reduce the overestimation, the sensitivity calculations of SSA are conducted by focusing on the physical properties of Black Carbon (BC), the inclusion of Brown Carbon (BrC), and the size distribution of dust. Large variations in the calculated SSA may result from slight changes of the geometric mean radius, geometric standard deviation, real and imaginary refractive indices, and density of BC. The inclusion of BrC and observationally-constrained dust size distributions also significantly affect the SSA, and result in a remarkable improvement for the simulated SSA at 440 compared with the AERONET observations."
Monday, 30 October 2017
Chemistry of peroxy radicals and soot formation at combustion conditions
Prof Sandeep Sharma
CU Boulder, PChem
"In this presentation, I will focus on the chemistry of peroxy radicals and soot formation at combustion conditions. Both these topics are of immense technological importance and will also allow me to present the latest theoretical techniques used to study them. These include the use of automated mechanism generators to build complex reaction mechanisms, the use of transition state theory to calculate rates of elementary reactions, and the use of the semiclassical method for calculating the partition function of anharmonic vibrational modes. The aim is to physically motivate these techniques and highlight the conditions under which we expect them to be predictive and also discuss their shortcomings. I will end the presentation with a short review of the latest work from my group that aims to address some of the shortcomings."
Monday, 23 October 2017
VOCs off-gassed by laser and inkjet printers, and CH4, CO2, and N2O gas fluxes in created wetlands
Wyatt Brown
CU Boulder, ANYL first year student
"Indoor air quality has become a more prominently researched subject in recent years, and indoor office environments are still poorly understood in terms of the volatile organic compounds (VOCs) associated with them. We have identified and quantified various VOCs off-gassed by laser and inkjet printers using solid phase micro-extraction in conjunction with GC/MS.
In the outdoor environment, it has been well-established that wetlands are important ecosystems for the planet and provide a multitude of ecological services. In order to combat the widespread destruction of wetlands, restoration efforts have recently seen a dramatic increase in prevalence. However, there is a complex, poorly understood relationship between the functions of created wetlands and the emission of greenhouse gasses. In this talk, the preliminary data on CH4, CO2, and N2O gas fluxes in created wetlands is presented. Preliminary dissolved organic matter (DOM) studies are also presented."
and
The reaction of acetyl peroxy radical with hydroperoxy radical
Marla DeVault
CU Boulder, ANYL first year student
"I spent three summers working in Prof. Keith Kuwata's computational chemistry lab at Macalester College. Primarily, my research focused on improving the steps taken to simulate reactions of organic peroxy radicals (OPR's) in the atmosphere. More specifically, I studied the reaction of acetyl peroxy radical with hydroperoxy radical, which is thought to be a source of hydroxy radical. These steps include determining which simple model chemistry could most accurately model the reaction, investigating the viability of non-reactive conformers, and testing methods for calculating the energy of transition structures."
Friday, 13 October 2017
Indoor air (photo)chemistry: A world-wide concern
Prof Sasho Gligorovski
State Key Laboratory of Organic Geochemistry, Chinese Academy of Sciences
"The first field campaign performed in a high school in Marseille, France (2011) confirmed the
existence of hydroxyl radicals (OH) that were more in line with typical outdoor concentrations
than indoor concentrations (Gomez Alvarez et al., 2013). It was demonstrated that photolysis of
nitrous acid (HONO) is the most important source of these highly reactive OH radicals in the
indoor air. This set of innovatory first direct measurements of OH radicals indoors was followed
with also avant-garde measurements of the solar actinic fluxes which can penetrate indoors and
photolysis frequencies of key indoor species (Gandolfo et al., 2016). The results obtained are of
enormous repercussions and need to be studied in the next few years much more profoundly due
to their still unexplored implications. The facts just mentioned need to be taken in combination
with the elevated concentrations of HONO present in the indoor air (Gligorovski, 2016). In
addition, HONO is an important indoor air pollutant, which can react with amines leading to
carcinogenic nitrosamines. While our understanding of photochemistry of the indoor surfaces is
still in its infancy, the recent results (Gomez Alvarez et al., 2014, Gandolfo et al., 2015, 2017)
based on the light induced heterogeneous NO2 reactions on domestic surfaces leading to HONO
formation, suggest that it is an area that should be pursued further. Another campaign carried out
in an office in Martigue, France (2016) was dedicated to evaluation of OH radical source
strength. Again, the photochemical reactions play an important role to the oxidation capacity of
indoor atmosphere. If we consider all these facts carefully, the concentration of OH radicals
indoors, could reach levels that would be of serious concern from the standpoint of public health.
For these reasons, it is very important to dedicate more efforts to determine the OH
concentrations that can be attained in various indoor settings, in relation to factors such as light
intensity, HONO concentrations, and humidity, among others."
Monday, 9 October 2017
Better Understanding Climate and Atmospheric Chemistry by Understanding the Formation of Mixed Phase Clouds
Prof Dan Cziczo
MIT
"Field and laboratory measurements using cloud chambers have been used to understand the atmospheric abundance of droplet and ice nucleating particles and to, in turn, construct parameterizations for mixed-phase and completely glaciated clouds in weather and climate models. This seminar investigates measurements of which particles act as the nuclei for droplets and ice crystals and how we can then mimic those particles in the laboratory to understand how clouds form in our atmosphere. We show here that assumptions about the source of the particles as well as uncertainty in the laboratory and field measurements propagate into uncertainty in our understanding of the Earth’s climate and the chemistry of our atmosphere.
When we consider cloud chambers, uncertainty is likely inherent to varying degrees in all instruments and is caused by a variety of factors including exposure of particles to different humidities and/or temperatures than predicated from theory. This can result in a variable underestimation of reported droplet and ice concentrations. This is a critical issue for models which relay on these data for correct parameterizations of cloud formation. For ice clouds in particular, we find that simulated long wave ice-bearing cloud forcing in a global climate model can vary up to 0.8 W/m2 and can change sign from positive to negative within the experimentally constrained bias range.
We’ll conclude with a discussion of possible instrument improvements and how these can improve our understanding of climate, precipitation and atmospheric chemistry."
Monday, 2 October 2017
Chemistry of Organic Compounds in the Atmosphere and Indoor Air
Prof Paul Ziemann
CU Boulder
"Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In addition to this we have recently conducted a number of studies of indoor air chemistry at CU. In this talk I will describe how we conduct the studies by using a diverse array of measurement techniques."
and
Recent Results and Upcoming Projects to Investigate Aerosol Sources, Properties, Processes, and Fate
Prof Jose Jimenez
CU Boulder
"Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, properties, and evolution are poorly understood. In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students. Ongoing projects include global aerosol measurements and analysis as part of the NASA ATOM project, which is sampling (almost) pole-to-pole across the vertical profile. Initial model comparisons suggest the importance of fast OA removal channels. We have used an Oxidation Flow Reactor (OFR) at multiple field studies and find consistent patterns, where SOA formation by O3 and NO3 are consistent with models, while formation by OH is substantially underpredicted unless semivolatile and intermediate volatility species are accounted for. We are using the GECKO-A fully explicit model to investigate this underprediction, as well as to characterize the similarities and differences of the chemical regimes across the OFR, large chambers, and the atmosphere. We are investigating gas/particle partitioning in the laboratory for different types of particles. Finally, we are continuing to explore the chemistry of indoor air with ToF-CIMS and other instruments. Future outdoor campaigns may include the study of emissions and chemical evolution of smoke from real fires in the western US (NASA FireChem) with AMS and soft-ionization EESI-TOF. Future indoor campaigns will involve sampling at locations such as dining halls, gyms, and art museums."
Monday, 25 Sept 2017 Ambient Ions in Planetary Atmospheres
Prof Eleanor Browne
CU Boulder
"Atmospheric ions influence the chemical composition of planetary atmospheres through ion-molecule reactions, ion-catalyzed reactions, and ion-particle interactions. Characterizing the chemical composition of ambient ions is necessary for a comprehensive understanding of these processes. In this talk, I will discuss our recent work using atmospheric pressure interface mass spectrometry to measure the chemical composition of ions in field and laboratory settings. I will discuss our measurements of ions in Earth’s atmosphere during the 2015 Holistic Interaction of Shallow Clouds, Aerosols, and Land-Ecosystems (HIS-CALE) II campaign in Lamont, OK as well as our laboratory investigation of ions resulting from the irradiation of CH4-N2 mixtures. "
and
Air Quality and Climate in the Anthropocene: Why we study remote pristine and polluted environments
Prof. Rainer Volkamer
CU Boulder
"Atmospheric chemistry is at the core of air pollution impacts on public health (ozone, air toxics, and aerosols) and climate (lifetime of greenhouse gases, radiation feedbacks). There is a need for techniques to better quantify emission fluxes of trace gases and aerosols from wildfires, which are deemed responsible for up to 40% of the global CO emissions. The Volkamer group develops instruments, conducts field and laboratory experiments to better probe, test and predict emissions, and study fundamental physical atmospheric chemistry principles of relevance in remote pristine and polluted air. We offer graduate research opportunities for one student in either of the following three areas: [1] Solar Occultation Flux spectroscopy (mobile SOF) is a new tool under development to better quantify emission fluxes of ozone, radical- and aerosol precursor gases (NH3, NO, NO2, HONO, VOCs, OVOCs, acids, etc) from wildfires. The CU airborne SOF instrument is currently being tested aboard the Wyoming King Air aircraft, in preparation for a field experiment to study emissions and plume chemistry from wildfires in the Pacific Northwest during summer 2018. [2] As part of the CLOUD consortium at CERN, Geneva we study nucleation and aerosol-cloud interactions in the pristine marine, polluted urban and remote free troposphere. For example, measurements of iodine monoxide (IO), iodine dioxide (OIO) radicals, molecular iodine (I2) and other species can constrain the rate of iodine atom release, and iodic acid formation that is deemed responsible for new particle formation in coastal environments, but this chemistry is currently poorly understood. [3] Field measurements of IO, bromine oxide (BrO) radicals and short live oxygenated hydrocarbons at Big Island, Hawaii (Mauna Loa Observatory, at 19.5°N, 155.6°W, 3.4 km AMSL) and La Reunion island, France (Maïdo Observatory, at 21.1°S, 55.4°E, 2.2 km AMSL) are part of an ongoing and larger effort to understand new particle formation, halogen chemistry and OVOC sources in the remote troposphere. An intensive operating period at Maïdo will bring together groups from Europe, Japan, and the US during spring/summer 2018. "
Monday, 18 Sept 2017
Comprehensive Analysis of the Gas- and Particle-Phase Products of VOC Oxidation
Julia Bakker-Arkema
ANYL 3rd-year student, Ziemann lab
"Controlled environmental chamber studies are important for determining atmospheric reaction mechanisms and gas and aerosol products formed in the oxidation of volatile organic compounds (VOCs). Such information is necessary for developing detailed chemical models for use in predicting the atmospheric fate of VOCs and also secondary organic aerosol (SOA) formation. However, complete characterization of atmospheric oxidation reactions, including gas- and particle-phase product yields, and reaction branching ratios, are difficult to achieve. In this work, we investigated the reactions of terminal alkenes with OH radicals in the presence of NOx in an attempt to fully characterize the chemistry of these systems while minimizing and accounting for the inherent uncertainties associated with environmental chamber experiments. Gas-phase products (aldehydes formed by alkoxy radical decomposition) and particle-phase products (alkyl nitrates, β-hydroxynitrates, dihydroxynitrates, 1,4-hydroxynitrates, 1,4-hydroxycarbonyls, and dihydroxycarbonyls) formed through pathways involving addition of OH to the C=C double bond as well as H-atom abstraction were identified and quantified using a suite of analytical techniques. The full product identification and quantitation, with careful minimization of uncertainties for the various components of the experiment and analyses, demonstrates our capability to comprehensively and accurately analyze the complex chemical composition of products formed in the oxidation of organic compounds in laboratory chamber studies."
Monday, 11 Sept 2017
Investigating emissions of ultrafine aerosols from consumer products: A study on 3D printers
Prof. Nina Vance
CU Boulder
"It is well established that inhalation of ultrafine aerosols (particulate matter < 100 nm) is associated with adverse health effects, however the extent of people’s everyday exposure to these pollutants is still poorly known. The introduction of novel consumer product applications and processes present the potential to introduce new ultrafine aerosols and nanoparticles to indoor environments such as homes, office buildings, schools, hotels, and hospitals. In this presentation, I will discuss the use of methods at the intersection of air resources engineering, nanotechnology, and exposure science to quantify and characterize people’s exposure to ultrafine aerosols and nanomaterials in everyday activities. This will be specifically demonstrated by a case study on aerosol emissions from 3D printers. The aerosol emission rates and size distributions that can be characterized by this type of work can serve as input to risk assessment models, to guide the selection of relevant particle doses in toxicity testing, and to engineer product improvements or develop regulations to ensure consumer safety."
Wednesday, 12 July 2017
Regional Influence of Wildfires on Atmospheric Aerosol in the Western US and Insights into Emission and Aging of Biomass Burning Organic Aerosol
Prof. Qi Zhang
UC Davis
"Biomass burning (BB) is one of the most important contributors to atmospheric aerosols on a global scale and a large source of emissions that impact regional air quality and global climate. In this study, aerosols in wildfire emissions in the Pacific Northwest region of the United States were studied from the Mt. Bachelor Observatory (MBO, ~ 2700 m a.s.l.) in Central Oregon and from the G-1 aircraft in summer 2013 during the DOE Biomass Burning Observation Project (BBOP) field campaign. Episodes of high aerosol concentrations were observed at MBO due to the impacts of plumes transported from large wildfire clusters upwind of the site. Organic aerosols (OA) were a dominant component of particles in these plumes and the regional enhancements of BBOA concentration normalized by amount of fuel burned were found to be mainly driven by the modified combustion efficiency (MCE), an index of the combustion processes of a fire. The ratio of the enhancement of OA mass to the enhancement of CO above their respective backgrounds (ΔOA/ΔCO) in fire plumes appeared to be constant independent of plume aging although BBOA composition was significantly influenced by atmospheric aging. Three types of BBOA were identified during this study, including a semivolatile BBOA-1 (~ 20% of OA mass) which appeared to represent BB POA and two more oxidized BBOAs (BBOA-2 and BBOA-3) that appeared to represent BB SOA. BBOA-3 was highly oxidized (O/C = 1.06; 31% of OA mass), contained no levoglucosan, showed very low volatility with only ~ 40% mass loss at 200°C, and had a similar mass spectrum as low-volatility oxygenated OA (LV-OOA) commonly observed in regional airmass. The chemical evolution of BBOA was examined for an episode when fire plumes originated from a single fire source were sampled continuously for 36 hours. Longer solar radiation evidently led to a higher mass fraction of the chemically aged BBOA-2 and BBOA-3 and substantially more oxidized OA although negligible net amount of OA production was observed in plumes photochemically aged compared to plumes transported primarily during night. These results suggest that SOA formation was balanced by BBOA volatilization, leading to almost no net amount of OA mass added during aging in wildfire plumes."
Spring 2017
Thursday, 27 April 2017
New Insights into Fossil Fuel Volatile Organic Compound Emissions and Chemistry Using H3O+ and NO+ Chemical Ionization Mass Spectrometry
Abigail Koss
ANYL PhD Candidate
"Volatile organic compounds (VOCs) are central to tropospheric chemistry. These species harm human health and contribute to formation of ozone and secondary organic aerosol. VOCs are challenging to measure because they are frequently present in small concentrations, they can have high spatial and temporal variability, and many different functional groups can be present. One of the most powerful techniques used to measure VOCs in the atmosphere is proton transfer reaction mass spectrometry (PTR-MS). In this talk, I discuss my contributions to the improvement and application of this technique.
We developed a new PTR-time-of-flight-MS (PTR-ToF-MS) instrument based on the Aerodyne ToF-CIMS. This instrument has higher sensitivity than its PTR-MS predecessors, and has enabled faster measurement and the measurement of many more VOCs. We extensively characterized this instrument, including the effect of ion guide components, and the response to changing humidity. The PTR-ToF-MS instrument was deployed on the NOAA P3 aircraft to measure VOCs associated with oil and natural gas producing regions. In the US, these regions have experienced a recent increase in fossil fuel production, sparking concerns about local and regional air quality. Few PTR-ToF measurements in these regions exist, and the interpretation of mass spectra can be quite different from that in urban or forested areas. I developed a guide to interpret these measurements. We detected several previously unreported species, including cyclic nitrogen-containing compounds, which may provide clues about emissions and could be important for overall nitrate reactivity. Comparison between basins answers major campaign science questions about mixing ratios of air toxics.
I expanded the capability of the ToF-CIMS instrument by adapting it to use NO+ reagent ion chemistry. Through a set of experiments using modular GC-CIMS and parallel NO+-CIMS and GC-EI-MS, I determined the selectivity and sensitivity of NO+ CIMS for several classes of compounds that PTR-MS cannot easily measure, including cyclic alkanes, long-chain alkanes, isomerically specific measurements of carbonyls, and alcohols. The NO+ CIMS instrument was deployed on a mobile laboratory to measure real-world highway vehicle emissions. Application of positive matrix factorization clearly identified the important emission sources, their VOC composition, and contribution to total VOC emissions."
Monday, 24 April 2017
Turning brown in the sun: Aldehydes, aqueous aerosol, and evaporating cloud droplets
Prof. David De Haan
University of San Diego
"Much of what we think we know about aqueous aerosol chemistry – reaction rates, products, mechanisms, and photolytic pathways – comes from extrapolating bulk aqueous-phase lab simulations to atmospheric conditions. Based on this approach, it is now commonly assumed that small, water-soluble aldehydes can react at night with ammonium salts to slowly form light-absorbing brown carbon (BrC). These BrC products are thought to be quickly destroyed by sunlight. When aqueous aerosol processes are studied in aqueous aerosol particles, however, this common narrative turns out to be only partially true. In this talk, results will be presented from recent chamber studies on ammonium and amine-containing aerosol particles as they interact with aldehyde species, solar simulator lamps, and clouds. In some cases, sunlight actually accelerates BrC formation during cloud processing. Chemical analysis of the aerosol produced in these experiments suggests that mechanisms initiated by photolytically-produced radical species are the dominant source of oligomers, and by extension, of BrC."
Monday, 17 April 2017
Products and yields from the gas-phase oxidation of benzenediols
Zachary Finewax
University of Colorado, Boulder
"Biomass burning (i.e. wildfires, prescribed burns) emits significant amounts of organic carbon to the atmosphere. However, the secondary processing of these emissions is poorly understood. In this work, catechol and resorcinol (1,2-benzenediol and 1,3-benzenediol, respectively) were reacted with hydroxyl radical (OH) in the presence of NOx, or with nitrate radical (NO3) in an environmental chamber. These compounds were previously identified and quantified as significant emissions from biomass burning. Both benzenediol isomers produced a significant quantity of secondary organic aerosol (SOA) after reaction with OH or NO3. 4-nitrocatechol was identified as the dominant product in the SOA from catechol oxidation by both OH and NO3, whereas the product distribution from resorcinol oxidation included benzenetriols, nitroaromatics, and hydroxybenzoquinones. Formation mechanisms of these products are discussed."
Monday, 10 April 2017
North American pollution measurements from geostationary orbit with Tropospheric Emissions: Monitoring of Pollution (TEMPO)
Dr. Kelly Chance
Harvard-Smithsonian Center for Astrophysics
"TEMPO is the first NASA Earth Venture Instrument. It launches between 2019 and 2021 to measure atmospheric pollution from Mexico City and Cuba to the Canadian oil sands, and from the Atlantic to the Pacific. It does this hourly at high spatial resolution, ~10 km 2 , to measure the key elements of air pollution chemistry. Geostationary daytime measurements capture the variability in the diurnal cycle of emissions and chemistry at sub-urban scale to improve emission inventories, monitor population exposure, and enable emission-control strategies. TEMPO measures UV/visible Earth reflectance spectra to retrieve O 3 , NO 2 , SO 2 , H 2 CO, C 2 H 2 O 2 , H 2 O, BrO, OClO, IO, aerosols, cloud parameters, and UVB radiation. It tracks aerosol loading. It provides near-real- time air quality products. TEMPO is the North American component of upcoming the global geostationary constellation for pollution monitoring, together with the European Sentinel-4 and the Korean Geostationary Environmental Monitoring Spectrometer (GEMS).
TEMPO science studies may include: Solar-induced fluorescence from chlorophyll over land and in the ocean to study tropical dynamics, primary productivity and carbon uptake, to detect red tides, and to study phytoplankton; measurements of stratospheric intrusions that cause air quality exceedances; measurements at peaks in vehicle travel to capture the variability in emissions from mobile sources; measurements of thunderstorm activity, including outflow regions to better quantify lightning NO x and O 3 production; cropland measurements to follow the temporal evolution of emissions after fertilizer application and from rain-induced emissions from semi-arid soils; investigating the chemical processing of primary fire emissions and the secondary formation of VOCs and ozone; examining ocean halogen emissions and their impact on the oxidizing capacity of coastal environments; measuring spectra of nighttime lights as markers for human activity, energy conservation, and compliance with outdoor lighting standards intended to reduce light pollution."
Friday, 7 April 2017
Dissertation Defense: Development and Application of an Oxidation Flow Reactor to Study Secondary Organic Aerosol Formation from Ambient Air
Brett Palm
University of Colorado, Boulder
"Secondary organic aerosols (SOA) in the atmosphere play an important role in air quality, human health, and climate. However, the sources, formation pathways, and fate of SOA are poorly constrained. In this dissertation, I will present development and application of the oxidation flow reactor (OFR) technique for studying SOA formation from OH, O3, and NO3 oxidation of ambient air. With a several-minute residence time and a portable design with no inlet, OFRs are particularly well suited for this purpose.
I will first introduce the OFR concept, and discuss several advances I have made in performing and interpreting OFR experiments. This includes estimating oxidant exposures, modeling the fate of low-volatility gases in the OFR (wall loss, condensation, and oxidation), and comparing SOA yields of single precursors in the OFR with yields measured in environmental chambers. When these experimental details are carefully considered, SOA formation in an OFR can be more reliably compared with ambient SOA formation processes.
I will then present an overview of what OFR measurements have taught us about SOA formation in the atmosphere. I will present a comparison of SOA formation from OH, O3, and NO3 oxidation of ambient air in a wide variety of environments, from rural forests to urban air. In a rural forest, the SOA formation correlated with biogenic precursors (e.g., monoterpenes). In urban air, it correlated instead with reactive anthropogenic tracers (e.g., trimethylbenzene).
In mixed-source regions, the SOA formation did not correlate well with any single precursor, but could be predicted by multilinear regression from several precursors. Despite these correlations, the concentrations of speciated ambient VOCs could only explain approximately 10-50% of the total SOA formed from OH oxidation. In contrast, ambient VOCs could explain all of the SOA formation observed from O3 and NO3 oxidation. Evidence suggests that lower-volatility gases (semivolatile and intermediate-volatility organic compounds; S/IVOCs) were present in ambient air and were the likely source of SOA formation that could not be explained by VOCs. These measurements show that S/IVOCs likely play an important intermediary role in ambient SOA formation in all of the sampled locations, from rural forests to urban air."
Monday, 3 April 2017
Measurements of emissions from agricultural fires and wildfires in the U.S
Dr. Xiaoxi Liu
University of Colorado, Boulder
"Biomass burning (BB) produces significant amounts of trace gases and aerosol, which play important roles in atmospheric chemistry and climate. This study presents detailed airborne measurements of emissions from 15 agricultural fires and 3 wildfires in the U.S. during the 2013 Studies of Emissions and Atmospheric Composition, Clouds and Climate Coupling by Regional Surveys (SEAC4RS) and the Biomass Burning Observation Project (BBOP). A detailed set of emission factors (EFs) for 25 trace gases and 6 components of submicron particulate matter (PM1) was reported for the agricultural fires located in the southeastern U.S. Observed EFs are generally consistent with previous measurements of crop residue burning, but the fires studied here emitted high amounts of oxygenated volatile organic compounds, sulfur dioxide, and fine particles. Filter-based measurements of aerosol light absorption implied that brown carbon was ubiquitous in the plumes. The rapid chemical evolution of the primary emissions in 7 out of 15 agricultural plumes was examined in detail for ~1.2 hr. A Lagrangian plume cross-section model was used to simulate the evolution of ozone, reactive nitrogen species, and organic aerosol (OA). For the western wildfires, we measured an extensive set of EFs for over 80 gases and 5 PM1 species. The wildfires emitted high amounts of PM1 (in which OA comprised most of the mass) with an average EF that is over two times of prescribed fire EFs. The EFs were used to estimate the annual regional emissions from agricultural fires and wildfires for CO, NOx, total non-methane organic compounds, and PM1. Our wildfire PM1 emission estimate from 11 western states is over three times that of the 2011 National Emissions Inventory (NEI) PM2.5 estimate, mainly due to our high EF(PM1), and also higher than the PM2.5 emitted from all other sources in these states according to NEI. This supports the practice of prescribed burning that could reduce fine particle emissions."
And
Atmospheric chemistry of aliphatic amines: reaction mechanisms and temperature effects
Dr. Derek Price
University of Colorado, Boulder
"Aliphatic amines come from both anthropogenic and biogenic sources, including agricultural/livestock emissions and biomass burning. Radical oxidation of aliphatic amines can produce organic aerosol as well as aminium salt aerosol. The extent to which organic/aminium-salt aerosol is formed is dependent on the type of radical and precursor amine. Temperature has an important impact on SOA formation from aliphatic amines. Temperature variations can occur both seasonally and through vertical mixing in the atmosphere. In this presentation, I will discuss (1) the reaction pathways identified in smog chamber studies of hydroxyl and nitrate radical oxidation of aliphatic amines through comparison of mass spectra from both aerosol (HR-ToF-AMS and PILS-ToF-MS) and gas phase (SIFT-MS) instrumentation, and (2) the effects of temperature changes on the system."
Monday, 20 March 2017
Atmospheric Organics: The Cubism of Atmospheric Chemistry
Colette Heald
MIT
"Reactive organic carbon (ROC) is the fuel of atmospheric chemistry: the oxidation of these species leads to the formation of ozone, aerosols, and CO2, with both air quality and climate impacts. Organic aerosol is an important, often dominant, contributor to atmospheric aerosol, and yet the complexity of its formation and evolution in the atmosphere preclude a good understanding of its impacts. In this talk I will highlight some recent work from my group on tropospheric organics, including: (1) the global budget of reactive organic carbon (2) the deposition of organic carbon and potential constraints on the lifecycle of ROC, and (3) the decadal trend in organic aerosol over the United States."
Monday, 13 Mar 2017
Science Diplomacy: Lessons from recent updates to the Montreal Protocol
Dr. Federico San Martini,
Secretariat for the Implementation of the Montreal Protocol
"On 15 October 2016, after 7 years of consultations, the Parties to the Montreal Protocol adopted an amendment in Kigali, Rwanda that added hydrofluorocarbons (HFCs) to the list of substances controlled under the Protocol. Under the Amendment, countries committed to phase down the production and consumption of HFCs by more than 80 percent, and agreed to provisions to control HFC-23 by-product emissions. Implementation of the Amendment will avoid more than 80 billion metric tons of carbon dioxide equivalent emissions by 2050. The Montreal Protocol has been called the most successful environmental treaty to date, and the Kigali Amendment the single largest contribution to date towards keeping global temperature rise below 2 oC. Why is the Montreal Protocol considered so successful, how will the amendment contribute to climate protection efforts, and how did science inform the policy process? This talk will explore how science informed policy in the Montreal Protocol, and what lessons could be applied to other fora. Time permitting, we will also discuss international air quality monitoring efforts at diplomatic facilities.
Dr. San Martini has worked for many years on science-policy issues related to air quality, short-lived climate forcers and stratospheric ozone protection. He obtained his PhD with Prof. McRae at MIT, and postdoced with Prof Mario Molina working in Air Pollution in Mexico City, before taking a position with the Board on Chemical Sciences and Technology at the National Academies. He moved to the State Department as a AAAS Science and Technology Policy Fellow, first working on water resources, and then on air quality, short-lived climate forcers and stratospheric ozone protection. While at the State Department Ico was instrumental in establishing the air quality monitoring network at U.S. diplomatic facilities and ensuring that air quality data is publicly available to researchers. More recently he joined the Montreal Protocol Multilateral Fund Secretariat where he works on projects related to ozone- and climate-protection."
Monday, 6 March 2017
Secondary Organic Aerosol Formation from Atmospheric Oxidation of Isoprene: Implications for Air Quality, Climate and Public Health
Prof. Jason Surratt
University of North Carolina
"Atmospheric fine particulate matter (PM 2.5 ) plays a key role in climate and is associated with adverse effects on air quality and human health. The largest mass fraction of PM 2.5 is organic, which is mostly derived from secondary organic aerosol (SOA) formed by the atmospheric oxidation of hydrocarbons. Atmospheric oxidation of isoprene (2-methyl- 1,3-butadiene), the most abundant non-methane hydrocarbon emitted into the atmosphere, is now recognized as one of the largest contributors to PM 2.5 . Despite its abundance, the exact manner in which isoprene- derived SOA is formed has been recently examined through the combination of synthetic organic and analytical chemistry with flow reactor, smog chamber, and field studies. Through these recent studies, we have identified reactive epoxides and hydroperoxides produced from the atmospheric oxidation of isoprene under low-nitric oxide (NO) conditions that are crucial to the formation of ambient SOA. Notably, certain isoprene-derived SOA constituents have been recently observed in cloud water samples and shown to contribute to light-absorbing aerosol, indicating that isoprene-derived SOA may be important for aerosol climate effects. We have found that anthropogenic pollutants, such as acidic sulfate aerosol, significantly enhance isoprene SOA. This is of great public health importance since isoprene is primarily emitted from terrestrial vegetation, and thus, is not controllable, whereas anthropogenic emissions are controllable. Whether SOA derived from this source contributes to the adverse health effects induced by exposure to ambient PM 2.5 reported in epidemiological studies is largely unknown. Using an in vitro model of human airway epithelial cells (BEAS-2B), we have also evaluated the potential early biological effects induced by exposure to isoprene-derived epoxides and hydroperoxides and their resultant SOA constituents. Exposure induced cytotoxicity and expression of oxidative stress and inflammation-associated genes have been assessed. Our initial findings suggest that isoprene-derived epoxides, hydroperoxides and the resultant SOA constituents induce altered oxidative stress and inflammation-associated gene expression in human lung cells under non-cytotoxic conditions. These recent findings highlight the importance of future work aimed at linking PM 2.5 source, composition, exposure biomarkers and health outcomes."
Monday, 27 February 2017
Cannabidiol-dependent modulation of cognitive learning and synaptic function
Prof. Jeff Smith
CSU Pueblo
"The National Institutes of Health, National Institute on Drug Abuse, currently lists Cannabidiol as having potential therapeutic value for treating neurological disorders that include a strong learning and memory component including; anxiety, psychosis, pain, and substance use disorders. It is also being studied for its potential to modulate various neurodegenerative disorders that profoundly affect learning and memory including Alzheimer’s disease. Despite this potential therapeutic importance, the current scientific understanding of exactly how Cannabidiol affects various forms of learning and memory, and the underlying cellular mechanisms that it targets, is inadequate to guide its most efficacious and least harmful use for treating such disorders. Our research advances knowledge in this area by showing that Cannabidiol modulates trace fear conditioning in mice. These experiments model cognitive learning and memory processes and involve multiple brain regions, including the hippocampus, which has a critical role in the learning and memory disorders listed above, and it is essential for achieving normal trace fear conditioning in rodents. Our work further shows that Cannabidiol modulates basal synaptic transmission in mouse hippocampal slices by affecting conduction velocity in the Shaffer collateral and Mossy Fiber pathways, and by modulating synaptic plasticity in these regions. Impulse propagation and synaptic plasticity are essential fundamental mechanisms that support learning and memory, therefore our results present a clearer picture of how Cannabidiol might be most useful, and least harmful for treating neurological disorders that have a strong cognitive learning and memory component."
Download the video here
Monday, 20 February 2017
Atmospheric Chemistry of Nitrogen Oxides at Soil-Air Interfaces
Prof. Jonathan Raff,
Indiana University
"There remain large uncertainties in the terrestrial sources and sinks of reactive oxides of nitrogen (NOy = NO, NO2, and HONO)—gases that play an important role in regulating the oxidizing capacity of the atmosphere. Most terrestrial surfaces are covered in soil particles, either as fine coatings of air-blown dust or as topsoil in which countless organisms live. In addition, soil particles possess large intrinsic surface areas and myriad reactive surface sites on which chemical reactions occur. These properties make soil a potentially important consideration in understanding the heterogeneous reactions that control the atmospheric NOy budget. In this presentation I will discuss results of laboratory experiments conducted on soil and individual soil constituents aimed at testing the hypothesis that redox couples involving soil organic matter and minerals mediate HONO conversion from NO 2 at night and from nitrate during the daytime. In addition, we carried out kinetics studies using a coated wall flow reactor and surface composition studies using nano-DESI and nanoSIMS [at the Environmental Molecular Sciences Laboratory (EMSL), Pacific Northwest National Laboratory] to explore the role of minerals and organic matter as sinks for HONO in soil."
Monday, 13 February 2017
Understanding the Molecular Signature of Atmospheric Organic Aerosols using Ion Mobility Mass Spectrometry
Dr. Xuan Zhang
NCAR
"I will present some recent developments on the Ion Mobility Mass Spectrometer (IMS) that measures the collision cross section () and mass-to- charge ratio (m/z) of charged molecules. A two-dimensional space based on these two quantities is developed to facilitate the comprehensive investigation of complex organic mixtures in the atmosphere. Species of the same chemical class, despite variations in the molecular structures, tend to develop a unique distribution pattern by following a trend line on the space. The characteristics of trend lines for a variety of functionalities that are commonly present in the atmosphere can be predicted by the core model simulations, which provide a useful tool to identify the chemical class to which an unknown species belongs on the space. Furthermore, molecular characterization of labile species such as multi-functional organic nitrates and highly oxidized organic molecules in the condensed phase using the IMS technique is discussed."
Monday, 6 February 2017
Aerosol Liquid Water: A Valentine to the Clean Air Act
Prof. Ann Marie Carlton
University of California, Irvine
"Water is a ubiquitous and abundant component of atmospheric particles. It influences light scattering, the hydrological cycle, atmospheric chemistry, and secondary formation of particulate matter (PM) in the atmosphere. Despite the critical importance of aerosol liquid water, actual mass concentrations are not well documented in the literature. Routine air quality networks that measure particle mass [e.g., the U.S. Environmental Protection Agency’ s Interagency Monitoring of Protected Visual Environments(IMPROVE)] and most particle measurement techniques [e.g., the aerosol mass spectrometer (AMS)] remove water and other semivolatile compounds during sampling and/or during filter equilibration. Using speciated ion and meteorological data we estimate water mass concentrations to better understand the geospatial patterns and historical trends of aerosol liquid water in the context of improved air quality and recently noted reductions in particulate organic carbon (OC) mass. We find a decrease in aerosol water mass concentrations that is correlated with decreasing organic particle mass, for which we can provide a plausible mechanistic explanation. These findings are consistent with the hypotheses that aerosol liquid water facilitates formation of biogenic secondary organic aerosol (SOA) and that biogenically derived SOA is modulated in the presence of anthropogenic perturbations. Decreasing aerosol liquid water mass can be explained, in part, by environmental regulations aimed at reducing sulfur emissions to alleviate environmental problems associated with acid rain and inorganic particle mass, suggesting the Clean Air Act is more successful than accounted for."
Monday, 30 January 2017
Closing the loop on phase equilibrium: connecting fundamental volatility measurements of complex fluids with applications of headspace detection
Megan Harries
ANYL 3rd Year Student, CU Boulder
"Almost all fluids that are useful to us are complex mixtures consisting of many components with differing properties. An understanding of the phase equilibrium of these fluids is essential to many scientific disciplines as well as industry. For example, knowledge gaps in this area affect applications ranging from the development of alternative fuels to the deployment of forensic methods based on vapor characterization. These applications depend on our ability to bridge the gap – governed by thermodynamics – between vapor and liquid composition. This is difficult for even a binary, but beyond a ternary mixture, it becomes a real hair-puller. To unite these two pieces of the complete picture, I am adapting two relatively new techniques: the advanced distillation curve (ADC) method for volatility measurement and PLOT-cryoadsorption for vapor sampling. A modification of the ADC method, in development, provides simultaneous determination of temperature, pressure, and the composition of both vapor and condensed phases of a fluid mixture, while PLOT-cryoadsorption provides the field-ready vapor analysis. I will present a preliminary study that demonstrates the method and our ability to reconcile the measurements with models. Finally, I will discuss two related projects demonstrating applications, one using ADC to evaluate two alternative fuels and the second demonstrating environmental applications of a field portable PLOT-cryoadsorption device."
Monday, 23 January 2017
UV Photochemistry of Carboxylic Acids: A source of unsaturated VOC and OVOC in marine air
Randall Chiu
ANYL 3rd Year Student, CU Boulder
"Photochemistry plays an important role in marine dissolved organic carbon (DOC) degradation, but the mechanisms that convert DOC into volatile organic compounds (VOCs) remain poorly understood. We irradiated carboxylic acids (C7-C9) on a simulated ocean surface with UV light (<320 nm) in a photochemical flow reactor, and transferred the VOC products into a dark ozone reactor. Glyoxal was detected as a secondary product from heptanoic, octanoic, and NA films, but not from octanol. Primary glyoxal emissions were not observed, nor was glyoxal formed in the absence of ozone. Addition of a photosensitizer had no noticeable effect. The concurrent detection of heptanal in the NA system suggests that the ozonolysis of 2-nonenal is the primary chemical mechanism that produces glyoxal. This source can potentially sustain few 10 pptv glyoxal over oceans, and helps to explain why glyoxal fluxes in marine air are directed from the atmosphere into the ocean."
Thursday, 12 January 2017
Secondary Organic Aerosols: Development and Application of New Techniques in Chamber and Field Experiments
Jordan Krechmer (Advisor: Jose-Luis Jimenez)
ANYL student PhD Dissertation Defense, CU Boulder
Secondary organic aerosols (SOA) have detrimental effects on human health and can influence the Earth’s climate by altering radiative forcing. Their sources, fates, and chemical composition across the globe, however, remain poorly constrained. In this talk I will present new developments in the analysis of SOA field and laboratory experiments. First, I will describe the development of a method to accurately quantify the loss of gaseous compounds to the Teflon walls of environmental chambers using real-time measurements. The method used short bursts of light to produce oxidants in situ, which in turn produced several gas-phase products with differing volatilities. Gas-phase products were observed in real time with a chemical ionization mass spectrometer (CIMS). The time scale of this process was short (< 700 s) enough to be on the order of other processes in SOA chamber experiments and is thus important enough to necessitate accounting for.
Second, I describe how the same method was then applied to chamber experiments with liquid organic seed aerosol present to quantify the effect of gas-wall partitioning on aerosol mass yield experiments. A well-characterized simple chemical system is used to produce low volatility organic compounds at a rapid rate, which are taken up by liquid organic seed particles and/or the Teflon chamber walls. Both gas-phase products and aerosol concentrations are continuously monitored. A simple but comprehensive box model is used to quantify gas-particle partitioning and α. We discuss the implications for quantifying gas-particle partitioning under more complex conditions.
Fall 2016
Monday, 14 November 2016
Industrial Hemp: Opportunities in R & D in Colorado’s Fastest Growing Industry
Bob Sievers
Department of Chemistry and Biochemistry, Environmental Program and CIRES
University of Colorado Boulder
“Industrial Hemp” is legally defined as Cannabis Sativa containing less than 0.3% THC, and “Marijuana” is defined as containing more than 0.3% THC. In 2016 in Colorado, the industrial hemp and marijuana industries created revenues totaling $1 billion dollars. To put this in context, this exceeds revenues from grain growing, sports and performing arts venues, or residential construction. It is now possible at CU (and other universities) to perform R & D on the growth, separation, purification, and applications of industrial hemp or marijuana under strict regulations, which will be discussed. Examples of possible uses range from precursors to novel materials and various medical applications such as those outlined by the National Institute for Drug Abuse. Examples are treatment of stress and pain, epileptic seizures, and substance abuse. Development of new separation, analysis methods, and pharmaceutical delivery are important to the success of this industry. Methods for improving bioavailability of cannabidiol have been invented.
Monday, 7 November 2016
Exploring the Evolution of Biomass-Burning Aerosol in Chambers and the Atmosphere
Jeff Pierce
Associate Professor, Department of Atmospheric Science
Colorado State University
I will discuss theoretical work on the evolution of biomass-burning organic aerosol in smog chambers and in ambient plumes that shows: (1) vapor wall losses appear to matter greatly in smog-chamber oxidation experiments of biomass-burning aerosol, and (2) differences in fire size and meteorology might impact the organic aerosol (and aerosol size distribution) evolution in ambient plumes as much as differences in emission factors or chemical mechanisms. Laboratory and field experiments should carefully consider these complicating factors when comparing across different experiments and plumes.
Monday, 31 October 2016
Cell Membrane Conditions on C-Reactive Protein Binding
Mitchell Alton, First Year Graduate Student
Department of Chemistry and Biochemistry
University of Colorado Boulder
C-reactive protein (CRP) is an important human protein involved in identification of apoptotic (dying) cells and acts as a general marker of inflammation. CRP functions by binding to the lipid, phosphatidylcholine (PC), which is present in all cells. However CRP does not bind to normal, healthy cells. It is theorized that CRP has different binding affinities for healthy versus dying cells due to differences in membrane curvatures and the presence of oxidized lipids in dying cells. Additionally, CRP has two different conformations that affect binding affinities. I will discuss how these conditions affect the binding of CRP to membranes.
Measurements of Peroxy Radical Loss Rates on Laboratory Surfaces
Benjamin Deming, First Year Graduate Student
Department of Chemistry and Biochemistry
University of Colorado Boulder
Peroxy radicals are important intermediates in the oxidative processing of volatile organic compounds in the atmosphere. Numerous instruments to measure atmospheric concentrations of these short-lived species are in active development. Wall losses of these compounds should be considered when designing such an instrument due to losses within the inlet. In this work, an ECHAMP peroxy radical detector was used to determine the wall-loss rates of HO 2 , CH 3 O 2 , C 2 H 5 O 2 , and isoprene peroxy radicals on PFA Teflon, quartz, halocarbon wax, and other materials. In addition to concentration and relative humidity dependencies, the use of FEP vs PFA Teflon was investigated.
Monday, October 17, 2016
Liquid water on Mars? Stable or metastable salt solutions formed via deliquescence
Raina Gough
Department of Chemistry and Biochemistry and CIRES
University of Colorado Boulder
Several different hygroscopic salts are known to exist in the Martian soil. Although Mars is a cold, dry planet where pure liquid water is not stable, deliquescence of perchlorate and other species can likely provide small amounts of liquid water temporarily. We use Raman microscopy and an environmental cell to probe the conditions under which such liquid brines can form and persist under low Martian temperatures. We will discuss the brine-forming ability of several different Mars-relevant salts and salt mixtures, as well as the potential habitability or toxicity of these aqueous solutions to bacterial spores. We discuss the relevance of these studies to the recently publicized “recurring slope lineae” (RSL) on Mars that may be evidence of liquid water flows. We also discuss the results of experiments we have done on salty sediment samples from the Dry Valleys of Antarctica, the only terrestrial analog site for the Martian RSL.
Electronic Properties and Composition of GaAs1-xPx Grown by Close-Spaced Vapor Transport
Allison Davis, First Year Graduate Student
Department of Chemistry and Biochemistry and CIRES
University of Colorado Boulder
Abstract
Semiconductor thin films are widely utilized in the production of photovoltaic devices. GaAs is a III-V binary semiconductor with exceptional optoelectronic properties such as a direct band gap of 1.42eV and a lattice parameter of 5.6Å, making it an attractive material for the construction of high efficiency solar cells. GaAs can be combined with GaP, another III-V semiconductor with a 2.25 eV band gap, which allows the band gap and the lattice parameter to be tuned. Combining these binary materials to make the ternary alloy GaAs1-xPx is useful for multi-junction cells. This study aims to produce and characterize GaAs1-xPx thin films grown via CSVT.
Monday, October 3, 2016
Characterization of Organic Nitrogen in the Atmosphere
Ellie Browne
Department of Chemistry and Biochemistry and CIRES
University of Colorado Boulder
Abstract
Organic nitrogen is a ubiquitous atmospheric component typically accounting for between one-quarter and one-third of reactive nitrogen deposition. The chemical complexity and reactivity of organic nitrogen, however, has made it challenging to study. Consequently, little is known about the atmospheric processing of organic nitrogen and the resulting implications for biogeochemistry, air quality, and climate. Research in my group uses mass spectrometry to identify the organic nitrogen compounds present in the atmosphere and to investigate the chemical processing of these compounds. This talk will describe the techniques that we use in the lab will discuss results from our recent field measurements.
Recent Results and Upcoming Projects to Investigate Aerosol Sources, Properties, Processes, and Fate
Jose-Luis Jimenez
Department of Chemistry and Biochemistry and CIRES
University of Colorado Boulder
Abstract
Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, properties, and evolution are poorly understood. In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students. Ongoing projects include global aerosol measurements and analysis as part of the NASA ATOM project, which is sampling (almost) pole-to-pole across the vertical profile. Initial model comparisons suggest the importance of fast OA removal channels. We have used an Oxidation Flow Reactor (OFR) at multiple field studies and find consistent patterns, where SOA formation by O3 and NO3 are consistent with models, while formation by OH is substantially underpredicted unless semivolatile and intermediate volatility species are accounted for. We are starting to use the GECKO-A fully explicit model to investigate this underprediction, as well as to characterize the similarities and differences of the chemical regimes across the OFR, large chambers, and the atmosphere. We are investigating wall losses of vapors on large chambers, and using the results to enable the investigation of gas/particle partitioning for different types of particles. Finally, we are continuing to explore the chemistry of indoor air with ToF-CIMS and other instruments, where we measured 60+ organic acids in a classroom, some of which are related to humans. Future campaigns will involve sampling at locations such as dining halls, gyms, and art museums.
Monday, September 19, 2016
Chemistry of Organic Compounds in the Atmosphere and Indoor Air
Paul Ziemann
Department of Chemistry and Biochemistry and CIRES
University of Colorado Boulder
Abstract
Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to understand atmospheric and indoor air chemistry and to develop models that are used to establish air quality regulations and predict the effects of human activities. Research in my laboratory focuses primarily on environmental chamber studies of the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form aerosol particles. In this talk I will describe how we conduct the studies by using a diverse array of measurement techniques
Volatile Organic Compounds in the Atmosphere
Joost de Gouw
Department of Chemistry and Biochemistry and CIRES
University of Colorado Boulder
Abstract
Volatile organic compounds (VOCs) in the atmosphere are emitted from many different natural and man-made sources. In the presence of nitrogen oxides, VOCs are precursors for the formation of secondary pollutants like ozone and fine particles, which are both important for climate and air quality. We measure VOCs in the atmosphere using mass spectrometry from research aircraft, mobile laboratories and ground sites. I will focus on measurements made with a newly developed instrument with a focus on compounds that have not been widely reported.
Monday, September 12, 2016
Going Through a Phase: Loss of aerosol water upon contact
Margaret Tolbert
Department of Chemistry and Biochemistry and CIRES
University of Colorado Boulder
Abstract
The water content of atmospheric aerosols controls many properties including their ability to catalyze heterogeneous chemical reactions, their impact on climate and visibility, and their ability to form clouds. In the atmosphere, the water content of the particles depends on their composition as well as the ambient relative humidity and temperature. Here we use a long working-distance optical trop to probe the deliquescence and efflorescence phase transitions of individual levitated salt particles. In addition, we probe how efflorescence is impacted by contact with external particles of varying composition. Implications for particle phase and water content will be discussed.
Tropospheric Chemistry in the context of Air Pollution and Climate
Rainer Volkamer
Department of Chemistry and Biochemistry and CIRES
University of Colorado Boulder
Abstract
The growing world population in the Anthropocene is impacting atmospheric composition on global scales. The last decade has marked a turning point in human history, as for the first time more people are now living in urban rather than rural environments on our planet. Atmospheric chemistry is at the core of the impacts of Air Pollution on human health (ozone and aerosols) and Climate (lifetime of greenhouse gases, aerosol radiation feedbacks). The Volkamer group studies remote pristine atmospheric environments to better understand how natural sources of tropospheric halogens and marine organic carbon destroy ozone, oxidize atmospheric mercury, modify oxidative capacity and aerosols. Why was ozone so low in pre-industrial times? We currently do not have the answer to this question, but it determines the baseline under which anthropogenic impacts have modified the radiative forcing associated with ozone as an important greenhouse gas. We also develop mobile Solar Occultation Flux spectroscopy (mobile SOF) to better quantify total emission fluxes of ozone and aerosol precursor gases (NH3, NO2, C2H6, etc) from agriculture, oil & natural gas production, urban areas and wildfires. The CU mobile SOF instrument is a unique prototype in the US, and limited by the need to develop retrievals to measure a large variety of gases that absorb at mid-infrared wavelengths. Finally, we are conducting laboratory experiments to understand the reaction mechanisms that determine the sources and sinks of our field observations, test and improve atmospheric models, and satellites.
Spring 2016
Monday, April 18, 2016
Effects of Atmospheric Conditions on the Composition of Secondary Organic Aerosol Formed from the Oxidation of Isoprene and Monoterpenes
Megan Claflin
Department of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
Motivated by the Southern Oxidant and Aerosol Study (SOAS) field campaign of 2013, a series of environmental chamber experiments have been conducted to study the effect of environmental conditions on aerosol composition formed from biogenic VOC precursors. The experiments, performed under conditions that mimic those observed during SOAS, study the secondary organic aerosol (SOA) formed from the oxidation of isoprene and select monoterpenes. The composition of the SOA formed was characterized using spectrophotometric functional group analysis methods to quantify the amount of carbonyl [C(O)], carboxylic acid [C(O)OH], hydroxyl [CHOH], ester [C(O)OR], peroxide [CHOOR] and nitrate [CHONO2] groups in the aerosol. Further molecular analysis was done on select systems using various mass spectrometry methods. By comparing the composition of the chamber aerosol with field samples collected during the SOAS campaign, we will attempt to identify the chemistry that leads to aerosol formation in the southeast US.
Monday, April 11, 2016
On the Lifetime of Nitrogen Oxides in the Continental Boundary Layer
Ronald C. Cohen
Miller Professor 2015-2016
Professor, Department of Chemistry
Professor, Department of Earth and Planetary Science
University of California, Berkeley
Abstract
Nitrogen oxides drive the chemistry of the troposphere, catalyzing production of oxidants, ozone and aerosol. Textbooks describe this chemistry as starting with emission of NO and terminating with the gas phase reaction of OH with NO2 to produce HNO3. Here, I will describe approaches to observing and characterizing the lifetime of nitrogen oxides using in situ and space based observations. In contrast to the textbook story we find the lifetime of nitrogen oxides on the continents are primarily controlled by organic nitrate formation.
Monday, April 4, 2016
Heterogeneous Efflorescence by Mineral Dust Particles
Shuichi Ushijima
Department of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
One of the key factors that determines particle growth and heterogeneous reaction efficiency of aerosols is whether the particle is liquid or solid. Salt aerosols transition between these phases through efflorescence and deliquescence. Although homogeneous efflorescence has been studied in detail, heterogeneous efflorescence is not well understood. Here we examined heterogeneous efflorescence by optically levitating single droplets of salt solutions and exposed the droplet to a flow of mineral dust particles. The impact of mineral dust on efflorescence and the difference between immersion and contact efflorescence will be discussed.
Monday, March 28, 2016
Chemistry of Multifunctional Hydroperoxides in Secondary Organic Aerosol
Demetrios Pagonis
Department of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
Multifunctional hydroperoxides have been identified as a product of autoxidation reactions and have been proposed as structures for extremely low volatility compounds (ELVOCs) observed in secondary organic aerosol. The chemistry of these hydroperoxides is complex and challenging to study, as many conventional analytical techniques disturb the peroxide functionality and cause additional chemistry to occur. In this work we are able to produce multifunctional hydroperoxides in environmental chamber reactions as well as in solution, allowing us to investigate their chemistry under a variety of conditions. Observed intramolecular reactions, reactions with water, acid-catalyzed acetal formation and acid-catalyzed peroxyacetal formation are all discussed.
Monday, March 14, 2016
The Seasonality of Tropospheric Ozone and Reactive Nitrogen: From Measurements to Models
Erin E. McDuffie et al.
Department of Chemistry and Biochemistry
University of Colorado Boulder
Abstract
Tropospheric ozone is a potent greenhouse gas that forms through the oxidation of volatile organic compounds (VOCs) in the presence of nitrogen oxides (NOx). Previous field studies have largely focused on summertime ozone photochemistry that varies non-linearly with NOx and VOCs. First I will present results from OH reactivity, ozone production efficiency, and box-model analyses that quantify the impacts of regional oil and natural gas (O&NG) emissions on photochemical ozone in the Northern Front Range (NFR) of Colorado. The NFR is unique in that it has been non-compliant with ozone air quality standards since 2007 and has ozone precursor emissions from urban-Denver in close proximity to a rapidly developing O&NG basin. Second I will present an initial analysis of winter field data and discuss planned work aimed at addressing scientific uncertainties in wintertime O3 proces
Monday, March 7, 2016
Effect of a Functional Group on SOA Yields and Particle Composition from OH Radical Initiated Reactions of Alkanes in the Presence of NOx
Lucas Algrim
Department of Chemistry and Biochemistry
University of Colorado Boulder
Abstract
In a series of laboratory experiments we have identified major products and determined SOA yields from the reactions of C10 alcohol and C12 ketone position-isomers with OH radicals in the presence of NOx. Reactions were studied with a particle beam mass spectrometer (PBMS), gas chromatograph with flame ionization detection (GC-FID), and a scanning mobility particle sizer (SMPS), among other instruments. Functional group position had a considerable effect on SOA yields, which is fully explainable by the fate of alkoxy radical intermediates and vapor pressures of resulting products. Important observed differences from alkane chemistry were enhanced decomposition via α-cleavage, ability of the preexisting ketone to play a role in cyclic hemiacetal formation, and significantly hindered isomerization when the ketone was part of the transition-state ring.
Monday, February 29, 2016
Supercooling and ice formation of perchlorate and chloride brines under Mars-relevant conditions
Katie Primm
Department of Chemistry and Biochemistry-CIRES
University of Colorado Boulder
Abstract
Perchlorate and chloride salts, discovered in the Martian regolith at multiple landing sites, may provide pathways for liquid water stability on current Mars. It has previously been assumed that perchlorate and chloride brines form in the Martian regolith via melting or deliquescence, they would be present only briefly because efflorescence into a crystal or freezing to ice would soon occur. Here, we used a Raman microscope to study the temperature and relative humidity (RH) conditions at which magnesium perchlorate and magnesium chloride brines will deliquesce into aqueous droplets, form ice, and effloresce back into crystalline particles.
Monday, February 22, 2016
Investigating hydrocarbon emissions in oil and gas basins using mobile platforms
Ingrid Mielke-Maday
Deparmtment of Chemistry and Biochemistry
University of Colorado
Cires Auditorium
Abstract
Methane emissions are of concern due to methane’s contribution to global climate change and tropospheric ozone formation. Sources of methane include landfills, agriculture, and oil and natural gas operations. Recent studies have focused on determining the extent to which oil and gas operations contribute to methane emissions. The National Oceanic and Atmospheric Administration’s (NOAA) Global Monitoring Division has conducted fieldwork in oil and gas basins in order to characterize and quantify emissions of methane and non-methane hydrocarbons. Field campaigns from the past two years will be presented. Measurement techniques and methods, including the use of a mobile laboratory and aircraft, will be discussed.
Monday, February 15, 2016
Chemistry in of the coldest places in the atmosphere: Impacts of updated NOx lifetime and fate on lightning NOx emission rates
Benjamin A. Nault
Postdoctoral Researcher
Department of Chemistry and CIRES
University of Colorado, Boulder
Abstract
Nitrogen oxides (NOx ≡ NO + NO2) produced from lightning are an important natural source of NOx. This is the most important NOx source for the middle and upper troposphere. Lightning produced NOx controls the chemical production of middle and upper tropospheric ozone, an important greenhouse gas, and the oxidative capacity of the troposphere. However, the uncertainty in the emissions rates range from 2 to 8 Tg nitrogen per year. Recent studies have provided evidence that the oxidation of NOx to pernitric acid and nitric acid is slower than currently assumed and that methyl peroxy nitrate is an important temporary sink of upper tropospheric lightning NOx. Also, the conversion of dinitrogen pentoxide to nitric acid may be slower than currently assumed. I investigate the impacts of this updated kinetics and chemistry on model and in-situ observations of lightning NOx production rates. I show that using these results, the uncertainty range decreases. Also, the emission rates for different regions of the world increase by as much as 33% with the updated upper tropospheric NOx lifetime and fate.
Insights into Submicron Aerosol Composition and Sources from the
WINTER Aircraft Campaign over the Eastern US
Jason Schroder
Postdoctoral Researcher
Department of Chemistry and CIRES
University of Colorado, Boulder
Abstract
The WINTER aircraft campaign was a recent field experiment to probe the sources and evolution of gas and aerosol pollutants in Northeast US urban and industrial plumes during the winter. A highly customized Aerodyne aerosol mass spectrometer was flown on the NCAR C-130 to characterize submicron aerosol composition and evolution. Work towards constraining wintertime secondary organic aerosol formation and evolution will be presented from a case study of urban outflow from NYC. Observations and results of wintertime aerosol nitrate, power plant plume acidity, as well as measurements made from an oxidation flow reactor, flown for the first time, will be discussed.
Monday, February 1, 2016
Measurement of volatile organic compounds in the atmosphere using NO+ chemical ionization mass spectrometry
Abigail Koss, C. Warneke, P. Veres, B. Yuan, M. Coggon, J.A. de Gouw
Department of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
The underutilized technique of NO+ chemical ionization mass spectrometry (NO+ CIMS) may improve volatile organic compound (VOC) measurement in the troposphere. In this talk I describe the development of an NO+ CIMS instrument and evaluate the usefulness of the NO+ technique. The evaluation is established through labwork using a gas-chromatography (GC) interface, in-situ measurement of urban air using a GC interface, and direct measurement of urban air. NO+ is useful for fast (1Hz) measurement of carbonyl isomers, for small aliphatics, and large (C13-C15) n-alkanes. The NO+ CIMS technique may be an extremely useful approach for studies of SOA formation, photochemistry, and emissions from fossil fuels and biomass burning.
Fall 2015
Monday, December 7, 2015
Parameterization and evaluation of airborne halogen oxide measurements in the tropical transition layer and lower stratosphere
Barbara Dix, Sr. Research Associate
group of Prof. Rainer Volkamer
Dep. of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
Tropospheric halogen oxides catalytically destroy ozone, modify oxidative capacity and oxidize atmospheric mercury. Ozone is an important precursor for OH, which determines the lifetime of methane, an important greenhouse gas. About 75% of the global tropospheric ozone and methane loss occurs at tropical latitudes, where further the ozone radiative forcing is most sensitive to changes in the ozone budget. We have measured bromine and iodine monoxide (BrO and IO) by Airborne Multi-Axis Differential Optical Absorption Spectroscopy (AMAX-DOAS) over the Western, Central and Eastern tropical Pacific Ocean. AMAX-DOAS measures solar scattered light along changing lines of sight to detect trace gases at different altitudes. Light path lengths at instrument altitude can reach up to a few hundred km in thin air, which enables detection limits of about 0.3 pptv for BrO and 0.05 pptv for IO for 60s and 30s integration time respectively. The initial result of a DOAS analysis is the integrated concentration along the line of sight, i.e. a column measurement. To derive volume mixing ratios from MAX-DOAS data typically involves the time consuming process of simulating the light path contributions to each measured spectrum by radiative transfer modeling. Here we present a method to parameterize radiative transfer, which allows for a fast conversion of column measurements into volume mixing ratios along the flight track. We will compare parameterized results with vertical profiles we retrieved by inversion techniques and discuss advantages and limitations of our new method.
Monday, November 30, 2015
Immediate fates of the world’s oldest pesticide in California’s most lucrative crop
Eve-Lyn S. Hinckley
Assistant Professor of Environmental Studies
Fellow of the Institute of Arctic and Alpine Research
Abstract
Elemental sulfur (S0) has been used as an effective pesticide in agricultural systems since ancient Egyptian civilization. Today, in California’s winegrowing regions, applications of S0 dust are used as a preventative against powdery mildew infestation. Throughout the growing season, average applications are 150 kg S ha-1 yr1, and over Napa Valley vineyards alone, the applications total 810 Mg S0. Based on decades of research in northeastern U.S. forests that documented devastating ecosystem consequences of inadvertent reactive S and nitrogen deposition, I was inspired to understand how intensive, widespread, purposeful additions of S0 affect local-to-regional scale soil and water quality in agricultural systems. In Napa Valley vineyards, I have explored (1) What are the immediate fates of S0 locally in soils? and (2) What are the unintended consequences of its intensive, widespread use for downgradient ecosystems? In this talk, I will show the connection between hydrologic controls and the fate of S0 in California vineyards, and discuss how the pattern and consequences of continued applications might change under current drought conditions in the State. Ultimately, decisions about S pesticide management and water use in California’s winegrowing regions have implications for the sustainability of this industry, as well as the function of surrounding terrestrial and aquatic ecosystems.
Monday, November 16, 2015
Light-absorbing impurities and spectral albedo of the Juneau Icefield
Jennifer Lynne Berry
Department of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
Light absorption by ice is extremely low in the visible and near ultraviolet wavelengths, so small concentrations of light-absorbing impurities can have a large effect on snow and ice albedo. Snow samples containing light-absorbing impurities were collected alongside measurements of spectral albedo on the Juneau Icefield in Alaska to investigate this effect. An Integrating Sphere/Integrating Sandwich Spectrophotometer (ISSW) was constructed to infer black carbon and non-black carbon light-absorbing particle concentrations in snow. Although calibration of the ISSW is incomplete, Nanoparticle Tracking Analysis and SEM-EDX images of particles from meltwater show a wide range of particle sizes and distribution across the icefield.
Chemical and Physical Alterations of Acid-Treated Aluminosilicate Clay Minerals and Impacts on Heterogeneous Ice Nucleation
Kevin Jansen
Department of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
Mineral dust aerosol is a major contributor to global ice nucleation, although atmospheric processing of mineral dust by sulfuric and nitric acids has been found to alter its ice nucleation ability. Samples of kaolinite and montmorillonite, two aluminosilicate clay minerals, were treated with aqueous sulfuric and nitric acid to simulate atmospheric processing. The samples were studied X-ray diffraction, transmission electron microscopy, and inductively coupled plasma–atomic emission spectroscopy were used to study the structural changes due to acid treatment, which were correlated to observations found in ice nucleation experiments. On the basis of lattice spacing arguments, the observed reduction in ice nucleation activity of acid-treated minerals were correlated to physical and chemical alterations to the mineral structure and the formation aqueous salts on the mineral surface.
Monday, November 9, 2015
The Genetics of Terpenoid Production in Cannabis
Nolan Kane
Associate Professor
Ecology and Evolutionary Biology
University of Colorado at Boulder
Abstract
The Cannabaceae family produces an astonishing diversity of cannabinoids and related terpenes, which are thought to collectively provide the complex flavor and aroma of hops, as well as the psychoactive and medical effects of Cannabis. New research provides important insights into the genetic variation underlying this phytochemical diversity, and sheds light on approaches that can be used to improve breeding efforts to up- or down-regulate the production of these compounds.
Monday, November 2, 2015
Diversity of bioaerosols in indoor and outdoor air across the U.S.
Noah Fierer
Associate Professor
Ecology and Evolutionary Biology
and Fellow of CIRES
University of Colorado at Boulder
Abstract
Every time we breathe we are inhaling thousands of bacterial cells, fungal cells, and pollen grains. Although most of these microbes are innocuous, it is well-established that some airborne bacteria, fungi, and pollen can have important effects on human health. However, we have a limited understanding of how these bioaerosols vary across different geographic regions or the factors that structure their biogeographical patterns. We conducted a citizen science project that involved collecting dust samples from inside and outside ~1,500 households located across the U.S. to understand the continental-scale distributions of bacteria and fungi in indoor and outdoor air. We used high-throughput DNA sequencing to assess the diversity and sources of these bioaerosols, yielding our first insight into the continental-scale distributions of bioaerosols and how they are influenced by climate, land-use, home occupants, and home design.
Size-Dependent Molecular-Level Characterization of Secondary Organic Aerosol from NO3 Initiated Δ-carene Oxidation using Nanospray Desorption Electrospray Ionization High-Resolution Mass Spectrometry
Hyungu Kang
Department of Chemistry and Biochemistry
Abstract
We have collected size-separated Δ-carene oxidation aerosol samples using a Micro-orifice Uniform Deposit Impactor (MOUDI) by injecting Δ-carene into a dark flow-through chamber under low RH conditions (< 30%) with O3 and NO2 so the dominant reaction pathway would be with NO3 to form highly oxidized products.
The samples were analysed with a Nanospray Desorption Electrospray Ionization (nano-DESI) high-resolution mass spectrometer in both the positive and negative modes to reveal that the faster growing, and thus larger diameter, branch had a higher oxygen-to-carbon (O:C) ratio when compared with the lower branch, suggesting that the faster growing branch is more oxidized. Furthermore, the most intense peaks from the two branches were compositionally different, but a significant proportion of the formulas identified had N:C ratios of ~0.1, which suggests that organic nitrates are a large component of the aerosol products.
Monday, October 26, 2015
High-resolution mass spectrometry: peak fitting and aerosol partitioning
Harold Stark
Department of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
When measuring oxidized organic molecules in the atmosphere, often thousands of compounds can be present and provide a challenge for conclusive assignment in high-resolution mass spectra, particularly when the goal is to retrieve data series over long time periods of weeks or months, as typical in field campaigns. We have developed algorithms to identify and assign peaks in complex mass spectra to produce reliable peak lists for analyzing large data sets. Simulation of mass spectra confirmed the validity of the assignment algorithms for calculation of bulk chemical properties such as carbon number and carbon oxidation state. A new method of deriving such chemical properties without the need for any peak assignment will also be presented.
Application of a peak list from the new algorithms to measurements from a chemical-ionization mass spectrometer deployed in a Pine forest in Colorado allowed measurement of gas-particle partitioning of hundreds of organic compounds. Comparing two methods of deriving saturation concentration distributions (“volatility basis sets”) from the same dataset highlights the importance of fragmentation in thermal desorption techniques.
Particle Size Resolution of the Aerodyne Aerosol Mass Spectrometer
Doug Day
Department of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
Real-time determination of chemically-resolved particles size distributions and their evolution in laboratory and ambient studies can be used to investigate changing source contributions, growth rates, secondary aerosol yields, cloud condensation nuclei potential and other physicochemical processes. The Aerodyne Aerosol Mass Spectrometers (AMSs) provides fast size and chemical composition measurements of particles from ~50 nanometers to ~1 micrometer. This work addresses the resolution of the size measurement via particle time-of-flight (PToF) in this instrument. An algebraic model is used to estimate size resolution for different configurations of the instrument as well as particle diameters and vaporization rates. Under typical AMS configurations, for a given particle size, resolution is primarily controlled by the uncertainties in particle time-of-flight due to the finite opening time of the particle gating chopper and particle vaporization. The model was tested with a range of chopper gate widths, particle sizes, and particle compositions, and agreements and disagreements are investigated. The methodology to estimate the size resolution can be applied to all instruments that use particle time-of-flight to infer particle size. Instrument and data acquisition configurations can be optimized for a range of different particle sampling conditions including ambient sampling under low or high loadings, for aircraft platforms, for laboratory applications where loadings may be controllable or narrow particle size ranges are present, and when single-particle chemical or light-scattering detection is possible.
Monday, October 19, 2015
Portable Air Pollution Monitors and the Global Ozone (GO3) Project
John Birks
President, 2B Technologies and Director, Global Ozone (GO3) Project, Boulder, CO
Abstract
Over the past 15 years we have developed highly portable instruments for measurements of ozone, NO, NO2 and black carbon. These instruments have enabled measurements in remote locations and extreme environments around the world. Our small, light-weight, low-power instruments have been widely used for atmospheric measurements on balloons, kites, UAVs, towers, trams and research aircraft, and at many remote sites, including Antarctica, the Galapagos Islands, the Greenland ice sheet, the summit of Mont Blanc, the Amazon rain forest, on buoys in the Arctic Ocean, on commercial airliners, in many U.S. National Parks and at numerous other locations throughout the world.
The Global Ozone or “GO3” Project was founded as a non-profit organization for educational outreach in 2009. In the GO3 Project, middle and high school students at more than 100 schools around the world measure air pollutants and meteorological parameters outside their schools and upload their data to a public database every 15 minutes. The data may be graphed online and displayed on Google Earth. Students interpret their results and discuss their findings in blogs and forums in a social network similar to Facebook. The GO3 Project includes an online, interactive curriculum where students learn about atmospheric environmental problems and how they are interrelated, including: ground level ozone, stratospheric ozone depletion ("ozone hole"), acid rain and global climate change. The GO3 Project also includes a hands-on Black Carbon Experiment where students measure this important air pollutant by collecting particles on a filter and measuring the optical transmission through the filter using a simple but accurate photometer. In our newest project, GO3 Treks, students hypothesize how air pollutants vary along treks of their own design, and then carry out the treks using pocket-sized ozone and black carbon monitors. Treks are displayed on Google Earth within blogs where students discuss
Monday, October 12, 2015
CE-DOAS at its Detection Limit
Henning Finkenzeller
1st Year Graduate Student
Abstract
Cavity Enhanced Differential Optical Absorption Spectroscopy (CE-DOAS) is an established method for the in-situ measurement of trace gas concentrations. Its detection limit is often not determined by noise but systematical effects.
I comment on the combined effect of high frequency intensity variations and the non-linearity of the spectrometer in intensity space on the detection limit, which is estimated from model calculations. I also present the new calibration method ICOM (Integrated Calibration by means of Optical Modulation) for the determination of light path lengths in optical resonators, which is promising because of its high performance concerning spectral resolution, absolute accuracy and precision.
Heterogeneous Chemistry in the tropical free troposphere
Rainer Volkamer
Professor of Chemistry and Biochemistry & CIRES Fellow, University of Colorado, Boulder
Abstract
Tropospheric halogens catalytically destroy ozone, oxidize atmospheric mercury, and modify the oxidative capacity of the atmosphere. Ozone is a potent greenhouse gas, and an important precursor for hydroxyl (OH) radicals that determine the lifetime of CH4 another important greenhouse gas. About 75% of the global tropospheric O3 and CH4 loss occurs at tropical latitudes, where O3 radiative forcing is further most sensitive to changes in O3. Oxygenated VOCs (OVOC) affect the atmospheric oxidative capacity, modify NOx, and O3, but recent first measurements of selected OVOC, like glyoxal, methylvinylketone, and butanal in the tropical free troposphere currently remain unexplained by atmospheric models. Water soluble OVOC are further a globally relevant source of secondary organic aerosol (SOA). This talk reviews recent advances in instrumentation, and their applications in field observations, laboratory experiments, and modeling results in the Volkamer group. Selected recent publications: Waxman et al. (2015): Glyoxal and Methyl Glyoxal Setschenow Salting Constants in Sulfate, Nitrate and Chloride Solutions: Measurements and Gibbs Energies. Environ. Sci. Technol., doi: 10.1021/acs.est.5b02782. Saiz-Lopez et al. (2015): Injection of iodine to the stratosphere, Geophys. Res. Lett., doi: 10.1002/2015GL064796. Wang et al. (2015): Active and Widespread Halogen Chemistry in the Tropical and Subtropical Free Troposphere, Proc. Natl. Acad. Sci., 112 (30), 9281–9286, 2015. doi: 10.1073/pnas.1505142112. Volkamer et al. (2015): Aircraft measurements of BrO, IO, glyoxal, NO2, H2O, O2-O2 and aerosol extinction profiles in the tropics: Comparison with aircraft-/ship-based in situ and lidar measurements, Atmos. Meas. Tech. 8, 2121-2148, 2015. doi:10.5194/amt-8-2121-2015.
Monday, October 5, 2015
Building molecular complexity with sunlight at aqueous interfaces
Veronica Vaida
Professor, Department of Chemistry and Biochemistry &
CIRES Fellow, University of Colorado-Boulder
Abstract
High energy, low entropy solar radiation can be used to initiate radical reactions leading to organic polymers and oligomers in environmental systems. In atmospheric chemistry, the role of the photochemically generated OH radical is well studied. However, the role of other radicals, especially those generated from organic precursors, is less well-understood. This presentation will discuss examples of photochemically-initiated organic radical reactions at the water surface. Specifically, the photochemistry of oxoacids, such as pyruvic acid as well as a series of oxoacids with longer aliphatic side chains, will be discussed. Photochemical processing occurs with solar simulators under both aerobic and anaerobic environments, characteristic of the contemporary and ancient Earth’s atmosphere, respectively. The polymers produced are investigated by mass spectrometry and NMR, while the supramolecular aggregates that spontaneously self-assemble during photolysis are monitored by microscopy and dynamic light scattering. Implications of these abiotic processes to atmospheric and environmental chemistry will be discussed, specifically as they affect aerosol nucleation and growth.
Atmospheric Reduced Nitrogen: Trends and Future Directions
Eleanor Browne
Professor of Chemistry and Biochemistry &
CIRES Fellow, University of Colorado, Boulder
Abstract
Organic nitrogen is a ubiquitous atmospheric component typically accounting for between one-quarter and one-third of reactive nitrogen deposition, however, its chemical complexity and its reactivity has made it challenging to study. Consequently, little is known about the atmospheric processing of organic nitrogen and the resulting implications for biogeochemistry, air quality, and climate. Organic nitrogen can be broadly separated into two groups: oxidized organic nitrogen compounds such as acyl peroxy nitrates and reduced organic nitrogen compounds such as amines. Historically, our knowledge of the chemistry of reduced organic nitrogen has lagged behind that of oxidized organic nitrogen due to the dominance of oxidized nitrogen sources. Recent enactment of air quality regulations in the United States and parts of Europe, however, has resulted in decreased emissions of oxidized nitrogen while emissions of reduced nitrogen have remained constant or have increased due to expansion of agriculture and increased use of fertilizer. Thus, it is timely to study the chemistry of reduced organic nitrogen. I will discuss trends in emissions of reduced nitrogen, its implications for reduced organic nitrogen, and future studies on the chemistry of reduced organic nitrogen.
Monday, September 21, 2015
Chemistry of Organic Compounds in the Atmosphere and Indoor Air
Paul Ziemann
Professor, Department of Chemistry and Biochemistry &
CIRES Fellow, University of Colorado-Boulder
Abstract
Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to achieve a deep understanding of atmospheric and indoor air chemistry and to develop detailed and accurate models that are used to establish air quality regulations and to predict the effects of human activities. Research in my laboratory focuses primarily on the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form microscopic aerosol particles. Studies are conducted in large-volume environmental chambers where experiments are designed to simulate but simplify atmospheric chemistry and conditions in order to obtain information on gas and particle chemical composition, reaction rates and equilibria, and particle properties. Obtaining such data is a challenge, but in this talk I will describe how we approach this problem by using a diverse array of measurement techniques. I will also discuss our more recent studies on the chemistry of indoor air.
Jose Jimenez
Professor of Chemistry and Biochemistry &
CIRES Fellow, University of Colorado, Boulder
Spring 2015
Monday, April 13, 2015
Optical properties of brown carbon aerosol in the near-ultraviolet spectral region</b>
Rebecca Washenfelder
NOAA Chemical Sciences Division
Abstract
Aerosol scattering and absorption are among the largest uncertainties in radiative forcing. Black carbon is a strong radiative forcing agent, and absorbs strongly throughout the ultraviolet and visible spectral regions. In contrast, brown carbon has a wavelength-dependent absorption that increases sharply in the ultraviolet spectral region and its importance in radiative forcing is more unknown. Part of this uncertainty arises from the need to characterize potential sources of brown carbon aerosol, which include fossil fuel combustion, biomass burning, and secondary organic aerosol aging processes.
We have developed a new method to measure aerosol optical extinction as a function of wavelength for in situ aerosol, using cavity enhanced spectroscopy. We have demonstrated this method over the 360-420 nm spectral region, and plan to extend it to 300 nm. We retrieve complex refractive indices as a function of wavelength from the measured extinction cross sections. In the laboratory, this technique has allowed us to examine a proposed mechanism to produce brown carbon aerosol from the reaction of ammonia or amino acids with carbonyl products in secondary organic aerosol.
During the Southern Oxidant and Aerosol Study in summer 2013, we acquired field measurements of aerosol optical extinction at 360-420 nm. We combined these data with direct absorption measurements of water-soluble organic carbon obtained from a UV/VIS-WSOC instrument, and with aerosol composition measurements. I will present the magnitude of brown and black carbon absorption and the relative contributions of biomass burning, anthropogenic, and secondary organic aerosol contributions to brown carbon absorption in the Southeast U.S. during the summer.
Monday, April 6, 2015
Forensics, Metabolomics and Molecular Imaging by Mass Spectrometry
Facundo M. Fernández, Ph. D.
Professor
School of Chemistry and Biochemistry
Georgia Institute of Technology
Abstract
Mass Spectrometry (MS) is one of the key analytical methods used to identify and characterize small quantities of biological molecules embedded in complex matrices. Although MS has found widespread use, technical improvements in its instrumentation are needed to extend its application to the grand challenges that face the environmental, chemical and biomedical sciences. In this talk I will present insights into new approaches for generating ions under atmospheric pressure in an “open air” format followed by mass spectrometric detection. That research has enabled our group to perform a series of experiments in the fields of forensics, imaging and metabolomics. I will describe how “open air” MS has helped us detect the components in and track the sources of counterfeit drugs in developing countries, perform high throughput metabolic fingerprinting of patients with cancer, and image a variety of surfaces. I will also describe more fundamental work involving finite element simulations and Schlieren imaging of ion transport processes at the atmospheric pressure interface of the mass spectrometer. Finally, I will describe new results where plasma ion sources are used for better coupling of LC to MS.
Monday, March 30, 2015
Advances in the Quantitation of Atmospheric Organosulfates
Elizabeth Stone
Assistant Professor
Department of Chemistry
University of Iowa
Iowa City, IA 52242
Abstract
Organosulfates are significant components of secondary organic aerosols (SOA) formed under acidic conditions. This class of compounds contains a characteristic sulfate ester functional group (R-O-SO3-) and is suggested to be a significant contributor to organic carbon in fine particles in the atmosphere. However, the quantification of individual organosulfates molecules has proven challenging due to the lack of authentic quantification standards and suitable methods of analysis. To overcome these challenges, a series of organosulfates standards was synthesized and used to develop and validate of a new analytical method for their quantification. Recent results from the application of this method to ambient aerosol collected in Centreville, Alabama during the Southeast Atmosphere Study (SAS) in 2013 will be discussed.
Monday, March 16, 2015
Tropospheric Reactive Chlorine: Observations, Sources and Effects
Lea Hildebrandt Ruiz, Ph.D.
Assistant Professor
The University of Texas at Austin
Cockrell School of Engineering
McKetta Department of Chemical Engineering
Austin, TX 78712-1589
Abstract
Ambient measurements have detected tropospheric reactive chlorine concentrations much higher than predicted by state-of-the-art air quality models, challenging current understanding of the emissions and atmospheric chemistry of chlorinated compounds. For example, measurements in the Dallas Fort Worth (DFW) area routinely observed HCl concentrations of 1 ppb or more, peaking in the late afternoon. Modeling work has investigated several hypotheses for the source of HCl in DFW, and results suggest emissions of an organic chloride, potentially from hydraulic fracturing activity, as the most likely explanation. In the presence of particulate chloride reactive chlorine can also be formed from heterogeneous reactions on the particles’ surface. Laboratory chamber experiments were conducted to quantify the rate of heterogeneous production of chlorine, and results suggest that this path could explain observed sources of Cl2. Higher concentrations of reactive chlorine can lead to increased production secondary organic aerosol (SOA). Mass yields of SOA formed from chlorine-radical-initiated oxidation of hydrocarbons were measured in laboratory chamber experiments. Volatility basis set parameters from these experiments can be incorporated in air quality models to more accurately represent reactive chlorine concentrations and its effects on atmospheric composition.
Monday, March 9, 2015
Secondary Organic Aerosol Modeling using the Statistical Oxidation Model
Shantanu Jathar
Colorado State University
Abstract
Multi-generational gas-phase oxidation of organic vapors can influence the abundance, composition and properties of organic particulate matter or organic aerosol. Most air quality and climate models lack or include an ad hoc treatment of multi-generational oxidation. In this seminar, I will highlight our recent work where we coupled a semi-explicit multi-generational oxidation model for organics (fully constrained by experimental smog chamber data that includes both functionalization and fragmentation reactions) with a gas-phase chemical mechanism in a regional 3-D air quality model. The seminar will discuss results where we (a) investigated the role of multi-generational gas-phase chemistry on the mass, composition, volatility and oxidation state of SOA and (b) explored the influence of vapor wall-losses on ambient concentrations and properties of SOA. Based on those results, I will argue that 3-D models need to include (this or similar) multi-generational oxidation schemes to accurately describe the atmospheric evolution of OA in air quality and climate models.
Monday, March 2, 2015
Optical Properties of Titan Haze Analogs Using Photoacoustic and Cavity Ring-Down Spectroscopy
Melissa S. Ugelow
3rd Year Graduate Student
Abstract
The organic haze that surrounds Saturn's moon Titan is formed through the photolysis and electron initiated dissociation of methane and nitrogen. Both the chemical pathways leading to the haze formation and the resulting haze optical properties are still highly uncertain. Here we examine the optical properties of simulated haze aerosol to better understand its scattering and absorption properties, and the impact of haze on Titan's radiative balance. To determine the complex refractive index of haze particles, we combine two spectroscopic techniques, one that measures absorption and one that measures extinction: photoacoustic spectroscopy coupled with cavity ring-down spectroscopy (PASCaRD). This technique provides the benefit of a high precision determination of the imaginary component of the refractive index (k), along with the highly sensitive determination of the real component of the refractive index (n) in a flow system set up. The Titan aerosol analogs studied are produced by two energy sources, UV excitation and spark discharge excitation. The refractive indices are determined at two wavelengths, 405 and 532 nm, using the PASCaRD system. I will present preliminary data on the complex refractive indices of laboratory generated Titan aerosol analogs at both wavelengths using both energy sources. The high precision values determined from this method should be useful for modelers and for data retrieval from spacecraft and remote sensing instruments.
Monday, February 23, 2015
Chemical Nucleation in the Atmosphere: Recent Discoveries Enabled by Instrument Development
Peter H. McMurry
Department of Mechanical Engineering
111 Church St. SE
University of Minnesota, Minneapolis, MN 55455 USA
Abstract
Observations throughout the atmosphere have shown that nucleation from the gas phase occurs every few days and that nucleation rates are correlated with collision rates of sulfuric acid vapor molecules. Our research team, which includes the groups led by Drs. Fred Eisele, Jim Smith and David Hanson, have developed three new instruments to study atmospheric nucleation: the Cluster Chemical Ionization Mass Spectrometer (Cluster CIMS), for measuring the concentrations of neutral molecular clusters formed by nucleation (1-10 ppqv sensitivity); the DEG Scanning Mobility Particle Spectrometer (DEG SMPS), for measuring the number distributions of freshly nucleated particles as small as 1 nm; and the Ambient Pressure Proton Transfer Mass Spectrometer (AmPMS), for measuring concentrations of basic organic and inorganic gases that react with and stabilize sulfuric acid-containing clusters (1pptv sensitivity).
Together, these instruments allow measurements of precursor vapor concentrations and the complete particle number distribution down to 1 molecule. This information is providing us with a new understanding of the physical and chemical processes that lead to nucleation.
Atmospheric observations and laboratory studies have confirmed that nucleation occurs due to a sequence of acid-base reactions that form stable clusters that subsequently grow by the further uptake of organic and inorganic compounds. Evaporation of sulfuric acid from clusters that contain 1 to 3 sulfuric acid molecules is the primary bottleneck to nucleation. Our measurements have provided estimates of these evaporation rates, leading to a simple analytic expression for nucleation rates that is in reasonable agreement with observations.
The seminar will describe the bases for these conclusions.
Monday, February 16, 2015
Rethinking Secondary Organic Aerosol formation and removal in 3D models based on explicit chemistry
Dr. Alma Hodzic, NCAR, Boulder, CO
Current secondary organic aerosol (SOA) parameterizations fail to explain the observed amounts and properties of tropospheric SOA, which results in large uncertainties in their effects on radiation and climate. We use the Generator of Explicit Chemistry and Kinetics of Organics in the Atmosphere (GECKO-A) to simulate SOA gas-phase chemistry in various environments (urban, forest, low/high NOx). Our results show that GECKO-A predicts significant SOA mass growth in urban plumes, and formation of less volatile and more soluble organic products, than predicted by parameterizations typically used in 3D models. The results also indicate that atmospheric processing of gas-phase oxidation intermediates, which are poorly represented in 3D models, can significantly modulate SOA formation and lifetime. We discuss the possible effect of dry deposition and organic photo-fragmentation reactions on condensable organic vapors and SOA. We derive new parameterizations that reproduce GECKO-A behavior in terms of volatility, yields and solubility for use in 3D models. These parameterizations are applied at regional and global scales to re-evaluate SOA concentrations, burden and removal.
Wednesday, February 11, 2015
Isoprene aerosol yields quantified with space-based relationships between aerosol optical depth and formaldehyde: contrasting regimes in Africa and the southeast US
E. A. Marais et al., Harvard University
Abstract: Isoprene is an important precursor of organic aerosol (OA), but yields of OA are uncertain. We obtain satellite-derived yields of isoprene OA for Africa and the southeast US by exploiting spatial and temporal consistency between aerosol optical depth (AOD) from MODIS and formaldehyde, ΩHCHO, from OMI. GEOS-Chem chemical transport model yields of isoprene OA using reversible partitioning of isoprene OA are low (<1%) and the resultant AOD is lower than MODIS AOD. GEOS-Chem shows the observed slope from regression of AOD against ΩHCHO is a sensitive indicator of yields of isoprene OA and reproduces observed slopes when partitioning to the aerosol phase is irreversible and regional mean isoprene OA yields are 5.5±1.8% for Africa and 8.6±2.4% for the southeast US. Gas-phase isoprene oxidation is predominantly by NO in the southeast US, and mixed NO and HO2 in Africa. Higher satellite-derived yields under NO dominant conditions is consistent with higher yields observed with chambers operating close to ambient NO2 to NO ratios. We infer lower yields of isoprene OA (3.0%) with boundary-layer southeast US SEAC4RS aircraft observations, as GEOS-Chem calculation of AOD is sensitive to vertical distribution of isoprene OA. Simulated yields of isoprene OA are uniform in GEOS-Chem, but SEAC4RS-derived yields increase to 4.2% at 2-4 km. The AOD we simulate in Africa and the southeast US with our satellite-derived yields implies larger direct radiative forcing from isoprene OA than previous model estimates.
Monday, February 9, 2015
Formation of Low Volatility Compounds and SOA from Isoprene Oxidation without IEPOX uptake
Jordan Krechmer
3rd Year Graduate Student
Department of Chemistry and Biochemistry
University of Colorado, Boulder
Abstract
Many of the sources and formation mechanisms of secondary organic aerosol (SOA) are still poorly understood. In this talk, I will present evidence of gas-phase low volatility organic compounds (LVOC), produced from the OH oxidation of isoprene hydroxy hydroperoxide (ISOPOOH), condensing and forming secondary organic aerosol (SOA) during the Caltech FIXCIT chamber study. Decreases in LVOC concentrations directly corresponded to the appearance and growth in organic aerosol, indicating that LVOC were condensing and forming SOA (at OA levels below 1 μg m-3) with a wall-loss corrected mass yield of roughly 1.5% (from isoprene). This previously uncharacterized formation pathway from isoprene could account for up to 8.0 Tg yr-1 of SOA, or 5% of the estimated global SOA source. The experimental conditions and AMS SOA spectrum indicate that SOA formation in this study is separate and not explained by previously described IEPOX uptake. Condensing species have 4-5 carbons and volatilities consistent with multiple hydroxyl groups, as well as carbonyl and possibly hydroperoxide groups. These species are not extremely low volatility VOCs (ELVOC) as previously observed from monoterpene oxidation by O3, but most likely LVOC with saturation concentrations ranging from 10-2 to 10¬ μg m-3. Their ability to condense at low OA levels at room temperature and to grow nanoparticles indicate that their importance may be largest in environments with lower OA concentrations. The same LVOC compounds were observed in the atmosphere during the SOAS campaign in the SE US. The results of efforts to extract mechanistic information from an ion mobility spectrometer-mass spectrometer (IMS-MS) during the SOAS field study and subsequent laboratory work will also be presented.
Monday, February 2, 2015
Photon and Water Mediated Sulfur Chemistry in Planetary Atmospheres
Jay Kroll, 3rd Year Graduate Student
Department of Chemistry and Biochemistry
Abstract
Sulfur compounds have been observed in the atmospheres of a number of planetary bodies in our solar system including Earth and Venus. The global cloud cover on Venus located at an altitude between 50 and 80 kilometers is composed primarily of sulfuric acid (H2SO4) and water. Planetary photochemical models have attempted to explain observations of sulfuric acid and sulfur oxides with significant discrepancies remaining between models and observation. I will report on recent laboratory studies attempting to measure the kinetics of reactions of SO2 and water to form sulfurous acid and progress made in instrument development.
Monday, January 26, 2015
Weeding Out the Myths: Introduction to Medical Marijuana
Laura Borgelt, FCCP, BCPS
Associate Dean for Administration and Operations
Professor, Departments of Clinical Pharmacy and Family Medicine
University of Colorado
, Skaggs School of Pharmacy and Pharmaceutical Sciences
Abstract
Medical marijuana laws and funded studies in Colorado make it increasingly important to understand the characteristics and appropriate use of medical marijuana. This presentation will use an evidence-based approach to introduce the endocannabinoid system and the pharmacology of marijuana as a potential treatment for various conditions. The proposed mechanisms of action for marijuana provide valuable information about its potential benefits and risks. Additionally, the pharmacokinetics of marijuana, including its absorption, distribution, metabolism, and elimination, will be discussed to help participants determine how various dosage forms may be used. The overall goal is to help participants learn more about how marijuana works and how that relates to the risks and benefits of using medical marijuana.
Fall 2014
Monday, Nov. 17th, 2014
Iodine Monoxide observations from CU AMAX-DOAS aboard the NSF NCAR GV research aircraft
Theodore Koenig 3rd Year Graduate Student Department of Chemistry and Biochemistry, University of Colorado
Abstract
The CU Airborne Multi-AXis Differential Optical Absorption Spectroscopy (CU AMAX-DOAS) instrument was deployed for the TORERO and CONTRAST field campaigns. These were in the Tropical Eastern Pacific in January-February 2012, and the Tropical Western Pacific in January-February 2014 respectively. We report the first scattered light limb measurements of IO. I will discuss a number of highlights from our observations: 1) instances of enhanced IO mixing ratios in the transition layer 2) a free troposphere background of IO that shows little variation with altitude 3) a hemispheric gradient in total IO column and 4) the first quantification of IO in the lower stratosphere. These measurements provide the best constraints to date on IO outside the planetary boundary layer. They offer the chance to begin assessing our understanding on iodine chemistry in the transition layer, free troposphere, and lower stratosphere and its impacts of climate, particularly tropospheric ozone.
Monday, Nov. 10th, 2014
ANALYTICAL & ENVIRONMENTAL CHEMISTRY DIVISION and ATMOSPHERIC CHEMISTRY PROGRAM SEMINAR
Jointly sponsored by the Department of Chemistry and Biochemistry, CIRES, and the Environmental Program
November 10, 2014 12:00 Noon - 1:00 pm Ekeley S274
University of Colorado, Boulder
Characterization of a Real-Time Tracer for Isoprene Epoxydiols-Derived Secondary Organic Aerosol (IEPOX-SOA) from Aerosol Mass Spectrometer Measurements
Weiwei Hu et al.
Cooperative Institute for Research in Environmental Sciences, and Department of Chemistry and Biochemistry, University of Colorado, Boulder
Abstract
Secondary organic aerosol (SOA) can be formed from isoprene epoxydiols (IEPOX), compounds that are produced from isoprene oxidation under low-NO conditions. IEPOX-SOA can account for a substantial fraction of organic aerosol (OA) in biogenic-influenced areas. In this study, IEPOX-SOA was identified from measurements at the forested Southeast U.S. supersite (Centreville, AL) during the Southern Oxidant and Aerosol Study (SOAS) using Positive Matrix Factorization (PMF) of aerosol mass spectrometer (AMS) measurements. The SOAS results clearly show that the fraction of OA measured at C5H6O+ (fC5H6O) in AMS spectra is a good tracer for IEPOX-SOA and correlates with the well-known methyltetrol tracers of this chemistry. Other field and chamber studies are included in the analysis to investigate the robustness of fC5H6O as an IEPOX-SOA tracer in AMS data. We observed clearly higher fC5H6O (3x10-3 - 25x10-3) in OA from regions with strong isoprene emissions vs those from urban and biomass-burning plumes (0 - 3.5x10-3 with an average of 1.75x10-3). In isoprene-influenced areas, fC5H6O in ambient OA positively correlates with the relative contribution of IEPOX-SOA to OA and decreases with OA aging. The kOH for destruction of IEPOX-SOA via heterogeneous oxidation in aerosol phase is approximately 5.3x10-13 cm3 molec-1 s-1, with life time is around 14.5 days (assuming average OH concentration=1.5x106 molecular cm-3). Volatility analysis using a thermal denuder shows that IEPOX-SOA is not more volatile than bulk SOA, contrasting with the high volatility of the tracers identified. Finally, we develop a simplified method to estimate ambient IEPOX-SOA mass concentrations as a function of fC5H6O, which is shown to perform well compared to the full PMF method. When only unit mass resolution data is available as in ACSM data, the method performs less well because of increased interferences from other ions at m/z 82. Estimated IEPOX-SOA concentrations in the southeastern U.S. from an aircraft campaign (SEAC4RS)correlate well with the IEPOX-related species detected by other techniques over a wide range of conditions, which confirms the usefulness of our method. IEPOX-SOA accounts for 14 - 17% of the OA in the SE U.S. during the summer according to both the SEAC4RS and SOAS results.
Oxidation flow reactors for the study of atmospheric chemistry systematically examined by modeling
Zhe Peng Cooperative Institute for Research in Environmental Sciences, and Department of Chemistry and Biochemistry, University of Colorado, Boulder
Abstract
Oxidation flow reactors (OFRs) using OH produced from low-pressure Hg lamps at 254 nm(OFR254) or both 185 and 254 nm (OFR185) are commonly used in atmospheric chemistry and other fields. OFR254 requires the addition of externally formed O3 since OH is formed from O3 photolysis, while OFR185 does not since O3 is formed in the reactor and OH can also be formed from H2O photolysis. In this study, we use a plug-flow kinetic model to investigate OFR properties under a very wide range of conditions applicable to both field and laboratory studies. We show that the radical chemistry in OFRs can be characterized as a function of UV light intensity, H2O concentration, and total external OH reactivity (e.g., from VOCs, NOx, and SO2). In OFR185, OH exposure is more sensitive to external OH reactivity than in OFR254, because injected O3 in OFR254 promotes the recycling of HO2 to OH, making external perturbations to theradical chemistry less significant. There has been some speculation in the literature about whether “non-tropospheric chemistry” (photolysis at 185 or 254 nm, and/or reactions with O(1D) and O(3P)) may play an important role in these OFRs. For field studies in forested regions or the urban area of Los Angeles, reactants of atmospheric interest are predominantly consumed by OH. Nontropospheric oxidants contribute to the degradation of some species under conditions of low H2O concentration and/or very high external OH reactivity. This appears to have been a problem in some laboratory and source studies, but can be avoided in future studies by experimental planning based on our findings. Some biogenic VOCs can have substantial contributions of reaction with O3 under some operating conditions, especially for OFR254. NO3 may have played an unexpectedly significant role in some past laboratory studies. RO2 fate is similar to that in the atmosphere under low-NOx conditions. A comparison of OFRs with typical environmental chamber studies using UV blacklights and with the atmosphere is presented. OFRs’ key advantages are their short experimental time scales, portability to field sites, enabling a direct connection of field and laboratory studies, and controllable and predictable radical chemistry. This study further establishes the usefulness of these reactors and enables better experiment planning and interpretation.
Monday, Nov. 3rd, 2014
Uncovering the structure and dynamics of a single nanoparticle using the world’s shortest laser pulses
Dan Hickstein, Kapteyn-Murnane Group, JILA, University of Colorado
Abstract
Modern femtosecond lasers can produce pulses of light that are shorter than the vibrational periods in molecules and generate electric fields stronger than the Coulomb field that binds electrons in atoms. These lasers are ideally suited to studying ultrafast processes in nanomaterials, such as electron transfer in photovoltaic nanostructures. However, all previous laser-nanoparticle experiments have been conducted on nanoparticles suspended in solvent, embedded in a bulk material, or attached to a surface, meaning that powerful gas-phase spectroscopy techniques cannot be used. In 2011 we embarked on a collaboration with the Jimenez group to construct a photoelectron–photoion spectrometer capable of examining isolated nanoparticles in the “gas phase.” To our surprise, the completed spectrometer was capable of recording the complete photoion distribution resulting from the interaction of a single nanoparticle with the femtosecond laser pulse. This breakthrough technique has allowed for the examination of localized light fields in nanostructures, the discovery of shock waves in nanoscale plasmas, and the observation of the evanescent wavefunction in quantum dots. In this talk I describe how we combined the tools of physical chemistry, laser physics, and atmospheric science to construct this new instrument, describe the discoveries to-date, and discuss our plans for future experiments, which include pushing the time-resolution towards the attosecond frontier.
Monday, October 27th, 2014
The Structure of Atmospheric Particles & Impacts on Atmospheric Chemistry and Climate
Miriam Freedman, Penn State University
Abstract
The interactions of aerosol particles with light and clouds are determined in part by the structure of atmospheric particles, which is the focus of research in my laboratory. My talk will focus on molecular-level studies of surfaces relevant for cirrus (ice) cloud formation and the phase separation behavior of submicron aerosol particles composed of organic and inorganic components. Through these projects, I will demonstrate the importance of characterizing aerosol structure in determining aerosol physical and chemical properties relevant to atmospheric chemistry and climate.
Monday, October 20th, 2014
Analysis of arson fire debris by low temperature dynamic headspace adsorption porous layer open tubular columns
Megan Harries, First Year Graduate Student, University of Colorado, Boulder
Abstract
We present results of the application of PLOT-cryoadsorption (PLOT-cryo) to the analysis of ignitable liquids in fire debris. We tested ignitable liquids, broadly divided into fuels and solvents, on three substrates: Douglas fir, oak plywood and Nylon carpet. We determined that PLOT-cryo allows the analyst to distinguish all of the ignitable liquids tested by use of a very rapid sampling protocol, and performs better (more recovered components, higher efficiency, lower elution solvent volumes) than a conventional purge and trap method. We also tested the effect of latency (the time period between applying the ignitable liquid and ignition), and we tested a variety of sampling times and a variety of PLOT capillary lengths. Reliable results can be obtained with sampling time periods as short as 3min, and on PLOT capillaries as short as 20cm. The variability of separate samples was also assessed, a study made possible by the high throughput nature of the PLOT-cryo method. We also determined that the method performs better than the conventional carbon strip method that is commonly used in fire debris analysis. Future work will center on improving the understanding of phase equilibria of complex mixtures and on the development of relevant equations of state.
Poly- and Per-Fluorinated Alkyl Substances in the Environment
Julia Bakker-Arkema, 1st Year Graduate Student, Department of Chemistry and Biochemistry
Abstract
This talk will explore the research of Dr. Scott Mabury and his work on poly and perfluorinated alkyl substances, or PFAS, in the environment. I will examine the ways that PFAS are transported and transformed in different systems, specifically the atmosphere, soil and water, and biological systems.
Monday, October 13th, 2014
Ozonolysis of a polyunsaturated acid and its primary oxidation products
Zachary Fineway, First Year Graduate Student, University of Colorado, Boulder
Abstract
Detection of glyoxal, the smallest α-dicarbonyl, in remote locations over the Pacific Ocean, has prompted research into its sources. Glyoxal is likely produced by oxidation of compounds within the sea surface microlayer. Reactions of linoleic acid, a polyunsaturated fatty acid found in the sea surface microlayer, and its primary gas phase oxidation products yielded glyoxal without an OH scavenger present. In the presence of an OH scavenger, glyoxal production is significantly reduced, and malondialdehyde, the smallest β-dicarbonyl, was detected. These findings elude to a mechanism of glyoxal formation, and a possible explanation of why malondialdehyde has not been detected in the atmosphere.
Pond Scum and Boiling Water: Water Chemistry at Yellowstone National Park
Randall Chiu, First Year Graduate Student, University of Colorado, Boulder
Abstract
Since the mid-1990’s, the Nordstrom lab at the US Geological Survey National Research Program (USGS NRP) has sampled numerous hot springs and rivers in Yellowstone National Park (YNP). The data, collected at least annually, provide a unique record of the inorganic chemistry (including major cations and anions, trace metals, and unusual species such as polythionates) of the geothermal waters at YNP. The data are used by collaborators at various universities to supplement microbiology research and also by USGS researchers investigating the geochemistry of YNP. This talk will summarize some of the current research efforts at YNP, with emphasis on USGS work that has public health and safety implications.
Monday, October 6th, 2014
Nitrogen Oxides and Aerosols (NOaA): Some applications of cavity enhanced spectroscopy in atmospheric chemistry
Stephen S. Brown
Chemical Sciences Division, NOAA Earth System Research Lab Department of Chemistry and Biochemistry, University of Colorado
Abstract
Nitrogen oxides (NOx) are emitted from both anthropogenic and natural processes and critically influence the oxidizing capacity of the atmosphere. Aerosols arise from a variety of sources and impact climate through their radiative effects and atmospheric chemistry through heterogeneous and multiphase reactions. Nitrogen oxides in particular undergo distinct heterogeneous reactions that link gas and aerosol phase chemistry. Both nitrogen oxides and aerosols are amenable to measurement by new, high sensitivity optical techniques known as cavity enhanced spectroscopy. I will survey some recent efforts and future projects toward development of this instrumentation, as well as several applications, including nighttime chemistry, wintertime photochemistry and aerosol optical properties.
Monday, September 29, 2014
A new mechanism for solid formation in the atmosphere: contact efflorescence
Margaret Tolbert Professor, Department of Chemistry and Biochemistry & CIRES Fellow, Univ. of Colorado-Boulder
Abstract
The phase state of atmospheric particulate is an important factor in the magnitude of both the direct and indirect radiative effect of aerosols on climate. While the homogeneous phase transitions of deliquescence and efflorescence have been studied for decades, there is far less information available on heterogeneous efflorescence. Here we describe a new mechanism of possible atmospheric importance, contact efflorescence, a process that occurs when a supersaturated droplet comes in physical contact with a solid particle. A newly constructed optical trap is used to measure contact efflorescence for single levitated droplets exposed to single collisions. This talk will describe the experimental technique and present preliminary data for contact efflorescence.
Laboratory Studies of the Chemistry of Secondary Organic Aerosol Formation
Paul Ziemann Professor, Department of Chemistry and Biochemistry & CIRES Fellow, Univ. of Colorado-Boulder
Abstract
In this talk I will describe for incoming graduate students the atmospheric chemistry research being conducted in my laboratory. Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to achieve a deep understanding of atmospheric chemistry and to develop detailed and accurate models that are used to establish air quality regulations and to predict the effects of human activities on global climate. Research in my laboratory focuses on the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form microscopic aerosol particles. Studies are conducted in large-volume environmental chambers where experiments are designed to simulate but simplify atmospheric chemistry and conditions in order to obtain information on gas and particle chemical composition; gas and heterogeneous/multiphase reaction rates and equilibria; thermodynamic, hygroscopic, and phase properties of particles; and gas-particle-wall interactions. Obtaining such data is a challenge, but in this talk I will describe how we approach this problem by using a diverse array of measurement techniques including real-time and offline mass spectrometry, temperature-programmed thermal desorption, gas and liquid chromatography, NMR, spectrophotometry, and scanning mobility particle sizing. I will then provide examples of our use of these methods to develop quantitative chemical reaction mechanisms of organic gas and aerosol chemistry and models of SOA formation and particle properties such as hygroscopicity and describe some ongoing research projects.
Monday, September 22, 2014
Volatile Organic Compounds in the Atmosphere
Joost de Gouw
Dept. Chemistry & Biochemistry
Abstract
Volatile organic compounds (VOCs) are released to the atmosphere from a large variety of sources both natural and man-made. In the atmosphere, VOCs are oxidized by different radicals on time scales of minutes to years. As a result of this chemistry, ozone and fine particles can be formed, which are two important air pollutants and which also play a role in the climate system. I will discuss ongoing research at the NOAA Earth System Research Laboratory that is aimed at quantifying the sources of VOCs, their chemical transformations, and the impact of these processes on air quality and climate. Also, new sources of energy lead to emissions of different VOCs, which is an emerging issue in our research.
Prof. Jose-Luis Jimenez
Dept. Chemistry & Biochemistry and CIRES, Univ. of Colorado-Boulder
Summary of Recent Results and Upcoming Projects to Investigate Organic Aerosol Sources, Properties, Processes, and Fate
Abstract
Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (POA, emitted in the particle phase) and secondary OA (SOA, formed by gas-to-particle conversion). In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group over the last year, as well as of upcoming projects of potential interest to 1st year students. Ongoing projects include analysis of SOA formation and aging from field studies in urban areas (CalNex-LA), forests (BEACHON-RoMBAS, SOAS, GoAmazon) and over regional scales from aircraft (DC3 and SEAC4RS), including direct measurements of the SOA formation and photochemical evolution from ambient air using an oxidation flow reactor (OFR). We are modeling the oxidation chemistry in the OFR in detail, and under most conditions we can show that non-tropospheric chemical pathways (e.g. reactions with O1D or photolysis at 185 or 254 nm) are negligible. SOA formation in LA is consistent with other urban sites. SOA formation and aging in the OFR is remarkably consistent across sites, showing peak formation at ~2 days of equivalent oxidation and loss of mass at ~2 weeks of oxidation. The detection and aging of SOA formed from isoprene epoxides in the Southeast US with the high-resolution AMS is shown to be robust, resulting in a ~15% contribution to the OA budget in summer and a lifetime of ~2 weeks against gas-phase aging. Gas/particle partitioning is measured directly using a FIGAERO-CIMS, with consistent results with other instruments at the SOAS site. Finally, I will introduce future projects in our group, including an update on the new shared smog chamber facility in the Cristol building (for which testing will start in October), development and application of chemical ionization mass spectrometry to laboratory research on OA gas/particle partitioning and to SOA formation from terpenes and the NO3 radical, and a NASA pole-to-pole aircraft field campaign on aerosol composition.
Monday, September 15, 2014
Research Overview of the Joint Center for Artificial Photosynthesis (JCAP):
Development of Scalably Manufacturable Solar-Fuels Generators
Carl Koval, Director
Abstract
JCAP was established in 2010 as one of the DOE’s Energy Innovation Hubs. JCAP’s mission is to build fully integrated solar-fuels generators that utilize earth-abundant semiconductors and catalysts for efficient conversion of water to O2 and H2 and for the reduction of CO2 to liquid fuels. JCAP prototypes are designed to enable separation of products and therefore require membranes and complex interfaces between various material components that will function under realistic operating conditions. JCAP’s long-term goal is to develop a commercially viable, solar-generation technology that simultaneously satisfies the following four criteria: high efficiency, multi-year stability, low module cost, and safe operation. JCAP’s approach to assembling complete artificial photosynthetic systems is to develop robust concepts for complete solar-fuels generators, then to break them down into essential assemblies of components, and finally to adapt or discover the materials needed to fabricate those assemblies. JCAP research bridges basic and applied sciences as well as engineering associated with prototype construction and consideration of scale-up challenges.
Monday, September 8, 2014
Reactive trace gases in tropospheric chemistry and climate
Rainer Volkamer -- Presentation given by Volkamer Group
Abstract
Reactive trace gases and aerosols are relevant components of tropospheric chemistry and climate. Bromine and iodine oxide radicals, and oxygenated VOC species modify the HOx radical abundance, influence the reactive chemistry and lifetime of climate active gases (e.g., ozone, methane, dimethyl sulfide) and can trigger the atmospheric deposition of mercury to ecosystems. Reactive trace gases are also a source for secondary aerosol mass that modifies aerosol optical properties, and cloud interactions (Earth albedo). The Volkamer group develops innovative optical spectroscopic instruments (in-situ and remote sensing) to measure trace gases and aerosols, and applies these instruments to conduct field measurements from mobile platforms (vehicles, ships, aircraft) and laboratory measurements in simulation chambers and flowtubes. This talk will present examples of current graduate student projects in the Volkamer group (MAD-CAT, CONTRAST, and FRAPPE projects). Future student opportunities exist in relation with the analysis of data from ongoing field projects, as well as laboratory studies that use in-situ cavity enhanced absorption spectroscopy and mass spectrometry to investigate the role of Setschenow ‘salting-in’ for aerosol formation. Prof. Volkamer is currently on sabbatical in Europe, and is best reached by email.
Tuesday, August 26, 2014
Explicit Modelling of the Multiphase Oxidation of Organic Compounds
Prof. Bernard Aumont, LISA and Univ of Paris XII
Atmospheric oxidation of organic matter is a gradual process involving a myriad of gaseous intermediates usually denoted as secondary organic compounds (SOC). These intermediates are typically species bearing one or more functional groups, such as ketone, aldehyde, alcohol, nitrate or hydroperoxide moieties. Secondary organic compounds play a central role in the chemistry of the atmosphere, being directly involved in the HOx/NOx/Ox tropospheric budget, in the production of particulate matter via the formation low volatility organic compound and in cloud chemistry via the formation of water soluble organic compounds. Explicit chemical mechanisms aim to embody the current knowledge of the transformations occurring in the atmosphere during the oxidation of organic matter. These explicit mechanisms are therefore useful tools to explore the fate of organic matter during its tropospheric oxidation and examine how these organic chemical processes shape the composition and properties of the gaseous and the condensed phases. Nevertheless, the explicit mechanism describing the oxidation of hydrocarbons with backbones larger than few carbon atoms involves millions of SOC, far exceeding the size of chemical mechanisms that can be written manually. Data processing tools can however be designed to overcome these difficulties and automatically generate consistent and comprehensive chemical mechanisms on a systematic basis. The Generator for Explicit Chemistry and Kinetics of Organics in the Atmosphere (GECKO-A) has been developed for the automatic writing of explicit chemical schemes of organic species and their partitioning between the gas and condensed phases. GECKO-A can be viewed as an expert system that mimics the steps by which chemists might develop chemical schemes. GECKO-A generates chemical schemes according to a prescribed protocol assigning reaction pathways and kinetics data on the basis of experimental data and structure-activity relationships. In its current version, GECKO-A can generate the full atmospheric oxidation scheme for most linear, branched and cyclic precursors, including alkanes and alkenes up to C25. Recent assessments and applications of the GECKO-A modeling tool will be presented, with a focus given to studies devoted to examine SOA formation and ageing, SOC phase partitioning and multiphase oxidation processes.
Spring 2014
Monday, May 19, 2014
Surface observations of VOCs over the temperate southern hemispheric oceans
Dr. Sarah Lawson
CSIRO MARINE AND ATMOSPHERIC RESEARCH
Aspendale, Australia
Abstract
VOCs fuel tropospheric ozone production, are precursors to secondary aerosol and influence the oxidative capacity of the atmosphere. The ocean is potentially a source (and sink) of many climatically important VOCs such as isoprene, monoterpenes and glyoxal, yet there are very few measurements over the remote oceans, particularly in the Southern Hemisphere. We present surface observations of VOCs over a very biologically active region of the South West Pacific Ocean (43ºS) during the 2012 Surface Ocean Aerosol Production (SOAP) voyage, and observations over the Southern Ocean from the Cape Grim Baseline Station (41ºS). VOCs were measured insitu using Proton Transfer Reaction Mass Spectrometry (PTR-MS) and also collected on 2,4-DNPH cartridges and VOC adsorbent tubes for later analysis by HPLC and GC/MS at CSIRO, Aspendale. Non-negligible mixing ratios of isoprene, monoterpenes, glyoxal and methyl glyoxal were all observed over the remote ocean indicating an oceanic source of these species. Very high mixing ratios of DMS were observed over phytoplankton blooms during SOAP alongside elevated levels of methanethiol and dimethyl sulfone . The significance of these observations is discussed and observations are compared with those elsewhere in the remote marine boundary layer.
Monday, May 8, 2014
Development of optical spectroscopic instruments and application to field measurements of marine trace gases
Sean Coburn
University of Colorado, Boulder
ABSTRACT
Halogens (X = Cl, Br, I) and organic carbon are relevant to the oxidative capacity of the atmosphere, and linked to atmospheric sulfur and nitrogen cycles, modify aerosols, and oxidize atmospheric mercury. The atmospheric abundance of halogen radical species in the atmosphere is very low, but even concentrations of parts per trillion (1 ppt = 10-12 volume mixing ratio) or even parts per quadrillion (1 ppq = 10-15 volume mixing ratio) in the atmosphere are relevant for the bioaccumulation of the neurotoxin mercury in fish. Halogen radicals can be traced through measurements of halogen oxides (XO, where X = Cl, Br, I), that are ~1-10 times more abundant. However, measurements of halogen oxides are sparse, partly due to the lack of analytical techniques that enable their routine detection. Global models are unable to accurately reproduce the few measurements that are available. I have developed a research grade Multi-AXis Differential Optical Absorption Spectroscopy (MAX-DOAS) instrument to measure bromine monoxide (BrO) and iodine monoxide (IO) routinely in the troposphere. I present autonomous measurements of BrO and IO in Pensacola, Florida that maximize sensitivity towards the detection of BrO in the free troposphere (altitudes >2km) from ground. The derived profiles are then coupled to a box-model to assess the impact of these measurements on the oxidation of mercury in the atmosphere. The second part of my talk describes first measurements of glyoxal diurnal cycles and Eddy Covariance fluxes of glyoxal in the marine atmosphere. Glyoxal is the smallest alpha-dicarbonyl, and a useful tracer molecule for fast photochemistry of hydrocarbons over oceans. The unique physical and chemical properties of glyoxal (i.e., high Henry’s Law coefficient, H = 4.2x105 M atm-1, and short atmospheric lifetime of ~2 hrs) pose challenges to explain this soluble gas over the remote ocean, and recent measurements over the open ocean currently remain unexplained by models. In order to better understand the sources and sinks of this molecule in the marine atmosphere, a Fast Light-Emitting-Diode Cavity-Enhanced DOAS (Fast LED-CE-DOAS) instrument was modified to measure eddy covariance fluxes of glyoxal. These are the first measurements of EC fluxes of glyoxal, and only the 7th molecule for which the EC flux technique has been used from ships. Results from a first cruise deployment over the tropical Pacific Ocean (TORERO field campaign) are presented.
Monday, April 28, 2014
Gas/Particle Partitioning of Organic Acids: instrument development and field deployment
Samantha Thompson
University of Colorado, Boulder
Abstract
Organic acids are common atmospheric oxidation products and can help our understanding of those mechanisms. In particular their partitioning between the gas and particle phase can lead to a better understanding of their contribution to SOA loadings and lifetime. In this talk I will be presenting the development a new instrument (the FIGAERO-HRToF-CIMS) for real time detection of both gas and particle phase organic acids. Results from lab characterization and a field campaign as part of the Southern Oxidant and Aerosol Study (SOAS) will be shown
Monday, April 21, 2014
Can Mass Spectrometry be More Radical?
Professor Yu Xia
Assistant Professor, Analytical Chemistry
Purdue University
Abstract
Radical induced damage to biomolecules is highly relevant to aging, neurodegenerative diseases, and cancers. Fundamental understanding of radical-biomolecule interactions is important for the development of new drugs and therapeutic procedures, but is often missing. We have taken a unique approach to address this issue by studying gas-phase ion/radical reactions. Instrumentation and methods have been developed to enable ion/radical reactions in an electrospray ionization (ESI) plume or inside a linear ion trap mass spectrometer. Accurate mass measurement, stable isotope labeling, tandem mass spectrometry, and theoretical calculations are utilized to provide insight into the reaction mechanisms.
Monday, April 14, 2014
Chemical Reactions Among Pollutants Indoors – The Human Touch
Charles J. Weschler
Rutgers University
Abstract
Regardless of whether a pollutant originates outdoors or indoors, most of our exposure to it occurs indoors. Indoor exposures to ozone and airborne particles of outdoor origin partially explain the mortality ascribed to these pollutants in epidemiological studies. The human body influences indoor concentrations of these and other chemicals in occupied indoor environments. Constituents of human skin oil react with ozone. Lower indoor ozone concentrations lead to less generation of secondary organic aerosols (SOA) from ozone-initiated reactions with terpenes and other unsaturated indoor pollutants. SOA levels influence the levels of co-occurring semivolatile organic compounds (SVOCs). Occupants also inadvertently transfer their skin oils and skin flakes to surfaces, impacting indoor surface chemistry, even when humans are no longer present. In brief, dynamic physical and chemical processes involving occupants and indoor pollutants markedly influence occupant exposures in indoor environments.
Monday, April 3, 2014
Novel phase transitions of optically levitated microdroplets: Contacts and glasses relevant to the atmosphere
Ryan Davis
University of Colorado, Boulder
Abstract
The phase state and water content of atmospheric particles influence the particle’s size, optical properties and chemical reactivity with important but poorly constrained effects on air quality and climate. One challenging problem is predicting the ambient conditions required to initiate a phase transformation of a particle from liquid to solid, particularly when consideration is given to multi-particle interactions as well as chemical composition of the liquid droplet. One such example is the phenomenon of contact nucleation. Presented here are the first direct observations of contact nucleation of crystalline ammonium sulfate using a recently developed long-working distance optical trap. Insight is given to the mechanism behind contact nucleation. The effect of amorphous or glassy phase states will also be discussed.
Monday, Feb. 24, 2014
Chemical and physical processes controlling organic aerosols and tropopause cirrus: An observational perspective
Dr. Andrew Rollins,
NOAA Chemical Sciences Division
Abstract:
Condensed mass in the atmosphere in the form of aerosol and cloud particles is a significant driver of climate and heterogeneous atmospheric chemistry. The processes that control the formation of these particles are complex, and understanding them is frequently limited by the ability to accurately measure the gas and particulate species required to test our theories. In this talk I will discuss results from two recent projects where new analytical instrumentation has been developed and used to inform our understanding of gas/particle partitioning processes involving organic aerosols and cirrus clouds. The first study presented exploits high time resolution measurements of organic nitrate aerosols using a laser induced fluorescence based technique. Laboratory and field measurements using this instrument have been used to probe the influence of nitrogen oxide chemistry on the formation of secondary organic aerosols (SOA) in the troposphere. Observations during the California Nexus (CalNex) field study demonstrate the significance of nitrate radical initiated SOA formation at night, and important nonlinearities in this chemistry.
In the second half of the talk I will discuss measurements of water vapor and ice water content in the tropical tropopause layer (TTL) using a new tunable diode laser based hygrometer operated on the NASA Global Hawk aircraft. Confidence in the accuracy of these historically challenging measurements is strengthened by use of an in-situ calibration system, and excellent agreement with a separate hygrometer operated in parallel. These measurements have provided evidence for the transport of water in excess of saturation through the TTL and into the stratosphere.
Thursday, Feb. 20, 2104
The role of oceanic halogen and sulfur compounds for the middle atmosphere
Dr. Susann Tegtmeier
GEOMAR Helmholtz Centre for Ocean Research Kiel, Kiel, Germany
The decline of anthropogenic chlorine in the stratosphere within the 21st century will increase the relative importance of naturally emitted, very short lived halocarbons on stratospheric ozone destruction. Such halocarbons play a significant role in present day ozone depletion, in particular in combination with enhanced stratospheric sulfate aerosol loading. There is a need to better understand how much of the observed stratospheric halogen and sulfur aerosol originates from natural sources, in particular from oceanic emissions, and how this will change and affect the middle atmosphere in a future climate.
In this talk, I will describe recent advances in our understanding of brominated and iodinated halocarbons based on ship campaign data, modelling studies, and aircraft measurements, including current and future halocarbon emission inventories and their contribution to the stratospheric halogen loading. Similarly, dimethylsulphide emissions are used to assess the impact of naturally emitted sulfur on the stratospheric aerosol loading. One focus of the presentation is on the tropical West Pacific, the main source region for stratospheric air, where highly localized halogen sources and a tropospheric OH minimum were identified. The potential of the latter to amplify the impact of oceanic halocarbons and South East Asian SO2 on the stratospheric composition is discussed. Finally, the ozone-depletion potential weighted emissions of halocarbons will be compared to those of other ozone depleting substances to quantify the overall impact of natural halocarbons on the ozone layer for current day conditions and future scenarios.
Monday, February 17, 2014
Prof. Tim Bertram, UCSD
Chemistry at atmospheric aqueous interfaces: In situ constraints on halogen activation at the air-sea and air-particle interface
Abstract:
Reactions occurring at atmospheric, aqueous interfaces can serve to catalyze reaction pathways that are energetically unfavorable in the gas phase. The reactive uptake of N2O5, a primary nocturnal nitrogen oxide (NOx) reservoir, serves as both an efficient NOx removal mechanism and regionally significant halogen activation process through the production of photo-labile ClNO2. Both the reaction rate and ClNO2 product yield are a complex function of the chemical composition of the reactive surface. To date, analysis of the impact of N2O5 chemistry on oxidant loadings in the marine boundary layer has been limited to reactions occurring on aerosol particles, with little attention paid to reactions occurring at the air-sea interface. Here, we report the first direct measurements of the air-sea flux of N2O5 and ClNO2 made via eddy covariance in the polluted marine boundary layer. The results are combined with in situ determinations of the N2O5 loss rates to aerosol particles and interpreted within a time-dependent coupled atmosphere- ocean model.
Thursday, Feb. 13, 2014
Chemical changes to light absorbing carbonaceous aerosol with oxidative aging
Dr. Eleanor Browne
MIT
Light absorbing carbonaceous aerosol (LAC) is one of only two atmospheric components that both degrade air quality and cause climate warming. Due to the short atmospheric lifetime (~days-weeks) of LAC, there has been considerable recent interest in quantifying the climate and health impacts of LAC and developing mitigation strategies that will simultaneously improve climate and human health. Despite this recent interest, large uncertainties in the quantification of LAC’s climate impact remain. In part this is due to the poorly characterized influence of “aging”, the complex atmospheric processes that alter the chemical and physical properties of aerosol over their lifetime.
In this talk, I discuss experiments using vacuum ultraviolet (VUV) ionization-high resolution aerosol mass spectrometry to quantify the kinetics and chemical composition changes to LAC (both black and brown carbon) upon oxidation. In brief, I find that heterogeneous oxidation by OH of the aliphatic compounds present on the surface of black carbon particles occurs quickly (lifetime of ~1 day) and produces oxygenated hydrocarbons. I also present results for the aging of brown carbon generated from the smoldering of pine needles. The primary aerosol emitted from smoldering is highly oxygenated and when it ages in the presence of the gaseous smoldering emissions, a chemically distinct secondary organic aerosol component forms. These results show that LAC undergoes substantial chemical changes on the timescale of a day. Since the chemical changes described here likely have important impacts on the climate-relevant properties of LAC (such as light absorption and hygroscopicity), further quantification of these aging mechanisms and timescales is needed in order to accurately estimate the climate effects of LAC.
Monday, Feb. 10, 2014
Mercury cycling in the atmosphere-ocean-land system: Insights from regional and global modeling
Dr. Yanxu Zhang
Harvard University
Abstract
Mercury is a neurotoxin that affects public heath primarily through consumption of marine fish and seafood. Anthropogenic mercury emission sources include coal burning and artisanal gold mining. Mercury undergoes long-range transport in the atmosphere and can cycle multiple times in the atmosphere-land-ocean system before eventually buried in the ocean sediment. To attribute the source of mercury in a certain region thus requires an appreciation of the contribution from legacy and external sources. However, our current understanding of the legacy mercury in the land and ocean subjects to large uncertainties. There are also unresolved questions in the atmospheric chemistry of mercury that affect its long-range transport potential. This talk will highlight recent advances in combining regional/global modeling in the atmosphere/ocean and measurements in different environmental compartments to improve understanding of the mercury cycle.
Monday, February 3, 2014
Tech Transfer in the Life Sciences
Brynmor Rees, University of Colorado Technology Transfer Office
Academic research is a creative field in which new ways of thinking often emerge. It is therefore not surprising that many inventions are born out of research. How is it that university inventions can best be transformed into real-world solutions, and what processes have been established to facilitate innovation at CU? Brynmor Rees, a Licensing Manager from CU’s Technology Transfer Office (TTO), will lead a discussion about inventions, intellectual property (including patents), and commercialization at CU. The presentation will include what to do with your invention, information about starting companies, how the patent system works, and real case studies.
Monday, January 13, 2014
Active Learning: Tips for Success.
Jenny Knight, MCD Biology.
Much evidence has been collected over the past 10 years supporting the efficacy of active learning in college science classrooms. However, many instructors who are interested in change still find it challenging to implement successfully. I will present reasons why it's worth trying active learning, and suggest some approaches and techniques that can be easily tried in any class.
Fall 2013
Monday, December 2, 2013
Fernando Rosario-Ortiz,
Professor,
Civil & Environmental Engineering,
University of Colorado
Photochemical formation of reactive oxygen species from wastewater organic matter
Abstract
The study of the physicochemical properties of organic matter has been of interest to researchers in the areas of chemistry and engineering for many decades. Organic matter influences many different processes in the natural and engineered environment, including fate and transport of organic contaminants and the formation of disinfection byproducts. Over the past decade, emphasis has been placed on the study of the physicochemical properties of effluent organic matter (EfOM), which represents the organic matter found in wastewater effluents. The presence of EfOM in wastewater effluents impacts not only the efficacy of engineered processes towards the removal of organic contaminants, but also the natural fate of these compounds in receiving streams. In this presentation, the photochemical formation of reactive oxygen species (ROS) from EfOM will be discussed. The ROS of interest include singlet oxygen (1O2) and hydroxyl radical (HO). The overall quantum yields for the formation of these species as a function of EfOM composition will be discussed, in addition to additional details with regards the photophysical processes responsible for the formation of these species in the complex mixture that is EfOM.
Monday, November 18, 2013
Roger Atkinson
Air Pollution Research Center, University of California, Riverside, CA 92521
Products of OH + furan reactions and some implications for aromatic hydrocarbon atmospheric degradation
Abstract
Unsaturated 1,4-dicarbonyls are important products of the atmospheric degradations of aromatic hydrocarbons such as benzene, toluene, xylenes and trimethylbenzenes, which comprise ∼20% of the non-methane volatile organic compounds present in urban air masses in the USA. However, in many cases the measured formation yields of the unsaturated 1,4-dicarbonyls are significantly lower than those of the presumed co-product 1,2-dicarbonyls. These discrepancies could be due to analytical problems and/or rapid photolysis of unsaturated 1,4-keto-aldehydes and unsaturated 1,4-dialdehydes, or to incorrect reaction mechanisms for the OH radical-initiated reactions of aromatic hydrocarbons. Since unsaturated 1,4-dicarbonyls are major products of OH + furans, with apparently simpler product distributions and mechanisms, we have investigated the reactions of OH radicals with furan, 2- and 3-methylfuran, and 2,3- and 2,5-dimethylfuran, in the presence of NO. Using direct air sampling atmospheric pressure ionization tandem mass spectrometry and gas chromatography, the unsaturated 1,4-dicarbonyls were observed and quantified. The measured unsaturated 1,4-dicarbonyl formation yields ranged from 8 ± 2% from OH + 2,3-dimethylfuran to 75 ± 5% from OH + furan. Other products were also formed. These data will be presented and discussed and, time permitting, a brief discussion of in situ nitro-aromatic and nitro-PAH formation from the atmospheric degradations of aromatic hydrocarbons and PAHs will also be presented.
Monday, November 11, 2013
Shuichi Ushijima, First Year Graduate Student, Department of Chemistry and Biochemistry, University of Colorado,
A Colorimetric Method to Determine Biological Production of Hydrogen Peroxide
Abstract
Hydrogen peroxide (H2O2) is the most stable reactive oxygen species (ROS) and is involved in reactions with dissolved organic matter (DOM) and biologically important metals in natural waters. A technique which uses horseradish peroxidase (HRP) and Amplex Red to measure the biological production of H2O2 is tested in this study. In this method, HRP catalyzes the reaction between H2O2 and Amplex Red to produce resorufin, a colored compound, which can be measured using UV-Vis spectrometry. This method is compared to another technique that utilizes the chemiluminescent reaction of H2O2 to acridinium ester. Results from analysis of fresh water samples and cultures of Chlamydomonas reinhardtii will be discussed.
Lucas Algrim, First Year Graduate Student, Department of Chemistry and Biochemistry, University of Colorado
Temperature-Dependent Kinetic And Mechanistic Study Of The Gas-Phase Oxidation Of Select Carbonyls
Abstract
Carbonyls are common intermediates in the overall oxidation of organic volatile compounds (VOCs), a chemical process that can influence tropospheric ozone production, aerosol formation, and climate change. Understanding carbonyl chemistry is imperative to fully understand and model such processes. To further the current knowledge of carbonyl chemistry, which is lacking for larger branched species, the temperature dependence of the gas-phase reaction of three ketones, 3-pentanone (DEK), 3-methyl 2-butanone (MIPK), and 2,4-dimethyl 3-pentanone (DIPK), with Cl atoms were investigated using an atmospheric smog chamber equipped with FT-IR, PTR-MS, and GC-FID. The ketones’ Arrhenius expressions were found to be: kDEK = 4 ±11 x 10-11 cm3 molecules-1 s-1 e(2 ± 7 kJ/mol)/RT, kMIPK = 7 ±23 x 10-11 cm3 molecules-1 s-1 e(0.2± 8 kJ/mol)/RT, kDIPK = 3 ±110 x 10-10 cm3 molecules-1 s-1 e(-3± 8 kJ/mol)/RT. Within the uncertainties the rate coefficients exhibit no temperature dependence. Product yield analysis of DIPK and MIPK consistently displayed a tertiary α-carbon: primary β-carbon (relative to carbonyl) branching ratio of approximately 70% : 30% for H-atom abstraction by Cl atoms over the investigated temperature range. This indicates a temperature-independent mechanism, consistent with the kinetic measurements.
Monday, November 4, 2013
Amanda McLaughlin, First Year Graduate Student, Department of Chemistry and Biochemistry, University of Colorado
Characterization of copolymers of vinylbenzyl thymine (VBT) and vinylbenzyl chloride (VBC) using size exclusion chromatography
Abstract
VBT, a versatile monomer, has successfully been copolymerized with vinylbenzyl triethyl ammonium chloride (VBA) to create a material that can be cast as a lasting coating with proven antimicrobial properties on a variety of surfaces. Molecular weight determination of (VBT)n(VBA)m, however, has been presented many difficulties due to the high charge density of VBA. Copolymerization of VBT with VBC will lead to a system that can be fully characterized and subsequently quaternized to yield a polymer with similar utility, but with higher tunability to that of the (VBT)n(VBA)m system. Results of the synthesis of (VBT)n(VBA)m copolymers will be presented.
Size exclusion chromatography (SEC) is a liquid chromatography technique, which allows for the separation and characterization of organic compounds based on size of the molecules. This technique will be utilized for the characterization of copolymers of VBT and VBC, using standard samples of poly-VBC to create a standard curve.
Ingrid Mielke-Malay,
First Year Graduate Student,
Department of Chemistry and Biochemistry,
University of Colorado
Water sensing by localized surface plasmon resonance on HKUST-1 and ZIF-8 multilayer metal organic frameworks
Abstract
Metal organic frameworks (MOFs) show potential for use in gas sensing and sequestration, with applications in industrial, defense, and biological settings, as well as environmental monitoring. Different gases can be adsorbed into these crystalline networks of organic ligands and metal ions or clusters by changing their sub-nanometer sized pores. An important practical consideration when developing MOFs for gas sensing is the requirement that they operate effectively under humid conditions. Layers of two MOFs, HKUST-1 (Cu3BTC2(H2O)n] and ZIF-8 (2-methylimidazole zinc salt), were formed on silver nanoparticles and the extent to which they adsorbed sample gases and water vapor was determined using localized surface plasmon resonance spectroscopy.
Monday, October 28, 2013
Prof. Jose-Luis Jimenez
Dept. Chemistry & Biochemistry and CIRES, Univ. of Colorado-Boulder
Instrument and Technique Development, Field Studies, and Computer Modeling to Elucidate Organic Aerosol Sources, Properties, Processes, and Fate
Abstract
In this talk I will present an overview and highlights of research on OA instrumentation, measurements, and modeling by our group since Fall 2008, as well as of upcoming projects of interest to 1st year students. Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere leading to important impacts on climate, human health, and other issues, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (POA, emitted in the particle phase) and secondary OA (SOA, formed by gas-to-particle conversion). Together with others in the community and contrary to the understanding at the time, we demonstrated in the mid-2000s that SOA dominates over POA at most locations. This paradigm shift has led to intense research on the sources, processing, properties, and fate of SOA. Because pre-existing and commercial instruments were very limited for the analysis of the complex mixtures of highly oxidized species comprising real SOA, we have developed or co-developed several experimental techniques aimed at extracting more information out of ambient and laboratory air, and pioneered their application in field experiments. These include measurements of eddy covariance fluxes, metals, and fast (millisecond) processes with the Aerodyne Aerosol Mass Spectrometer (AMS), development and application of soft-ionization techniques, measurements of the volatility and gas/particle partitioning of organic species, and measurement of the SOA formation potential of ambient air in real-time. We have also pioneered many data analysis techniques for aerosol mass spectrometry data, including 2 and 3-dimensional factor analysis, techniques for constrained high-resolution ion fitting, and elemental analysis of bulk organics with detection limits 6 orders-of-magnitude better than commercial techniques. We have applied these techniques to characterize POA sources (such as biomass burning, motor vehicles, and cooking) and to analyze ambient air at ground sites and from several aircraft in major field studies such as MILAGRO, SOAR, AMAZE, ARCTAS, DAURE, FLAME-3, CalNex, BEACHON-RoMBAS, DC3, SEAC4RS, and SOAS. We have proposed a new paradigm (Jimenez et al., Science, 2009) that is consistent with worldwide measurements and in which OA and OA precursor gases evolve continuously by becoming increasingly oxidized, less volatile, and more hygroscopic, leading to the formation of oxygenated organic aerosol (OOA), with concentrations comparable to those of sulfate aerosol throughout the Northern Hemisphere. We have used computer box modeling and collaborated closely with several regional and global modelers for deeper analysis and interpretation of field study data. Upcoming projects include development of hyphenated soft-ionization techniques, laboratory research on SOA formation from the NO3 radical and of indoor air chemistry, and a field study in the Amazon.
Monday, October 21, 2013
Demetrios Pagonis, First Year Graduate Student, Department of Chemistry and Biochemistry, University of Colorado
Isoprene Ozonolysis: Secondary Organic Aerosol Formation Pathways and Heterogeneous Aging
Abstract
Isoprene is the second most abundant volatile organic compound in our atmosphere, behind methane. Isoprene reacts with oxidizing agents in the atmosphere to form secondary organic aerosol (SOA), impacting climate and adversely impacting human health. After formation, SOA particles undergo heterogeneous reactions that age the particles, changing their composition as well as environmentally relevant properties such as size, composition, density, and potential to serve as cloud condensation nuclei. In this research we studied the pathway of isoprene SOA formation as well as the heterogeneous aging of SOA particles using FTIR. The role of the first-generation products methacrolein and methyl vinyl ketone in isoprene SOA formation was studied by comparing SOA formed from these first-generation products to isoprene SOA. Mass spectral data on particle composition were obtained using an Aerodyne Aerosol Chemical Speciation Monitor and illustrated the heterogeneous nature of the reactions that age particles.
Abigail Koss, First Year Graduate Student,
Department of Chemistry and Biochemistry,
University of Colorado
Measurements of Volatile Organic Compounds by GC-MS in Rural Alabama during the 2013 SOAS Campaign
Abstract
Volatile organic compounds (VOCs) are a large class of chemicals that are emitted into the atmosphere by both human and natural biological activity. VOCs are comprised of both precursor compounds that drive oxidation chemistry and oxidation products. Extensive measurements of VOCs can help determine the relationships between precursor and secondary compounds, and the relative effects of anthropogenic and biogenic emissions on climate and air quality.
The Southeastern US is a region of particular research interest, as it is strongly affected by both anthropogenic and biogenic VOCs. As part of the 2013 Southern Oxidant and Aerosol intensive study (SOAS), an in-situ gas-chromatograph mass spectrometer (GC-MS) was deployed at a forested site in rural Alabama. This site was dominated by biogenic emissions, but was also subject to anthropogenic influence.
The GC-MS measured a large number of primary and secondary anthropogenic and biogenic VOCs in the C2 to C11 range, with a time resolution of 30 minutes. Measured compounds of particular interest include isoprene, speciated monoterpenes, methylvinylketone (MVK), methacrolein, C2 to C11 alkanes, lightweight unsaturated hydrocarbons including ethene, propene, and acetylene, C6 to C9 aromatics, C1 to C7 oxygenated VOCS (alcohols, ketones, aldehydes), halogenated VOCs, acetonitrile, and several sulfur-containing compounds. A summary of these measurements will be presented. This summary will include characterization of various anthropogenic and biogenic sources sampled at the site, relationships of the most important VOCs to basic meteorological conditions, and diurnal profiles that illustrate shifts in photochemistry and emissions. These GCMS measurements will provide key information for constraints in models and to aid in the interpretation of data from other instruments.
Monday, October 14, 2013
Joost de Gouw, Cooperative Institute for Research in Environmental Sciences, University of Colorado at Boulder & NOAA Earth System Research Laboratory
Organic Carbon in a Changing Atmosphere: How Our Energy Choices Affect Air Quality and Climate Change
Recording of Seminar (Password-protected; Request password via email from Joost.deGouw@noaa.gov)
Abstract
Atmospheric emissions associated with the use of energy affect the chemical composition of the atmosphere, and have led to such diverse societal issues as air quality degradation and global climate change. Over the pas decade, resource limitations and concerns over energy security and the environment have led to significant changes in the use of energy in the U.S. The domestic production of oil and natural gas has strongly increased as a result of advances in directional drilling and hydraulic fracturing. Also, ethanol made from corn now constitutes approximately 10% of the volume of gasoline sales in the U.S. In this presentation, we will look at some of the atmospheric implications of these changing energy sources and how they are affecting atmospheric chemistry, air quality and climate change.
Monday, October 7, 2013
Daniel James Cziczo, Victor P. Starr Career Dev. Associate Professor of Atmospheric Chemistry, Massachusetts Institute of Technology
Combining Field and Laboratory Studies to Understand the Dominant Sources and Mechanisms of Cirrus Cloud Formation
Abstract
The formation of clouds is dependent on the availability of aerosols to initiate the condensation of water vapor. In the case of cirrus clouds, ice nucleation can occur homogeneously at low temperature and high saturation on the vast majority of particles or heterogeneously at high temperature and low saturation on the small fraction of atmospheric aerosols that are efficient ice nuclei (IN). The critical properties that make for a good IN are slowly being established. This work focuses on those particles which have been shown to be important IN through in situ studies: mineral dust and metallic particles are the dominant source of residual particles, whereas sulfate/organic particles are underrepresented and elemental carbon and biological material are essentially absent. We are now undertaking ice nucleation studies using the Droplet Measurement Technologies SPectrometer for Ice Nuclei (SPIN) on mineral dust and metallic aerosol and report ice nucleation conditions. A comparison to the types that are not observed in cirrus and to the onset of homogeneous freezing is made.
Monday, September 30, 2013
Rainer Volkamer, Department of Chemistry and Biochemistry and CIRES Fellow, University of Colorado, Boulder
Marine trace gases and aerosols: novel chemistry at atmospheric interfaces
Abstract
Discoveries about chemistry in the lower atmosphere are often driven by advances with analytical techniques to measure atmospheric composition. Oceans cover 70% of the Earth surface, yet the remote marine atmosphere remains one of the most poorly probed atmospheric environments on Earth. Ocean emissions of organic carbon molecules (gas- and aerosol phase) and halogens are relevant to climate discussions, because they modify oxidative capacity, i.e., the rate at which climate active gases such as ozone, and methane are removed from the atmosphere. They can further modify aerosols that influence Earth albedo. I will present optical spectroscopic instruments that my group has designed, assembled, and deployed over the past 6 years in laboratory studies of aerosol formation, and field measurements in the tropical atmosphere. These innovative instruments enable measurements of halogen oxide radicals (bromine and iodine oxide), and oxygenated small molecules, and allow us to study their chemical formation pathways, multiphase chemistry, distributions and relevance in the tropical atmosphere. We find that novel chemistry is operative at atmospheric interfaces (ocean surface, and aerosols). Marine organic carbon gases and halogens are particularly relevant in the tropical free troposphere, where most of the tropospheric ozone mass resides, 75% of the global methane destruction occurs, and mercury oxidation rates are accelerated at low temperatures.
Monday, September 23, 2013
Paul Ziemann Professor of Chemistry and Biochemistry & CIRES Fellow, Univ. of Colorado-Boulder
Comprehensive Laboratory Studies of the Chemistry of Secondary Organic Aerosol Formation: Scientific Questions, Techniques and Open Graduate Student Projects
Abstract
In this talk I will describe for incoming graduate students the atmospheric chemistry research being conducted in my laboratory. Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to achieve a deep understanding of atmospheric chemistry and to develop detailed and accurate models that are used to establish air quality regulations and to predict the effects of human activities on global climate. Research in my laboratory focuses on the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form microscopic aerosol particles. Studies are conducted in large-volume environmental chambers where experiments are designed to simulate but simplify atmospheric chemistry and conditions in order to obtain information on gas and particle chemical composition; gas and heterogeneous/multiphase reaction rates and equilibria; thermodynamic, hygroscopic, and phase properties of particles; and gas-particle-wall interactions. Obtaining such data is a challenge, but in this talk I will describe how we approach this problem by using a diverse array of measurement techniques including real-time and offline mass spectrometry, temperature-programmed thermal desorption, gas and liquid chromatography, NMR, spectrophotometry, and scanning mobility particle sizing. I will then provide examples of our use of these methods to develop quantitative chemical reaction mechanisms of organic gas and aerosol chemistry and models of SOA formation and particle properties such as hygroscopicity, and describe some future research projects for which I am recruiting CU graduate students.
Erin E. McDuffie
1sr Year Graduate Student
University of Colorado, Boulder
A New Instrument for the Analysis of Single Aerosol Particles: The Coupling of ATOFMS and LIBS
Abstract
Knowledge of aerosol particle composition is crucial in order to gain an understanding of health and environmental effects of atmospheric particulate matter, and requires measurements that can provide detailed information about chemical components. An Aerosol Time-of-Flight Mass Spectrometer (ATOFMS) is used for the semi-quantitative analysis of the composition of individual atmospheric particles upon laser-induced desorption and ionization. Integration of the spectroscopic technique of Laser-Induced Breakdown Spectroscopy (LIBS), in which light emitted by the elemental components of the particle upon irradiation with a laser is collected, should increase the quantitative data provided by this instrument. Due to the similarities between both techniques, integration of the two should be straightforward and requires only minor additions to the ATOFMS. This presentation will focus on the advantage of coupling these two techniques as well as the inherent physical and electronic challenges, including: plasma generation (excitation laser pulse energy), plasma dynamics in an electric field and vacuum conditions, emission detection (collection angle and distance from the light source), and software program design and implementation. These challenges continue to be systematically addressed in Dr. Deborah Gross’s Lab at Carleton College and are presented here as evidence of the range of issues that arise in producing a new single-particle analysis instrument.
Monday, September 16, 2013
Margaret Tolbert Department of Chemistry and Biochemistry and CIRES
Deliquescence of calcium perchlorate: A route to liquid water on Mars and other possibly habitable planets
The Wet Chemistry Laboratory (WCL) aboard the Phoenix Mars Lander identified the presence of 0.5% perchlorate (ClO4-). Reanalysis of the Viking gas chromatography-mass spectrometry results also suggests perchlorate was present in the soil and the Mars Science Laboratory (MSL) rover has potentially found perchlorate as well. Perchlorate salts are known to readily absorb water vapor from the atmosphere and deliquesce into an aqueous solution. We have previously performed laboratory studies to better understand the deliquescence (crystalline solid to aqueous salt) and also efflorescence (aqueous salt to crystalline solid) of several perchlorate salts at low temperatures. We found that NaClO4 and Mg(ClO4)2 are highly deliquescent, forming aqueous solutions at humidity values as low as 40% RH and at temperatures as low as 223 K. We also observed a significant hysteresis that occurs during efflorescence of these salt solutions, expected due to the kinetic inhibition of crystal nucleation. The efflorescence relative humidity values of sodium and magnesium perchlorate solutions are 13% RH and 19% RH, respectively, indicating that perchlorate salts could exist as stable or metastable aqueous solutions over a wide range of Martian RH and temperature conditions.
Although the low temperature deliquescence of several perchlorate salts is now well characterized, instruments onboard Phoenix and MSL have identified calcium perchlorate (Ca(ClO4)2) as the likely parent salt. Calcium perchlorate is known for its highly deliquescent properties and low eutectic point; however, the deliquescence and efflorescence of this salt have not yet been quantified as a function of temperature. We have used Raman microscopy to examine the deliquescence and efflorescence of Ca(ClO4)2 under relevant Martian temperatures. Results for these experiments will be presented and implications for the availability of liquid water on Mars and similar planets will be discussed.
Robert E. Sievers and team of 35 collaborators sponsored by FNIH and the Bill and Melinda Gates Foundation GCGH
Sievers Group Studies of Needle-free Delivery of Dry Powder Aerosol Vaccines and Pharmaceuticals
Scientists from the University of Colorado and Aktiv-Dry LLC have formulated dry powder vaccines effective against measles. We have developed and patented a system known as CO2-Assisted Nebulization with a Bubble Dryer® (CAN-BD) that stabilizes, micronizes, and dries these and various other biologicals and pharmaceuticals with great success. Based on our success with a variety of biopharmaceuticals, we have demonstrated that Aktiv-Dry’s CAN-BD system can be used to manufacture stable and active dry powders for inhaled vaccines and antibiotics. These dry inhalable powders can be administered through multi-dose or single-dose inhalers (such as Aktiv-Dry’s PuffHaler® or the BD Technologies’ Solovent) and are easy to handle and administer in situations in resource-poor countries. The measles live-attenuated, inhalable dry powder aerosolizable vaccine has been tested in Phase I clinical trials recently successfully completed in Pune, India; the 60 subjects were all dosed by inhaling a single breath of dry powder aerosol beginning in March 2012, and no serious adverse events have been reported to date. No serious adverse events were observed in the studies of the inhaled powder vaccine in macaques or in 60 human volunteers in a Phase I clinical trial.
Wednesday, September 4, 2013
Characterizing the influence of multi-generational chemical processing on organic particles
Christopher D. Cappa, Associate Professor, Dept. of Civil and Environmental Engineering, University of California, Davis
Abstract:
Reactions of organic compounds in the atmosphere with common oxidants (e.g. the hydroxyl radical and ozone) can lead to the formation of secondary organic aerosol (SOA) and can engender transformations within the particle-phase through heterogeneous reactions. The resulting specific chemical composition of the particles can influence their ability to take up water, activate into cloud droplets and absorb and scatter sunlight. Most organic compounds emitted into the atmosphere that are capable of forming SOA are subject to multi-generational oxidation, which simply means that they can react more than once, with each reaction having some probability of functionalizing or fragmenting the parent species. In this seminar, two aspects of this multi-generational ageing will be discussed: SOA models and SOA optical properties. Many models of SOA formation, especially those most commonly used to simulate regional and global SOA burdens do not account for such multi-generational ageing, instead parameterizing SOA formation as a one-step process. And those SOA models that do account for ageing tend to do so in a very ad hoc manner and with little validation (with chemically explicit models being the exception). The Statistical Oxidation Model (SOM) is a reduced complexity SOA model that explicitly accounts for multi-generational ageing in both the gas and particle phases and has been developed to capture the countering effects of fragmentation and functionalization. The basic framework of the SOM will be introduced along with an overview of next-generation developments of the model to make it amenable to use in 3D models. In the second part of this seminar, a series of SOA formation and ageing experiments will be discussed in which particle optical property measurements (light extinction and light absorption) have been made and correlated with particle composition. Our results demonstrate that SOA optical properties are not static and depend explicitly on the identity of the gas-phase precursor.
Spring 2013
Monday, May 6, 2013
How can we improve therapy for Tuberculosis?
Mercedes Gonzalez-Juarrero,
Mycobacteria Research Laboratories,
Microbiology, Immunology and Pathology Department,
Colorado State University
Tuberculosis [TB] is one of the leading killer infectious diseases today. Eradication of TB depends on the development of shorter and more effective treatment regimens, including novel treatment alternatives combined with classical TB therapies. At present patients with TB are treated daily with high concentrations of multidrug treatment administered by oral route, subcutaneous or intravenous injections. To improve TB control and treatment it is necessary to ease and shorten anti-TB drug regimens. One approach under study is the use of aerosolized drugs and use of drugs the target the host instead of the bacteria also known as host targeted therapy. In our laboratories we use an experimental murine model that allow for aerosolized anti-TB drug efficacy testing. The uses of aerosol delivery of anti-TB drugs which only option at present are to be used as injectables in addition to the use of lung immune modulators as adjuvants for TB chemotherapy will be discussed.
Monday, April 29, 2013
High-Altitude Aircraft Measurements of the Tropical Tropopause Layer from the NASA ATTREX Campaign
Eric Jensen, NASA Ames Research Center
The NASA Airborne Tropical TRopopause EXperiment (ATTREX) is a five-year airborne science program focused on understanding physical processes occurring in the tropical tropopause layer (TTL, ~13-20 km), with an emphasis on understanding the control of the humidity of air entering the stratosphere. The long-duration Global Hawk Unmanned Aircraft System is used to make measurements of water vapor, clouds, meteorological conditions, numerous chemical tracers, radiative fluxes, and BrO. During February-March, 2013, six 24+ hour flights were conducted from Dryden Flight Research Center to the tropical eastern and central Pacific. Our primary mode of operation was to repeatedly ascend and descend through the TTL (between about 45 and 60 kft). Over 100 vertical profiles were obtained during the six flights, providing a unique, high spatial-resolution dataset of TTL composition. Some of the flights provided surveys of TTL composition, extending well into the southern hemisphere. Other flights targeted thin cirrus near the tropopause forming in anomalously cold regions. Examples of ATTREX measurements will be presented with an focus on TTL relative humidity and clouds. Plans for deploying the Global Hawk to Guam for western Pacific measurements in 2014 will also be discussed.
Monday, April 22, 2013
An Overview of the OASIS 2009 Campaign, with Focus on Halogen, OVOC and Relative Nitrogen Chemistry
John Orlando and Frank Flocke, UCAR, Boulder, Colorado
This talk will present results from the Ocean-Atmosphere-Sea Ice-Snowpack (OASIS) intensive, conducted in Barrow, AK in the spring of 2009. A brief overview of the campaign and the unprecedentedly detailed data set collected in Barrow will be presented.
We have developed a detailed chemical mechanism to simulate the photochemistry during the campaign, which is often dominated by halogen chemistry. In addition, surface production and fluxes of VOC are assessed and compared with ambient measurements both in the gas phase and the snow phase.
In light of these findings, we will discuss “the PAN conundrum,” a long-known discrepancy between predicted and observed PAN mixing ratios in the Arctic coastal boundary layer. We have been working to solve this problem using our detailed zero-D chemistry model. Preliminary results will be shown and discussed.
Monday, April 15, 2013
Real-time organic aerosol formation and oxidative aging using a flow reactor in a ponderosa pine forest
Brett Palm, University of Colorado, Boulder, Department of Chemistry and Biochemistry
A Potential Aerosol Mass (PAM) flow reactor is used in combination with an Aerodyne High Resolution Time-of-Flight Aerosol Mass Spectrometer (AMS) to characterize biogenic secondary organic aerosol (SOA) formation and aging in a terpene-dominated forest during the July-August 2011 Bio-hydro-atmosphere interactions of Energy, Aerosols, Carbon, H2O, Organics & Nitrogen - Rocky Mountain Biogenic Aerosol Study (BEACHON-RoMBAS) field campaign at the U.S. Forest Service Manitou Forest Observatory, Colorado. Ambient air is sampled through the PAM reactor, inside which a chosen oxidant (OH, O3, or NO3) is generated and controlled over a range of values up to 10,000 times ambient levels. High oxidant concentrations accelerate the oxidative aging of volatile organic compounds (VOCs), inorganic gases, and existing aerosol, which leads to repartitioning with the aerosol phase. PAM oxidative processing represents from 1 to more than 20 days of equivalent atmospheric aging during the ~3 minute reactor residence time. During BEACHON-RoMBAS, PAM photooxidation enhanced SOA at intermediate OH exposure (1-10 equivalent days) but resulted in net loss of OA at long OH exposure (10+ equivalent days), demonstrating the competing effects of functionalization vs. fragmentation (and possibly photolysis) as aging increases. Similarities in common SOA oxidation tracers (f44 vs. f43 and Van Krevelen diagram [H/C vs. O/C] slopes) between ambient and PAM-processed aerosols suggests the PAM reactor employs oxidation pathways similar to ambient air. A simple model calculating expected SOA formation from complete consumption of the most abundant VOCs measured at the forest site (monoterpenes, sesquiterpenes, and toluene) consistently underpredicts the amount of SOA formed in the PAM reactor in the field observations. This suggests one or more issues, such as additional SOA sources or formation pathways.
Monday, April 8, 2013
Investigating and improving the freeze-thaw stability of vaccines with aluminum-containing adjuvants: Case studies with model HBsAg vaccines
Prof. LaToya Jones-Braun, School of Pharmacy, University of Colorado Health Sciences Center
Vaccines with aluminum-containing adjuvants, the most prevalent class of adjuvant in childhood vaccines, are susceptible to reduced efficacy if frozen. Accidental freezing of such vaccines have been documented globally, including in the US. This seminar will focus on research related to the freeze-thaw stability of model hepatitis B vaccines. Efforts to both understand the causes of the freeze-thaw instability as well as approaches to improving the robustness of the vaccine following exposure to subzero temperatures will be discussed.
Thursday, April 1, 2013
Special Analytical & Environmental Chemistry Seminar CIRES Auditorium, noon, Thu 3-April *** note unusual day and location ***
Nicole Riemer, Univ. of Illinois @ Urbana-Champaign
Understanding black carbon climate impacts using a stochastic particle-resolved model
Abstract: Black carbon (BC) aerosol influences climate in a number of complex ways. This includes the direct absorption and scattering of sunlight as well as impacts on the cloud microphysical properties. A key factor in quantifying the BC-climate impact is understanding the mixing state evolution of BC aerosol. The mixing state describes whether BC exists as pure particles or in combination with other species within one particle. Experimental evidence shows that BC can exist in very complicated mixtures, and that the precise mixture composition dramatically alters the effects and lifetimes of BC in the atmosphere. For example, sulfate coatings can enhance the CCN activation properties of BC, which facilitates the removal of BC through rainout, lessening the BC impact. On the other hand, coatings can focus radiation on BC nuclei, increasing the absorption effects of BC, thereby enhancing the BC impact. Tracking the mixing state in conventional aerosol models requires treating a multidimensional size distribution, which is computationally prohibitive. Therefore current models usually assume an internal mixture within one mode or size section. The uncertainties associated with this assumption, which artificially coats freshly emitted particles instantly, are not well quantified. This results in current aerosol models using or predicting widely varying rates of “aging” of BC particles from fresh emissions to coated aged particles.
In this seminar I will present an aerosol model of a new type, the stochastic particle-resolved model PartMC-MOSAIC. This model tracks individual particles as they undergo chemical and physical transformations in the atmosphere. Hence aerosol impacts that depend on per-particle composition can be represented in detail. I will illustrate the usefulness of this new approach by focusing on the aging process of black carbon. Using PartMC-MOSAIC we are able to quantify the individual processes that contribute to the aging of black carbon and demonstrate the effect of aerosol mixing state on aging time scales, optical properties, and CCN activation properties.
Monday, March 18, 2013
Ivan Ortega, University of Colorado, Boulder, Department of Chemistry and Biochemistry
Maximizing the information of ground based remote sensing measurements: trace gases and aerosol properties
Over the past few years the Atmospheric Trace Molecule Spectroscopy Laboratory (ATMOSpeclab) at CU Boulder has designed and deployed portable, high-sensitivity Multi Axis Differential Optical Absorption Spectroscopy (MAX-DOAS) instruments with a two-fold purpose: 1) to retrieve profile information of trace gases (i.e., NO2, HCHO, HONO, CHOCHO) and 2) to determine multi wavelength aerosol properties. Past MAX-DOAS measurements have extensively focused on the unique ability to derive information about trace gases; however, this technique can be expanded towards the retrieval of aerosol optical and microphysical properties. This talk presents the evaluation of a novel 2 dimensional MAX-DOAS instrument. The new instrument measures solar scattered light in the horizontal (varying azimuth angle), as well as the vertical plane (varying the elevation angle) which can be used to obtain effective aerosol microphysical properties, such as aerosol size distribution, phase function and refractive index; in addition to the trace gas and aerosol extinction profiles. The 2D MAX-DOAS was deployed and operated for 6 weeks at Cape Cod, MA as part of the Two Column Aerosol Project (TCAP). The TCAP deployment provides unique opportunities for comparisons with aerosol extinction profile measurements by the NASA Langley airborne High Spectral Resolution Lidar (HSRL), Aerosol Optical Depth by a co-located NOAA Multifilter Rotating Shadowband Radiometer (MFRSR) instrument, as well as in-situ measured optical properties aboard DoE’s G1 aircraft. A retrieval was developed to invert effective radius and complex refractive index of particles for monomodal logarithmic normal distribution derived from Mie-scattering calculations in the size range of 0.1-1um. Aerosol extinction retrieved from MAX-DOAS measurements can be compared with extinction predicted by Mie theory to test the assumption of particle homogeneity and sphericity.
Monday, March 4, 2013
Megan L. Melamed, IGAC Executive Officer, University of Colorado, CIRES, Boulder, CO
Overview of the International Global Atmospheric Chemistry (IGAC) Project
The International Global Atmospheric Chemistry (IGAC) Project was formed in 1990 to address growing international concern over rapid changes observed in Earth's atmosphere. IGAC operates under the umbrella of the International Geosphere Biosphere Programme (IGBP) and is jointly sponsored by the international Commission on Atmospheric Chemistry and Global Pollution (iCACGP). IGAC coordinates and fosters atmospheric chemistry research towards a sustainable world by facilitating international collaboration of atmospheric chemistry and multi-disciplinary research, coordinating the synthesis, assessment, and summary of research, cultivating the next generation of atmospheric chemist, and acting as the liaison between the atmospheric chemistry community and the broader Earth System Sustainability community. In July 2012, the IGAC International Project Office relocated to the Cooperative Institute for Research in Environmental Studies (CIRES) at the University of Colorado. Current IGAC activities and how to get involved in IGAC will be presented.
The Impact of Megacities on Air Pollution and Climate Change
As of 2008, for the first time, the majority of the world’s population is living in urban areas [1], many in megacities (with populations over 10 million). Currently, urban population fractions are higher in developed than developing countries. However, with most of the world population growth expected to occur in developing countries, urbanization is increasing at a higher rate in developing countries. These dramatic increases in population and urbanization, especially in the developing world, have been accompanied by technological and economic growth and development, yielding changes in land use, energy use, and transportation. The resulting changes due to urbanization have dramatic impacts on anthropogenic and biogenic emissions and have notably altered local to global-scale atmospheric composition, increasing the importance of understanding the impacts of urbanization on atmospheric chemistry. In light of the importance of megacities on atmospheric chemistry, the International Global Atmospheric Chemistry (IGAC) Project and the World Meteorological Organization (WMO) released in January 2013 the first comprehensive assessment of Atmospheric Chemistry in Megacities. An overview of this assessment will be presented.
Monday, February 25, 2013
Prof. Waleed Abdalati, Geography Department and CIRES, University of Colorado Boulder
From the Terrestrial to the Celestial: My Perspectives and Experiences as NASA Chief Scientist
For decades, NASA has been an iconic inspiration to the nation and the world, exploring our planet, the space environment, the solar system, and the universe. Success in these endeavors has been built on a foundation of science, technology and engineering that is producing such remarkable achievements as: landing a one-ton rover on the surface of Mars, developing a telescope that will peer 13.5 billion years into the past, and most importantly, providing comprehensive insights into the behavior and evolution of planet Earth. Enabling these capabilities, and the associated research, requires much more than technical and scientific advances, however. It involves a complex balancing of priorities and resources, as well as alignment among a diverse group of passionate stakeholders – all against a backdrop of political and policy turmoil. As chief scientist, it was my responsibility to help shape NASA’s direction, and work to achieve alignment among the interests and objectives of the scientific community, the White House, Congress and others. In this talk, I will discuss some of these challenges and the associated rewards as well as the role science plays at NASA, and the role NASA science plays in the broader national framework.
Monday, February 18, 2013
Prof. Brian Majestic University of Denver
Using iron isotopes as a tool to identify sources of atmospheric iron: an analysis of desert, urban, and oceanic environments
Abstract
Atmospheric transport followed by deposition of aerosol Fe is the primary source of Fe for much of the surface ocean. In many parts of the ocean, Fe serves as a limiting nutrient for phytoplankton growth, as well as other aquatic organisms. Most aerosol Fe, by mass, is crustally-derived, the majority of which is generated in the Sahara and other deserts. However, solubility measurements show that this crustal-derived Fe is highly insoluble (< 1%), possibly limiting its bioavailability. Two other sources of Fe, biomass burning and combustion-derived anthropogenic aerosols, are characterized by a much higher solubility, so in spite of their lower mass of Fe, they may represent an important contribution to overall bioavailable Fe in the surface oceans.
A new tool to determine the relative importance of these sources is Fe isotope analysis. Previous work has shown that crustal material has an isotopic composition equal to ~ 0.1 ‰. Therefore, isotopic deviations from 0.1 ‰ may indicate the presence of non-crustal Fe. In this talk, I will present Fe isotope data from the outskirts of Phoenix, AZ, a highly crustal environment as well as Bermuda, a pristine oceanic site. Bermuda is an ideal site to test this method because, during the summer months, the air masses reaching Bermuda originate from West Africa (crustal) and, during the winter months, the air masses originate from North America (more anthropogenic). Striking similarities between these two sites, as well as other Fe isotope studies, will be highlighted.
Monday, February 11, 2013
Professor Neil M. Donahue
Departments of Chemistry, Chemical Engineering and Engineering and Public Policy Director, Center for Atmospheric Particle Studies Carnegie Mellon University
Old Aerosols Never Die, They Just Get Oxidized
Abstract
Organic aerosols comprise a highly dynamic system of multiphase oxidation chemistry. A major element in the organic-aerosol lifecycle is the progressive oxidation of semi-volatile vapors. Oxidation influences the total aerosol mass by forming products with different vapor pressures than their precursor molecules, but the overall effect on the aerosol mass is nuanced. Oxidation (aging) can clearly enhance aerosol mass via formation of more heavily functionalized products, but it can also reduce the aerosol mass as carbon backbones are fragmented, especially later in the oxidation sequence. We have investigated a number of model systems as well as representative bulk aerosol systems to investigate this process, and developed a reduced chemical mechanism to describe it. In addition to influencing the overall mass, oxidation chemistry can produce small amounts of extremely highly oxidized organic vapors. These highly functionalized molecules can and do participate in the formation of molecular clusters (typically involving sulfuric acid in the atmosphere) and their subsequent growth to ultrafine particles and then to particles large enough to have significant health and climate effects. We have recently joined the CLOUD consortium at CERN to study this particle physics.
Monday, February 4, 2013
Dick and Jean Bedell, Boulder, Colorado
India From an Elephant's Back
Abstract
This presentation reflects observations of a fascinating country and culture by a Boulder couple. Dick and Jean Bedell have volunteered their medical services for several months each year over three decades. Through Rotary Clubs in India and United States, they helped establish a home for girls whose prostitute mothers died of AIDS, and a Hospice Centre staffed by 300 volunteers. They provide seminars for doctors, midwives and nurses in urban and rural facilities. Through Dr. Robert Sievers, they toured the amazing facility in Pune linked with his research on measles vaccine. Their presentation is informal with time for questions.
Monday, January 28, 2013
Roy (Lee) Mauldin, ATOC/INSTAAR, University of Colorado, Boulder
The X Files: Oxidation via Criegee radicals – From reactions to global impacts
Abstract
Oxidation processes in Earth’s atmosphere are tightly connected to many environmental and human health issues and are essential drivers for biogeochemistry. Oxidation processes remove natural and anthropogenic trace gases and air pollutants from the atmosphere, and produce low-volatility vapours that control secondary aerosol formation and atmospheric production of cloud condensation nuclei. These atmospheric oxidation processes are typically thought to be dominated by three main oxidants: ozone, hydroxyl radical (OH) and nitrate radical. Field measurements however have shown discrepancies between measurements and models indicating the presence of other mechanisms. Our group has recently shown that products formed from the ozonolysis of biogenic alkenes (most likely stabilized Criegee radicals) have a substantial oxidizing capacity, with the ability to oxidized compounds such as SO2 or dimethyl sulphide. This new “X-chemistry” can have significant, or even dominant, contribution to formation of low-volatility inorganic (sulfuric acid) and, most strikingly, very highly oxidized condensable organic vapours. This new route to the formation of sulfuric acid and extremely low-volatility organic compounds can have a considerable impact to global budgets of cloud condensation nuclei. Our observations point toward a highly important, yet unexplored area of atmospheric chemistry and indicate the discovery of a new natural negative feedback mechanism that could partially counteract climate warming.
Fall 2012
Monday, December 10, 2012
Callie Cole
Laboratory Astrochemistry: Kinetics Studies and Instrument Modifications
Abstract
Reactions involving organic molecules are important to astrochemistry and astrobiology alike. Several carbon- and nitrogen-containing anions (CN–, C3N–, and C5N–) as well as trans methyl formate have been spectroscopically detected in the interstellar medium (ISM), and this work focuses on relevant gas phase reactions involving these species. The tandem flowing afterglow-selected ion flow tube (FA-SIFT) was used to experimentally determine the rate constants and products of these reactions, and theoretical calculations were conducted to further understand their mechanisms. Additionally, an ion trap mass spectrometer has been modified to allow for the study of ion-neutral reactions. The building of this modification as well as future plans for this instrument with respect to astrochemistry will be discussed.
Monday, November 26, 2012
Siyuan Wang
SOA formation from volatile OVOC
Abstract
Glyoxal has potentially large sources from remote to polluted environments. Glyoxal is highly water-soluble; it may undergo a series of aqueous-phase processes in aerosol liquid water and lead to the formation of highly oxidized, high-molecular-weight compounds. In the present work, the multi-phase behavior of glyoxal is probed from chamber experiments (PSI chamber, Switzerland) to ambient environments (sub-urban Hong Kong). Phase transfer and subsequent aqueous-phase processes are simulated using a box-model. In both cases glyoxal is found to form secondary organic material under humid condition, which may be affected by various factors such as aerosol acidity and sulfate. This modeling work provides reasonable interpretations for observations and insights into the complicated processes of such a simple molecule.
Monday, November 12, 2012
Theodore Konstantinos Koenig
Separation of Water Spin Isomers by Adsorption
I will be presenting on work to determine whether water spin isomers can be enriched in the gas phase by selective adsorption. I will begin by briefly outlining the motivations and previous literature on the topic. I will then give information on the construction and testing of an instrument for the purpose of examining any effect by a variety of spectroscopies. Finally, initial FT-IR results will be presented to give a preliminary answer to whether selective adsorption does in fact occur.
Monday, October 29, 2012
Christopher Kampf
Effective Henry’s Law Constant Measurements for Glyoxal in Model Aerosols Containing Sulfate
Abstract
I am going to present results of an atmospheric simulation chamber study on the reversible partitioning of glyoxal, which was studied by direct measurements of gas-phase and particle-phase concentrations for the first time in model aerosols containing sulfate. Two complimentary methods for the measurement of glyoxal particle-phase concentrations are compared: 1) An offline method utilizing filter sampling of chamber aerosols followed by HPLC-MS/MS analysis and 2) Positive Matrix Factorization (PMF) analysis of AMS data. Ammonium sulfate and internally mixed ammonium sulfate/fulvic acid seed aerosols were used to study the influence of seed chemical composition on effective Henry’s law constants (KH,eff) of glyoxal under varying Relative Humidity (RH) and radiation conditions. KH,eff was found to increase exponentially with ammonium sulfate concentration or sulfate ionic strength, I(SO42-), respectively. A Setschenow type plot revealed that glyoxal is subject to a “salting-in” effect in aerosols. Further, the temporal behavior of the reversible uptake of glyoxal on seed aerosols can be described by two distinct reservoirs for monomers and higher molecular weight species filling up at two different characteristic time constants.
Christoph Knote
3D model simulations of glyoxal-SOA formation over California
Abstract
Most studies investigating the formation of SOA from glyoxal so far employed simple box model and pseudo-lagrangian approaches, which lack a comprehensive description of emissions variability and transport phenomena. We investigate regional scale variability of glyoxal SOA using the online-coupled regional chemistry transport model WRF/Chem with a focus on the summer 2010 over California. In my talk I will highlight improvements made to the model to better represent glyoxal formation pathways, discuss model performance when compared against a variety of measurement data available from the CalNex and CARES field campaigns, and show how we extend it by two different methods to describe formation of SOA from glyoxal. Finally I will present first results of simulations made for a selected period in June 2010.
Monday, October 22, 2012
Yong Liu
University of Colorado at Denver
Investigating O3-initiated heterogeneous oxidation of linoleic acid using ATR-IR flow reactor
Abstract
Despite recent extensive studies towards heterogeneous oxidation of unsaturated organics (mostly oleic acid) initiated by ozone, little is known about effects of environmental conditions such as ambient temperature and relative humidity; physical state; and oxidant concentration on heterogeneous reaction kinetics. In this work, we used linoleic acid as a proxy for atmospheric unsaturated organics to investigate its heterogeneous oxidation by O3 over a wide range of temperatures (258-314 K), relative humidities (0-80% RH) and ozone concentration (50 ppb -50ppm). Our experiments were carried out using a flow reactor coupled to an attenuated total reflection infrared spectrometer (ATR-IR). Pseudo-first order rate constants and overall reactive uptake coefficients were acquired from absorbance changes in peaks located near 1743 cm-1; 1710 cm-1; 1172 cm-1 and 1110 cm-1, which can be assigned to C=O in ester; C=O in acid; C-C and C-O stretching modes, respectively. Results showed that the kapp and γ increased with increasing temperatures. It was noted the temperature enhancement effect on the reaction kinetics was much more pronounced at lower temperatures. Such behavior can be explained by change in physical state of LA at lower temperatures. In addition, kapp and γ were enhanced by 2-fold as RH increased from 0 to 80%. Moreover, the effect of ozone concentration on the reaction kinetics was reported for the first time. kapp was found to display a Langmuir-Hinshelwood dependence on ozone concentration. Furthermore, yields and hygroscopic properties of reaction products were also investigated by FTIR spectroscopy. The intensity ratio of two C=O stretching bands, A1743/A1710, which was utilized as an indicator of the product yields, increased sharply with increasing temperatures in the lower temperature region (258-284 K), and then remained nearly constant in the higher temperature region (284-314 K). The product yields showed no significant variation with RH, for the intensity ratio of A1743/A1710 barely changed in the wide RH range of 0-80%. Water uptake studies showed that the LA thin film absorbed water with an increasing RH, and the hygroscopicity of the thin film was enhanced after ozone exposure.
Monday, October 15, 2012
Katherine Primm
Formation of Gold Nanoparticles and Nanorods Analyzed by SEIRA
Abstract
The goal of this research was to develop the best gold nanostructures for applications in a range of biological, medical, catalytic, environmental, and nanotechnological applications, these were then analyzed using surface-enhanced infrared absorption spectroscopy (SEIRA). Various nanostructures were formed by evaporating gold in vacuum onto CaF2 substrates at deposition angles ranging from incident to 85. Nanostructures were characterized with AFM, SEM, and UV/Vis-NIR spectroscopy. A monolayer of p-nitrobenzoic acid was deposited onto the gold nanostructures to determine the degree of vibrational enhancement in SEIRA. SEIRA enhancement factors of x20-50 were obtained from metal nanostructures formed by the evaporation of 5-7 nm of gold at incident angle. Gold nanorods aggregated during formation at grazing angles and did not yield larger enhancement factors. Further studies on the gold nanorods are being performed to form nanorods with better enhancement factors, which include different techniques in vapor deposition.
Melissa Ugelow
Tailoring Porous Silicon for Analyte Response
Abstract
To better assess and characterize the sensing properties of porous silicon (pSi), we have developed a strategy to create small spatially heterogeneous areas on a pSi surface that exhibit different degrees of surface oxidation by pin printing 9:1 H2O2:glycerol on the prepared pSi surface. The pSi surface chemistry and the heterogeneity of these pin printed regions were assessed using FT-IR microscopy and epi-luminescence microscopy, coupled with multispectral imaging. The results show that the pin printed areas are chemically heterogeneous across their ca. 300 μm diameter and the photoluminescence response to analyte depends on the analyte type and on the spatial position within the printed region (i.e., on the exact extent of oxide formation (Si-O-Si, O2SiH, O3SiH and Si-OH vs. SiH, SiH2 and SiH3) at the pSi surface). The combination of pin printing and multispectral microscopies allows us to create the entire range of pSi chemical compositions and simultaneously assess their performance all within a single 300 μm diameter printed feature. This can lead to more sophisticated pattern recognition schema to allow simultaneous analyte identification and quantification.
Monday, October 8, 2012
Jay Kroll
Investigating the Chemical Complexity of the Interstellar Medium
Abstract
I have collected and analyzed to rotational spectrum of methyl ethyl ketone (MEK) from 8 GHz to 1THz. This molecule contains similar functional groups to other known interstellar molecules such as acetone, acetaldehyde, and ethanol, and is therefore a likely product of interstellar organic chemistry. The microwave spectrum for MEK was acquired using the chirped-pulse waveguide Fourier Transform Microwave (FTMW) spectrometer at New College Florida, and the millimeter and submillimeter spectrum was acquired using the direct absorption flow cell spectrometer at Emory University. I will report on the preliminary results of the laboratory spectral analysis.
Jordan Krechmer
Development of Novel Sampling Systems for Direct Analysis in Real Time Ionization Sources
Abstract
Direct Analysis in Real Time (DART) is an ambient ionization source for mass spectrometry (MS) that is utilized in a wide variety of applications including food science, forensics, homeland security, and environmental analysis. Introduced in 2005, DART-MS does not require sample preparation and allows the analysis of solid and liquid samples in their native state. This talk will discuss the development of novel methods of sampling with the DART source to enhance its capabilities. Such methods include rapid heating of samples of metal substrates, ambient ion transfer tubes for non-proximate sensing, and magnetic automated sampling systems for the analysis of powders.
Monday, October 1, 2012
Paul Ziemann
Professor of Chemistry and Biochemistry, Univ. of Colorado-Boulder (starting June 2013)
Professor, Univ. of California Riverside (present)
Comprehensive Laboratory Studies of the Chemistry of Secondary Organic Aerosol Formation: Scientific Questions, Techniques and Open Graduate Student Projects
In this talk I will describe for incoming graduate students the atmospheric chemistry research being conducted in my laboratory. Laboratory studies provide much of the fundamental data on reaction kinetics, products, and mechanisms that are needed to achieve a deep understanding of atmospheric chemistry and to develop detailed and accurate models that are used to establish air quality regulations and to predict the effects of human activities on global climate. Research in my laboratory focuses on the atmospheric chemistry of organic compounds emitted from natural and anthropogenic sources and the physical and chemical processes by which oxidized organic reaction products form microscopic aerosol particles. Studies are conducted in large-volume environmental chambers where experiments are designed to simulate but simplify atmospheric chemistry and conditions in order to obtain information on gas and particle chemical composition; gas and heterogeneous/multiphase reaction rates and equilibria; thermodynamic, hygroscopic, and phase properties of particles; and gas-particle-wall interactions. Obtaining such data is a challenge, but in this talk I will describe how we approach this problem by using a diverse array of measurement techniques including real- time and offline mass spectrometry, temperature-programmed thermal desorption, gas and liquid chromatography, NMR, spectrophotometry, traditional elemental analysis, pycnometry, and scanning mobility particle sizing. I will then provide examples of our use of these methods to develop quantitative chemical reaction mechanisms of organic gas and aerosol chemistry and models of SOA formation and particle properties such as hygroscopicity, and describe some future research projects for which I am recruiting CU graduate students to start in my group in June 2013.
Monday, September 24, 2012
Robert E. Sievers and team of 30 collaborators sponsored by FNIH and the Bill and Melinda Gates Foundation GCGH
CU’s Inhalable Dry Powder Aerosol Measles Vaccine Now In Clinical Trials
Abstract
Several hundred children die every day from measles-related disease throughout the world and particularly in poor contries. We have developed a Measles Vaccine, Dry Powder (MVDP) and PuffHaler® a novel dry powder inhaler for respiratory delivery to overcome the safety and vaccine wastage problems associated with the current injectable vaccine. Phase I clinical trials of our new inhalable dry aerosol measles vaccine are now underway in 60 adult volunteers. So far there has not been observed or reported any serious adverse events, but the observation interval is not yet ended. MVDP was manufactured from bulk liquid, live attenuated, measles vaccine using CAN-BD® to yield a stable, dry powder, with fine particles suitable for aerosolization and delivery to the pulmonary tract by inhalation. This was achieved by substituting myo-inositol for sorbitol as a stabilizing sugar that is less hygroscopic. Contact with water is detrimental to the stability of measles vaccine and the microparticles are reconstituted only when they deposit in the moist surfaces of the respiratory tract. There is no need for purified water to be carried to remote locations. The stabilization and CAN-BD drying of other vaccines are now being explored. The measles vaccine shelf life is excellent at 2 to 8 °C with only about 0.5 log loss of activity after more than 2 years. At 25 °C, there is approximately only 0.8 log loss after 6 months of storage.
Rainer Volkamer
Reactive trace gases in tropospheric chemistry and climate – recent results and upcoming projects
Abstract
Reactive trace gases and aerosols are relevant components of tropospheric chemistry and climate. Bromine and iodine oxide radicals, and oxygenated VOC species modify HOx radical abundances, influence the reactive chemistry and lifetime of climate active gases (e.g., ozone, methane, dimethyl sulfide) and can trigger the atmospheric deposition of mercury to ecosystems. Reactive trace gases are also a source for secondary aerosol mass that modifies aerosol optical properties, and cloud interactions (Earth albedo). In this talk I will summarize research in the development of innovative optical spectroscopic instrumentation (in-situ and remote sensing) to measure trace gases and aerosols, their applications in field and laboratory measurements conducted by our group in 2012. These include ongoing data analysis and modeling of the TORERO, TCAP, WACS field projects, mechanistic studies in simulation chambers of secondary organic aerosol (SOA) formation, and heterogeneous chemistry of OVOC volatilization from surfaces coated with organics in flow tubes. Future opportunities exist as part of these ongoing data analysis and modeling activities, with simulation chamber experiments at facilities in Boulder and Switzerland (PSI 2013), and to further develop the University of Colorado Airborne Multi AXis Differential Optical Absorption Spectroscopy instrument (CU AMAX-DOAS) in preparation for field projects aboard research aircraft over the western tropical pacific ocean (CONTRAST 2014).
Monday, September 17, 2012
Alison Craven
The Importance of Dissolved Organic Matter to the Binding of Copper and the Release of Trace Elements from Coal Ash
ABSTRACT
The bioavailability and toxicity of copper to aquatic life depends on its speciation. Dissolved organic matter (DOM) plays an important role in the speciation of copper, but there is still much uncertainty about what controls the strength and formation of the Cu2+-DOM complex. The ratio of copper to DOM is known to affect the strength of Cu2+-DOM binding, but previous methods to determine Cu2+-DOM binding strength have generally not measured binding constants over the same Cu:DOM ratios, making these results difficult to directly compare. A competitive ligand exchange-solid phase extraction (CLE-SPE) method and a copper ion-selective-electrode (Cu-ISE) method were used to determine conditional stability constants for Cu2+-DOM binding at near neutral pH and 0.01 M ionic strength over a range of Cu:DOM ratios that bridge the detection windows of previous measurements reported in the literature. As the Cu:DOM ratio increased from 0.0005 to 0.1 mg Cu (mg DOM-1), the measured conditional binding constant (cKCuDOM) decreased from 1011.5 to 105.6 M-1. This behavior is consistent with the presence of Cu2+ binding sites of higher affinity and lower abundance that become filled as the total copper concentration increases. A comparison of the binding constants measured using CLE-SPE with those measured by Cu-ISE and voltammetry methods demonstrates that the Cu:DOM ratio is an important factor controlling the Cu2+-DOM binding strength for a variety of DOM isolates and whole water samples.
Using correct Cu2+-DOM binding constants to accurately model copper speciation is important for predicting copper toxicity in cases like the waters draining the Pebble deposit in southwestern Alaska, where small increases in the dissolved copper concentration may be harmful to salmonids and other aquatic biota. Experimentally determined Cu2+-DOM binding constants were used as inputs in Visual MINTEQ to model copper speciation and calculate Cu2+ concentrations. The results were then compared to results from the biotic ligand model (BLM), a speciation and toxicity model recommended by U.S. EPA for calculation of stream copper standards, which uses the Windermere Humic Aqueous Model (WHAM) to model metal interactions with DOM. The BLM was found to over-estimate Cu2+ at low total copper concentrations and under-estimate Cu2+ at high total copper concentrations, which may result in over- or under-estimations of toxicity.
DOM also has also been shown to enhance the dissolution of soils, sediments and minerals, which could result in the release of toxic trace elements into aqueous systems. Coal ash contains high concentrations of toxic trace elements that may have the potential to be released into the water. Releases of coal ash to rivers and streams, such as that which occurred from the Kingston Fossil Plant in Kingston, TN, into the Emory and Clinch Rivers in December 2008, are a concern because of the potential for human health problems, as well as ecological effects. In order to understand the effect that DOM has on the release of trace elements from coal ash, a series of release experiments were performed using coal ash generated from the Kingston Fossil Plant as a function of DOM concentration, DOM aromaticity, and calcium concentration. Various DOM isolates and filtered site-water samples collected near the Kingston Fossil Plant were used to produce coal ash suspensions at a fixed ash:water ratio of 1:1000 and a near-neutral pH. The major and trace elemental composition of the solution, and specific trace metals (mercury, lead, copper, aluminum) and metalloids (arsenic and selenium) were measured. The results indicate that DOM enhances the release of mercury, lead, copper, and aluminum from coal ash. The concentration of mercury, copper and aluminum released from the coal ash was positively correlated with the SUVA254 (ultraviolet absorbance at 254 nm divided by the dissolved organic carbon concentration) of the DOM. The release of arsenic and selenium from the coal ash was not dependent on the DOM concentration or SUVA254. Calcium was shown to inhibit the release of mercury, copper and aluminum from the coal ash
Monday, September 10, 2012
Jose L. Jimenez
Organic aerosol sources and processing in the atmosphere: recent results and upcoming projects
ABSTRACT
Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (emitted in the particle phase) and secondary OA (formed from chemical reactions of gas-phase species). In this talk I will summarize research in OA instrumentation, measurements, and modeling by our group over the last year. This includes ongoing analysis and modeling of the recent CalNex-LA, BEACHON-RoMBAS, and DC3 field projects, direct measurements of the SOA formation and photochemical evolution of ambient air using the Potential Aerosol Mass (PAM) flowtube, model/measurement comparisons to constrain the OA budget, and results from our new MOVI-CIMS instrument which allows molecular-level analysis of OA with high sensitivity and mass resolution. Finally, I will introduce future projects in our group, including an update on the design of the new shared smog chamber facility in the Cristol building (to be completed in May 2013), upcoming field projects in the Alabama (SOAS 2013) and the Amazon (GoAmazon 2014), and a new ion mobility spectrometer – CIMS instrument coupling.
Margaret Tolbert
The Ins and Outs of Ice Nucleation
ABSTRACT
Depositional ice nucleation on atmospheric aerosols has been identified as a potential pathway for cirrus cloud formation. Upper tropospheric aerosols as well as sub-visible cirrus residues are known to be enhanced in both sulfates and organics. While solid ammonium sulfate is an efficient depositional ice nucleus, the role of organics remains largely uncertain. I will discuss recent experiments in our group examining ice nucleation one particle at a time using Raman microscopy. We will probe ice nucleation at low upper tropospheric temperatures on mixed particles containing organics with ammonium sulfate. Raman mapping will be used to probe the composition, phase and structure of the mixed particles. Ice nucleation experiments reveal that at low temperatures, some organic containing particles nucleate ice on the outside while others nucleate ice from the particle interior.
Spring 2012
Tuesday, July 17, 2012
Professor Nathalie Mahowald
Cornell University
Aerosol indirect effect on biogeochemistry and climate
Abstract:
The net effect of anthropogenic aerosols on climate is usually considered the sum of the direct radiative effect of anthropogenic aerosols, plus the indirect effect of these aerosols through aerosol-cloud interactions. However, an additional impact of aerosols on a longer time scale is their indirect effect on climate through biogeochemical feedbacks, largely due to changes in the atmospheric concentration of CO2. Aerosols can affect land and ocean biogeochemical cycles by physical forcing or by adding nutrients and pollutants to ecosystems. The net biogeochemical effect of aerosols is estimated to be equivalent to a radiative forcing of –0.5 +/- 0.4 watts per square meter, which suggests that reaching lower carbon targets will be even costlier than previously estimated.
Tuesday, June 26, 2012
Professor Jean-Francois Doussin
University of Paris-East at Creteil (UPEC)
Interuniversitary Laboratory of Atmospheric Systems
Experimental simulation of the secondary aerosol production and ageing from the photo-oxydation of biogenic species
Abstract:
Secondary organic aerosols account for the majority of organic aerosols and play an important role in climate on both the global scale and regional scale. While significant progress has been made on the understanding of SOA formation, the transformations it undergoes during its atmospheric transit are still far from being understood. At the University of Paris East (France), a new indoor atmospheric simulation chamber has been built and its physical characteristics make it particularly well-suited to study SOA ageing processes involving oxidant, sunlight or clouds and their links with aerosol properties. In this talk, a brief description of this new tool, its analytical environment and its capabilities will be given. Then, results on on-going research involving biogenic species (such as a-pinene and isoprene) will be presented. In particular, results about ageing processes at the molecular level and their impact on aerosol properties as well as identification of field tracers for aged SOA will be shown.
Monday, May 14, 2012
Lucy Carpenter, U York, UK
Oxygenated volatile organic compounds (OVOCs) in the remote marine troposphere: Results from the Cape Verde Atmospheric Observatory (CVAO)
Abstract
Oxygenated volatile organic compounds (OVOCs) in the atmosphere are precursors to peroxy acetyl nitrate (PAN), affect the tropospheric ozone budget, and in the remote marine environment represent a significant sink of the hydroxyl radical (OH). The sparse observational database for these compounds, particularly in the tropics, contributes to a high uncertainty in their emissions and atmospheric significance. Here we show multi-annual measurements of acetone, methanol and acetaldehyde in the remote marine boundary layer made at the Cape Verde Atmospheric Observatory (CVAO) (16,848°N, 24.871°W), a subtropical marine boundary layer global GAW station situated on the island of São Vicente. Simulations of OVOCs at Cape Verde using the CAM-Chem global chemical transport model are markedly different from the observations in both concentration and variability and we explore reasons for this including oceanic and terrestrial primary and/or secondary OVOC sources. Finally, preliminary isoprene data from Cape Verde is discussed in the light of recent suggestions that marine isoprene emissions could contribute significantly to the sub-micron OC fraction of marine aerosol in the tropics.
Monday, May 7, 2012
Christa Hasenkopf
Fullbright Scholar & NSF International Research Fellow, National University of Mongolia and CIRES, Univ. of Colorado-Boulder
The story behind an upcoming field campaign in Ulaanbaatar, Mongolia – one of the world’s most PM-polluted cities
Abstract: Mongolia’s capital city of Ulaanbaatar, where wintertime daily mean PM10 concentrations can be measured in milligrams and black carbon (BC) concentrations in micrograms, contains some of the world’s most polluted air. Yet the remote city of 1.3 million people, isolated from other major anthropogenic PM sources, is severely understudied in the atmospheric science literature.
This seminar will present the story of Ulaanbaatar’s current air pollution crisis, as well as describe current and upcoming mitigation efforts, which may provide a novel and temporary opportunity to observe the effect of relatively fast PM/BC reduction on regional climate. It will also describe an upcoming year-long field campaign designed to help fill the current information-gap on Ulaanbaatar air pollution, and provide a better foundation for future studies. Lastly, the seminar will discuss opportunities in which others can get involved with both the scientific and outreach components of this project.
Monday, April 30, 2012
Gregory Schill
The Role of Organic Species on the Ice Nucleation Capabilities of Atmospheric Aerosol
Abstract
Depositional ice nucleation on atmospheric aerosols has been identified as a potential pathway for cirrus cloud formation. Upper tropospheric aerosols as well as sub-visible cirrus residues are known to be enhanced in both sulfates and organics. While ammonium sulfate is known to be an efficient depositional ice nucleus, the role of organics remains largely uncertain. To explore this role, we used a Knudsen cell flow reactor and Raman microscopy to study the ice nucleation efficacy of crystalline organic films and ammonium sulfate particles coated by liquid or glassy organics respectively. In the Knudsen cell studies, it was found that the ice nucleation efficiency of a series of monocarboxylic acids films was dependent on their O:C ratio. For the Raman experiments, we find that if the organic coatings are liquid, water vapor diffuses through the shell and ice nucleates on the ammonium sulfate core. Here, the organics minimally affect the ice nucleation efficiency of ammonium sulfate. In contrast, if the coatings become glassy, ice instead nucleates on the organic shell. These glassy coatings can alter the ice nucleation efficiency of ammonium sulfate.
Monday, April 23, 2012
Known and Unexplored Organic Constituents in the Earth’s Atmosphere: Instrument Development to Enhance Exploration
Allen Goldstein, Professor, Department of Environmental Science, Policy, and Management and Department of Civil and Environmental Engineering, University of California at Berkeley, USA
A substantial fraction of the atmospheric organic chemicals in both gas and particle phases have not been, or have very rarely been, directly measured. Even though our knowledge of them is limited, these compounds clearly influence the reactive chemistry of the atmosphere and the secondary formation, transformation, and likely the climate impact of aerosols. A continuing challenge in the coming decade of atmospheric chemistry and aerosol research will be to elucidate the sources, structure, chemistry, and fate of these clearly ubiquitous yet poorly constrained organic atmospheric constituents. Critical questions include: What atmospheric organic compounds do we know about and understand? What organic compounds are present as gases and in aerosols? What evidence exists for additional organic compounds in the atmosphere? How well do we understand the transformations and fate of atmospheric organics?
The complex chemical composition of atmospheric aerosols, particularly the organic carbon portion, presents unique measurement challenges. We developed the Thermal Desorption Aerosol Gas chromatograph (TAG) system for hourly in-situ speciation of a wide range of primary and secondary organic compounds in aerosols. This instrument combines a particle collector with thermal desorption followed by gas chromatography and mass spectrometric detection to provide separation, identification, and quantification of organic constituents at the molecular level. Observed compounds include alkanes, aldehydes, ketones, PAHs, monocarboxylic acids, and many more. The hourly time resolution measurements provided by TAG capture dynamic and frequent changes in aerosol composition. We have incorporated a two-dimensional chromatography (GC×GC) capability into TAG with a time of flight (TOF) MS detector. Two-dimensional chromatography provides two types of compound separation, most typically by volatility and polarity using two columns with different stationary phases connected in series separated by a modulator. The modulator periodically traps analytes eluting from the first column, and injects fractions of this effluent onto the second column in the form of narrow pulses providing additional separation for co-eluting peaks. The approach is especially useful for distinguishing polar compounds that would otherwise be buried in the unresolved complex mixture (UCM). We are developing a semivolatile collection system that allows simultaneous measurement of chemically specific semivolatile organics in the gas and particle phases, enabling in-situ analysis of speciated organic partitioning in the real atmosphere. We have also developed and deployed a combined TAG-AMS (Aerosol Mass Spectrometer) instrument for simultaneous measurements of the total and speciated aerosol composition. This talk will review our recent developments (TAG, 2DTAG, SVTAG, TAG-AMS), and present new observations of speciated semi-volatile separations between the gas and particle phases, 2DTAG and TAG-AMS observations in ambient air and controlled chamber source oxidation studies. New experiments using soft ionization techniques to more fully separate the UCM and to identify more of the organic species in aerosols will also be presented.
Monday, April 9, 2012
Sunil Baidar
Airborne MAX-DOAS for measurement of atmospheric trace gases
Abstract
We have developed a new motion-stabilized scanning airborne multi-axis differential absorption spectroscopy (CU AMAX-DOAS) instrument to retrieve column and profile information of trace gases like NO2, HCHO, CHOCHO, IO, BrO, O4 and aerosol extinction coefficient. Column observations of reactive trace gases provide means to bridge spatial scales between ground-based measurements, and satellite observations, and enable a more direct comparison with atmospheric models for improving emission inventories. This instrument was deployed aboard the NOAA Optical Remote Sensing Twin Otter Research Aircraft during the CalNex 2010 and CARES field campaigns. A total of 52 flights (48 research + 4 transfer flights) were carried out in California between May 19 and July 19, 2010. The focus of this deployment is to map the horizontal and vertical distribution of NO2, HCHO, CHOCHO and O4.
Here we describe the CU AMAX-DOAS instrument, give an overview of the profile retrieval algorithm, and present some results from the CalNex and CARES campaigns. Comparison of measured NO2 vertical columns with CMAQ and WRF-Chem model simulation results will also be presented. Comparisons show strong evidence for decreasing NOx emissions to be widespread in California.
Monday, April 2, 2012
Lia Rebits
Selection of DNA Aptamers That Would Improve Diagnostic Tests for Tuberculosis
Abstract
Despite the drastic decline in tuberculosis-related mortality due to the development of a vaccine and the discovery of effective anti-mycobacterial antibiotics during the middle part of the twentieth century, there has been a resurgence of the disease since the 1980’s primarily due to the advent of HIV/AIDS and drug-resistant Mycobacterium tuberculosis strains. The need for improved diagnostic tests that are capable of greater specificity, sensitivity, and rapidity than the current tests is apparent and such tests would contribute significantly to the number of lives saved annually. A DNA aptamer-based biosensor has the potential to meet these challenges; aptamers have proven to have similar dissociation constants to antibodies but provide additional benefits such as increased stability, ease of synthesis and a greater amenability to labeling. The SELEX process will be used to selectively evolve DNA aptamers that bind specifically to antigens particular to Mycobacterium tuberculosis. Aptamers discovered in this way may be used to selectively remove the mycobacterium or antigen from a biological matrix, as well as serve as the detection molecule via conjugation with a reporter in a similar fashion as that of a sandwich-ELISA test.
Monday, March 5, 2012
Sarah Brooks
Texas A&M University
Chemical Transformations of Atmospheric Aerosols and Their Cloud-Nucleating Abilities
Abstract
Emissions of biogenic volatile organic compounds (VOCs) lead to formation of secondary organic aerosol, contributing to a major fraction of the global tropospheric aerosol loading. The extent to which VOC oxidation products condense onto preexisting primary aerosols and modify their cloud-nucleating properties, however, remain highly uncertain. In chamber studies we show that water-soluble organic acids produced from the reaction between α-pinene and ozone rapidly accumulate onto preexisting soot particles. In less than 30 minutes, initially hydrophobic aerosols are converted to aerosols containing a mass fraction of 80-90% organics which activate efficiently as cloud condensation nuclei under representative atmospheric conditions. Our results imply that the microphysical properties of aerosols present in continental air masses are largely controlled by the composition and thickness of coatings (~30 nm in 30 minutes) formed during aerosol aging processes, rather than by the size or composition of original primary particles. In addition, chemical changes in aerosols impact their ice nucleating ability. Our results suggest that atmospheric models underestimate the impacts of chemical transformations of aerosols leading to inaccurate predictions of the indirect effects of aerosols on climate.
Monday, Feb. 20, 2012
Informal Seminar by Anna Wonaschuetz, University of Arizona (Group of Armin Sooroshian)
Measurements of Aerosol-Cloud Interactions
Ekeley S274, Monday Feb 20 @ noon
In this talk, I will present a brief tour of recent studies related to organic aerosols and cloud-aerosol interactions. The impact of shallow cumulus clouds on the vertical distribution of aerosol properties is investigated with the help of an airborne data set from the 2006 Gulf of Mexico Atmospheric Composition and Climate Study (GoMACCS). Vertical profiles of aerosol volume concentrations and chemical composition (in particular organics, sulfate and oxalate) give evidence of aerosol re-distribution from near the surface into high altitudes. I will also show first results of aerosol hygroscopicity in connection with measurements of WSOC and AMS chemical composition from last summer's Eastern Pacific Aerosol and Cloud Experiment (E-PEACE) field campaign and give a brief summary of two regional aerosol studies in the Los Angeles Basin and the deserts of Southern Arizona.
Monday, Feb. 13, 2012
Susan Doughty, Ph.D., J.D.
Greenlee Sullivan P.C., Intellectual Property Law
IP:50 Intellectual Property in 50 Minutes or Less
Abstract
It is important for everyone to have an awareness of Intellectual Property (IP) since the legal and financial stakes for missteps are potentially serious and costly. A summary of the types of Intellectual Property protection available as well as a case-study on the intersection of IP with University researchers will be provided.
Tuesday, Jan. 17, 2012
Center for Atmospheric Particle Studies, Carnegie Mellon University
Linking tailpipe to ambient: Quantifying the contribution of motor vehicle emissions to ambient fine particulate matter concentrations
Motor vehicles are an important source of air pollution in urban environments. Although tailpipe emission standards have become progressively tighter over the past few decades, this approach has not achieved the expected air quality benefits. To investigate the contribution of motor vehicle emissions to ambient fine particulate matter, we have conducted a series of smog chamber studies of varying complexity from individual compounds to actual vehicle emissions. The results reveal a dynamic picture in which fine particulate matter formed in the atmosphere dramatically exceeds the direct particle emissions, especially for low emitting sources. The talk concludes with some comparisons to ambient data from recent field studies and a discussion of the implications of these recent findings on human exposures and the design of regulations to control pollutant emissions.
Allen Robinson: Dr. Allen Robinson is a Professor in the Departments of Mechanical Engineering and Engineering and Public Policy at Carnegie Mellon University. His research examines the impact of emissions from combustion systems on urban, regional and global air quality. He received his Ph.D. from the University of California at Berkeley in Mechanical Engineering in 1996 and his B.S. in Civil Engineering from Stanford University in 1990. He was awarded the Carnegie Institute of Technology Outstanding Research Award in 2010, the Ahrens Career Development Chair in Mechanical Engineering in 2005 and the George Tallman Ladd Outstanding Young Faculty Award in 2000.
Friday, Jan. 13, 2012
Prof. Paul Ziemann, University of California-Riverside
Comprehensive Studies of the Chemistry of Aerosol Formation from Alkane Oxidation
Much of what is currently understood about the chemical and physical processes involved in the atmospheric oxidation of organic gases and particles and the formation of secondary organic aerosol (SOA) has been obtained from experiments carried out in environmental (smog) chambers. Achieving a level of understanding from such studies that is sufficient for extrapolating results to the atmosphere and for developing accurate models of SOA formation requires the acquisition of detailed information on a variety of gas and particle properties and processes, such as gas and particle phase reaction kinetics, gas and particle composition, particle phase, compound vapor pressures, and gas-particle-wall interactions. Obtaining such data is a challenge, but in this talk I will show results of studies carried out in our laboratory on the OH radical-initiated reactions of alkanes that are approaching this goal. I will demonstrate that by using a diverse array of measurement techniques including mass spectrometry, gas and liquid chromatography, spectrophotometry (with and without compound derivatization), traditional elemental analysis, pycnometry, temperature-programmed thermal desorption, and scanning mobility particle sizing it is possible to develop quantitative chemical reaction mechanisms of organic gas and aerosol chemistry and models of SOA formation. Doing so, however, requires a more comprehensive approach to chamber studies that attempts to account more fully for the effects of walls.
Fall 2011
Monday, Nov. 28, 2011
Reddy Yatavelli and Harald Stark
Gas and particle-phase organic acids measurement at a forest site using chemical ionization high-resolution time-of-flight mass spectrometry during BEACHON-RoMBAS campaign
Abstract
Organic acids are important constituents of aerosol particles. We will present preliminary analysis of organic acids in gas and aerosol particles measured in a ponderosa pine forest during July - August 2011 as part of the BEACHON-RoMBAS field campaign. We will also introduce the new measurement technique, based on chemical ionization, high-resolution time-of-flight mass spectrometry, utilizing a micro-orifice volatilization impactor [MOVI-ToF-CIMS], that was used to collect this data. Acetate anion (CH3C(O)O-, 59 Th) was chosen as the reagent ion for detection of organic acids. The high-resolution mass spectra, in combination with the temperature-dependent desorption information, can be used to identify specific compounds and estimate their gas/particle partitioning
Monday, Nov. 14, 2011
Juliane L. Fry
(Reed College, Portland OR; currently on sabbatical at CIRES & NCAR ASP)
Role of organic nitrates in secondary organic aerosol (SOA) production in forests
Abstract
Biogenic hydrocarbons, such as isoprene (C5), monoterpenes (C10) and sesquiterpenes (C15), react rapidly with nitrate radical (NO3) to form low-volatility products. We hypothesize therefore that nitrate oxidation may be an important source of secondary organic aerosol in pollution-impacted forest environments. In this talk I will present new data from the BEACHON-RoMBAS field campaign at the Manitou Forest Observatory in the Colorado front range (July/August 2011) relevant to this hypothesis. I will also describe a set of experiments underway as a collaborative project with NCAR’s Atmospheric Chemistry Division and NOAA’s Chemical Sciences Division to explore nitrate-initiated aerosol formation in laboratory chamber and flow tube experiments.
Monday, Nov. 7, 2011
Nick, Wagner
NOAA
Nighttime chemistry: Field measurements N2O5 uptake and ClNO2 production
Abstract
Nighttime chemistry accounts for up to half of the NOx removal from the atmosphere and produces atomic chlorine in the early morning through photolysis of ClNO2. NOx is removed through the production and heterogeneous loss of N2O5. The heterogeneous loss of N2O5 typically produces nitric acid which is removed through deposition. However, it has recently been discovered that in the presence of aerosol chloride, the N2O5 uptake produces ClNO2. The rate N2O5 heterogeneous loss been the subject of several laboratory studies; however, it has rarely been studied using ambient measurements. In this work, N2O5 uptake coefficients are determined from ambient wintertime measurements at the BAO tower in Erie, CO. The uptake coefficients are determined using an iterative box model. These uptake coefficients were found to be anti-correlated with the nitrate fraction of aerosol confirming suppression of uptake by aerosol nitrate. Additionally, a plume of chloride was observed in measurements of aerosol chloride, HCl and ClNO2. The N2O5 uptake coefficient was enhanced in this plume due to the competition between aerosol chloride and nitrate to react with N2O5.
Monday, Oct. 17, 2011
Ryan Davis
Isotopic Substitution in the Atmosphere and Solar Nebula: Cavity Ring-Down Spectroscopy of CO2 and VUV Photodissociation of CO
Abstract
Cavity ring-down spectroscopy (CRDS) is an ultra-sensitive spectroscopic technique capable of detecting trace gases in the atmosphere. In the Mark Thiemens group at UC-San Diego, a CRDS instrument was developed for the potential application of source apportionment of CO2 based on its isotopic fingerprint, which is an indicator of the chemical/physical processes the molecule has undergone. The theory behind and experimental design of the instrument is described here. Also, the oxygen isotope effect dominated by VUV photodissociation of CO, its implications for CO photolysis in the solar nebula, and the experimental test of the CO self-shielding theory is briefly discussed.
Samantha Thompson
Temporal Dynamics and Sources of Particle Types in Milwaukee, WI Studied with Single-Particle Mass Spectrometry
Abstract
Industrial processes and combustion sources release aerosols into the atmosphere that often contain components that are harmful to human health and the environment. In order to understand the sources and dynamics of these aerosol emissions, single-particle mass spectra of ambient aerosols were collected in Milwaukee, Wisconsin using ATOFMS during the summer of 2010. The ATOFMS data provided temporal trends of particulate metals, which can be correlated with wind direction and local source information to pinpoint their origins. Additional insight was gained into the particle emissions and dynamics in the local area by looking at the temporal behavior of particle classes.
Monday, Oct. 3, 2011
Margaret A. Tolbert
Importance of aerosol mixing state for ice nucleating efficiency: combined laboratory and field study
Abstract
Cirrus clouds composed of water ice are ubiquitous in the tropical tropopause region and play a major role in the Earth’s climate. Any changes to cirrus abundance due to natural or anthropogenic influences must be considered to evaluate future climate change. To understand cirrus cloud formation, it is necessary to determine the factors that control ice nucleation. In this work we have used optical microscopy coupled with Raman spectroscopy to examine water uptake, phase changes and ice nucleation on individual aerosol particles that have been impacted onto a hydrophobic surface. Studies are performed on both laboratory-generated particles of known composition and field-collected particles of unknown composition. Raman mapping experiments provide insight into the mixing state of the various components within single ice-nucleating particles. Results show that although overall chemical composition is important for ice nucleation, particle mixing state must be examined as well.
Rainer M. Volkamer
Oceans, Atmospheric chemistry, and Climate: Novel processes at the air-sea interface
Abstract
The Volkamer group develops optical spectroscopic instruments for use in laboratory experiments, and field studies to measure reactive trace gases and radicals that are relevant to tropospheric ozone, mercury cyling, and the formation of secondary organic aerosol (SOA). This talk gives an overview about group activities in the recent past and near future. The TORERO field experiment will study the Tropical Ocean Troposphere Exchange of Reactive halogen species and Oxygenated VOC by means of a comprehensive chemical payload of instruments centered around the CU Airborne Multi-AXis DOAS instrument aboard the NSF/NCAR GV aircraft in the area of Gallapagos Islands. It combines aircraft, ship and island based measurements, to probe chemical gases that form by destroying heat trapping ozone and can form new aerosols that cool climate. The group also participates in the Ganges Valley Aerosol Experiment (GVAX) in India, deploying the innovative CU Cavity Enhanced DOAS instrument, and a novel 2D scanning ground-based MAX-DOAS instrument. Future applications at the planned simulation chamber facility as part of the Center for Atmospheric Chemistry are briefly discussed.
Monday, Sep. 19, 2011
Bob Sievers
INHALABLE DRY POWDER AEROSOLS OF VACCINES AND ANTIBIOTICS
ABSTRACT
Dry powder aerosol inhalable vaccines are generally inherently more stable than liquid injected vaccines, and they can be administered through the moist respiratory tract along the same mucosal pathway used when wild-type virus infects a patient (PNAS, Jan. 31, 2011). Using unit dose high barrier packaging, there is no "vaccine wastage," which consumes about 40 % of all liquid measles vaccines worldwide, after multidose vaccines are reconstituted with water for injection. Eliminating needles also eliminates accidental needle sticks and the cost of sharps disposal. Because the large surface area of the alveoli of the subject is moist and dissolves the myo-inositol sugar-stabilized rapidly, there is no need to purify and carry water for injection into the field in developing country vaccine campaigns. With high-barrier individually sealed blister packs, contamination can be reduced, and shelf-life increased to 4 years. We are planning a joint study with Doctors Without Borders.
Jose L. Jimenez
Organic Aerosol Sources and Processing in the Atmosphere
Abstract
Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (emitted in the particle phase) and secondary OA (formed from chemical reactions of gas-phase species). In this talk I will summarize research in OA by our group over the last year, including a summary of the recent CalNex-LA and BEACHON-RoMBAS field projects in Pasadena (CA) and Woodland Springs (CO) respectively, direct measurements of the SOA formation and photochemical evolution of ambient air and collaborative work with modelers to constrain the OA budget. I will also discuss our new MOVI-CIMS instrument which allows molecular-level analysis of OA with high sensitivity and mass resolution and some first results from BEACHON-RoMBAS. Finally, I will introduce future field projects in India, Thailand, Southeast US, and the Amazon, and the outlook for the new smog chamber facility in Cristol Bldg.
Monday, August 29, 2011
Thomas J. Wenzel
Chiral Receptor Compounds as Enantioselective NMR Shift Reagents and Catalysts for Curricular Reform
Abstract
The use of crown ethers, calix[4]resorcinarenes and ionic cyclodextrin derivatives as enantioselective NMR shift reagents will be described. The cyclodextrins and calix[4]resorcinarenes are unusual because they are water-soluble reagents. A crown ether system that is useful for the analysis of secondary amines will be described. The beneficial learning that occurs when undergraduates conduct research was a major factor in causing me to restructure the way I teach my courses. The use of collaborative in-class learning activities and semester-long laboratory projects will be described, as will efforts to enact similar curricular changes at a consortium of over 20 schools.
Friday, August 19, 2011
SPECIAL SEMINAR
Arwa Mustafa, Center for Analysis and Synthesis, Department of Chemistry, Lund University, Lund, Sweden
Formulation of Phytonutrients Micro‐particles using Carbon Dioxide Assisted Particle Formulation Targeting Alzheimer’s disease
Abstract
Alzheimer’s disease (AD) is the result of a progressive aggregation of ß-amyloid peptide (ß‐AP) leading to the formation of extracellular fibrillar that deposits in selected areas of the brain. Polyphenols are efficient fibril inhibitors in AD by destabilizing beta‐amyloid fibrils. Our research aims to develop a drug carrier system that targets the brain via the nasal route to improve the treatment of Alzheimer’s disease (AD) by formulating well‐defined micron‐sized particles (10‐50µm) using supercritical carbon dioxide technology. Chitosan is a natural polymer that has a great potential for nasal delivery system facilitating the passage of hydrophilic molecules through the nasal mucosa. The technique of choice is the Carbon Dioxide Assisted Nebulization with a Bubble Dryer® (CAN-BD) since chitosan is soluble in water that would facilitate the micronization of oleuropein into chitosan.
Spring 2011
Monday, May 23, 2011
Ph.D. Defense, Daniel Bon, CIRES Aud., 23-May-11, 2 pm
Measurement of Atmospheric Volatile Organic Compounds from Urban, Industrial, and Biogenic Soruces by Proton-Transfer Ion-Trap Mass Spectrometry
Abstract
Volatile organic compounds (VOCs) are released into the atmosphere through a variety of natural and human-related processes. Understanding the atmospheric chemistry of VOCs is critical because of the role VOCs play in air pollution and climate. The diversity of VOCs found the atmosphere challenges even modern analytical methods. The Proton-Transfer-Reaction Ion-Trap Mass Spectrometer (PIT-MS) is a unique instrument for real-time measurement of VOCs in ambient air. In PIT-MS, VOCs are ionized by proton-transfer reactions with hydronium (H3O+) ions, and the product ions are detected by ion trap mass spectrometry. The PIT-MS instrument provides a mass spectrum every 1-10 seconds and has the capability for collision-induced dissociation (CID).
The analytical capabilities of the PIT-MS method are evaluated in this study through measurements of air impacted by different VOC sources: biogenic emissions in a California forest, urban emissions in Mexico City, industrial emissions in Houston, Texas, and emissions from controlled biomass burning experiments at the Fire Sciences Laboratory in Missoula, Montana. Through the use of CID, a gas chromatographic pre-separation method and through measurement comparisons with other instruments, the specificity of PIT-MS with regard to VOCs was studied in detail. These results are invaluable to researchers that use mass spectrometry and proton-transfer reactions with H3O+ ions to ionize VOCs.
Data from the MILAGRO campaign in Mexico City are used to characterize the urban emissions of VOCs. Hydrocarbon emission ratios relative to an inert tracer such as carbon monoxide (CO) are found to be about a factor of 2 larger in Mexico City than in the United States. The Mexico City data are also used for VOC source apportionment by the Positive Matrix Factorization (PMF) method. PMF analysis identifies three major VOC sources in Mexico City: vehicular traffic, liquid propane gas (LPG) usage and chemical formation.
PIT-MS instrument data from the TexAQS 2006 campaign in Houston, Texas are used to evaluate an industrial emissions inventory. The complexity of measured VOCs in industrial plumes is significantly larger than described in the inventory. For many VOCs, emission fluxes inferred from the measurements are significantly higher than in the inventory.
Thursday, May 12, 2011
Prof. Jon Abbatt, Univ. of Toronto
Studies in Multiphase Oxidation Chemistry in the Atmosphere: From Halides to PAHs to SOA
Abstract: Although the kinetics and mechanisms of gas phase reactions in the atmosphere are relatively well defined, the processes that occur via aerosol particles are much less well understood. In particular, a general issue persists of the degree to which condensed phase material in the atmosphere is subject to oxidative processes. Such multiphase chemistry may either occur at the surfaces of particles or films in the atmosphere, or in the bulk phase. This seminar will present three examples of multiphase chemistry, moving from a system for which we feel we understand the kinetics well, i.e. bromide oxidation by ozone in ice and water, to progressively less well understood systems, i.e. PAH heterogeneous reactions and the oxidation of SOA materials by hydroxyl radicals. In the case of SOA oxidation, a combination of experiments are presented using both laboratory surrogates for atmospheric SOA, and experiments with field samples. The potential atmospheric impacts of this work will be presented.
Thursday, April 28, 2011
Thesis defense for Ingrid Ulbrich
Characterization of Positive Matrix Factorization Methods and Their Application to Ambient Aerosol Mass Spectra
Thursday, April 28, 2:30 pm CIRES Auditorium
Abstract
Atmospheric aerosol has impacts on health, visibility, ecosystems, and climate. The organic component of submicron aerosol is a complex mixture of tens of thousands of compounds, and it is still challenging to quantify the direct sources of organic aerosol. Organic aerosol can also form from a variety of secondary reactions in the atmosphere, which are poorly understood. Real-time instrumental techniques, including the Aerosol Mass Spectrometer (AMS), which can quantitatively measure aerosol composition with high time and size resolution, and some chemical resolution, produce large volumes of data that contain rich information about aerosol sources and processes. This thesis work seeks to extract the underlying information that describes organic aerosol sources and processes by applying factor analytical techniques to organic aerosol datasets from the AMS. We have developed a custom, open-source software tool to compare factorization solutions, their residuals, and tracer-factor correlations. The application of existing mathematical techniques to these new datasets requires careful characterization of the precision in the data and the factorization models' behavior with these specialized datasets. We explore this behavior with synthetic datasets modeled on AMS data. The synthetic data factorization has predictable behaviors when solved with "too many" factors. These behaviors then guide the choice of solution for real aerosol datasets. The factor analyses of real aerosol datasets are useful for identifying aerosol types related to sources (e.g., urban combustion and biomass burning) and secondary atmospheric processes (e.g., semivolatile and low-volatility oxidized organic aerosol). We have also factored three-dimensional datasets of size-resolved aerosol composition data to explore the variability of aerosol size distributions as the aerosol undergoes processing in an urban atmosphere. This study provides evidence that primary particles are coated with condensed secondary aerosol during photochemical processing, shifting the size distribution of the primary particles to larger sizes. Application of these three-dimensional factorization techniques to other complex aerosol composition datasets (e.g., that use thermal desorption or chromatography for further chemical separation) has the potential to yield additional insights about aerosol sources and processes.
Monday, April 25, 2011
Frank N Keutsch Department of Chemistry University of Wisconsin-Madison
Measurements of Tracers of Biogenic Volatile Organic Compound Oxidation in Forests: Implications for Missing Sinks and Sources of the OH Radical
Abstract
The photochemical oxidation of volatile organic compounds (VOCs) is central to the formation of tropospheric ozone and secondary organic aerosol. Particularly in regions dominated by biogenic VOCs (BVOCs), our understanding of the chemistry of the OH radical, the most important tropospheric oxidant, is limited. In these biogenic regions, modeled OH concentrations are often dramatically lower than measurements, demonstrating unknown OH sources. In addition, measured OH sinks are often substantially larger than can be explained, and the presence of unknown, highly-reactive BVOCs have been proposed to explain this missing sink.
We have developed robust instrumentation for the ultra-sensitive measurement of BVOC oxidation products as tracers of BVOC oxidation by combining spectroscopic techniques that are new for field instrumentation with novel lasers. We propose that glyoxal is ideally suited as tracer of OH-driven BVOC oxidation and present rural measurements of glyoxal. We discuss these measurements within the context of the discrepancy between modeled and measured OH radical concentrations. We also present results from a study using the first formaldehyde flux measurements via eddy correlation obtained during the BEACHON-ROCS 2010 campaign at Manitou Forest. Different scenarios that can explain the unexpectedly large daytime fluxes of formaldehyde out of the forest canopy are discussed with respect to implications for the proposed unknown, highly-reactive BVOCs
Monday, April 18, 2011
Prof. Jesse H. Kroll Assistant Professor Civil and Environmental Engineering and Chemical Engineering MIT
Laboratory studies of the photochemical aging of organic aerosol
Abstract
The multigenerational oxidative processing (“aging”) of atmospheric organic aerosol is poorly understood, despite its likely influence on particle loadings and properties. In these experiments, n-alkanes are exposed to elevated levels of OH, in order to access the equivalent of several days’ worth of atmospheric oxidation. Measurements of the abundance and oxidation state of particulate organic carbon demonstrate the important role of fragmentation (C-C bond-breaking) reactions as oxidation progresses. Such reactions are highly efficient pathways for the formation of oxidized carbon; however, the corresponding changes to carbon number also tend to offset any decreases in volatility from the addition of functional groups. Thus particulate organics have increasingly smaller carbon numbers as they become more oxidized, with the continual escape of some fraction of the carbon to the gas phase during aging.
Monday, April 11, 2011
J’aime Manion
The Development of Inhalable Antibiotics for the treatment of TB
Abstract
The goal of this project is the development of unit-dose, inhalable, antibiotic microparticles for use in primary and combined therapy approaches to treating tuberculosis (TB) and highly drug-resistant strains of Mycobacterium tuberculosis (Mtb) that result in multi drug-resistant (MDR-TB) and extensively drug-resistant TB. Targeted to the alveolar space by merit of high fine particle fractions (FPF) in the < 3.3 µm range, these antibiotic microparticles utilize the same inhalation pathway as Mtb, to deliver antibiotic to protected TB lesions. This study explores strategies of combining microparticles with excipients to improve the fine particle fraction and deliver a larger fraction of antibiotic to the deep lung.
David McAdams
CAN-BD Processed Vaccines, Safer Vaccination in a Breath of Fresh Air
Abstract
In a modern world where the media and fringe groups seem to be touting the supposed dangers of vaccination, the scientific observations remain that many lives are saved and epidemics prevented through the use of vaccines. In an effort to improve the safety of vaccinations in the developing world the Sievers group is currently researching the production of inhaled, needle-free vaccines. This talk will cover the design of a needle-free dry powder HPV vaccine, collaboration with an overseas vaccine producer (Serum Institute of India) in the production of a needle-free Measles vaccine and tests of uniform activity throughout the particle size ranges of the aforementioned vaccine.
Monday, April 4, 2011
Charles S. Henry Departments of Chemistry, Chemical and Biological Engineering, and School of Biomedical Engineering Colorado State University
Microfluidic Tools for the Analysis of Aerosol Chemistry
Abstract
Ambient aerosols have significant impacts on both our environment and human health. From an environmental perspective, aerosols are one of the most significant unknowns for predicting the impact of human activity on the earth's climate. From a health perspective, aerosols are known to contribute to a wide range of diseases and cause significant changes in mortality and morbidity, however, the mechanisms for these health effects are not clear. It is clear for both environmental and health effects that knowing chemical composition is important in developing a better understanding of the impact of aerosol. This talk will focus on new microfluidic tools our lab has create to measure the chemical composition of aerosols. Microfluidic devices are attractive as an alternative to traditional aerosol analyzers because they can provide fast measurement times, high sensitivity, are compatible with a range of measurement chemistries, and can be relatively inexpensive. This talk will focus on recent results from our laboratory where microfluidic tools were used to make measurements of key chemical components of aerosols.
Monday, March 28, 2011
Dr. Erika Dawson InDevR Boulder, CO
Translating Academic Research into New Products for Virus Quantification and Identification
Abstract
This talk will describe my experiences at InDevR during the development of our two existing product lines, the ViroCyt Virus Counter and ampliPHOX Colorimetric Detection Technology. Starting with two separate technologies originally developed at CU, InDevR has advanced the original ideas into two unique instruments that are currently in different stages of development. I will share some recent data comparing the Virus Counter to standard virus quantification methods for applications in bioprocessing and vaccine development. I will also describe how ampliPHOX has been utilized to quickly and inexpensively detect and characterize influenza viruses using the FluChip.
Monday, March 14, 2011
Highly Concentrated Dispersions of Stable Submicron Therapeutic Protein Particles For Subcutaneous Injection
Keith P. Johnston
Dept. Chemical Engineering, Univ. of Texas Austin
Abstract
Protein and peptide therapeutics address needs in a wide range of diseases including cancer, infectious and inflammatory diseases. Despite their successes, administration of these therapeutics is limited by the lack of efficacious alternatives to intraveneous delivery of the required 100-500 mg doses. Here, we report a novel strategy compatible with subcutaneous injection that preserves IgG activity: aqueous-based, dispersions of submicron particles at 300 mg/ml IgG concentrations with apparent viscosities of below 50 cP. These dispersions may be prepared by generation of IgG particles, formed with thin film freezing (TFF) or a novel rapid spiral wound in-situ freezing technology (SWIFT) that preserve protein stability by limiting the protein’s exposure to liquid/gas and liquid/ ice interfaces. Dispersions of these particles in a solution containing PEG300 and n-methyl-2-pyrrolidone in buffer at the protein isoelectric point restricted protein solubility to <40 mg/ml and prevented complete particle dissolution. The low apparent viscosities of these dispersions result from the low viscosity of the initial solution and the low intrinsic viscosities ([η]). Additionally, antibody stability against denaturation and aggregation (as measured by HPLC SEC and ELISA) was enhanced by low solvation and decreased protein mobility of the solid state. High protein stability and biological activity is maintained upon subcutaneous delivery into mice(Jennifer Maynard laboratory, UT). The ability to form active, highly concentrated dispersions of therapeutic proteins with low viscosities, offers novel opportunities in subcutaneous injection for more effective treatment of disease.
Monday, March 7, 2011
Optical constants of model SOA formed by cloud processing
Kyle Zarzana
Abstract
Aerosols play an important but poorly understood role in affecting the Earth’s climate, and a better understanding of their origins and properties is required. A significant fraction of organic aerosol is composed of large light-absorbing species, but the formation and properties of these species are not well known. Recent work has shown that reactions between small organic molecules in cloud water can form species with chemical and physical properties very similar to the oligomers found in aerosols. We used cavity ring-down aerosol extinction spectroscopy and atomic force microscopy to examine the optical and physical properties of these model aerosols. The effects of these particles on direct radiative forcing were modeled to determine the significance of these particles on climate.
A case study for SOA formation by glyoxal processing in aqueous aerosol in Mexico City
Eleanor Waxman
Abstract
The role of heterogeneous chemistry as a source of Secondary Organic Aerosol (SOA) remains difficult to quantify. SOA is the portion of organic aerosol that forms in the atmosphere as a result of atmospheric transformations. Glyoxal is a building block for SOA formation as a result of heterogeneous chemistry. Measurements from the MCMA-2003 campaign in Mexico City showed that the gas phase glyoxal concentrations were lower by a factor of two to three than predicted by the Master Chemical Mechanism, and that this missing gas phase glyoxal was equivalent to the formation of about 5 μg/m3 of organic aerosol mass over the course of eight hours. Numerous recent laboratory studies have found that glyoxal can form significant amounts of SOA due to uptake, by both dark (equilibrium and irreversible) processes and by rapid photochemical uptake to aerosols. These processes have recently been reviewed, and a model framework has been developed based on these laboratory experiments to numerically describe glyoxal processing in aqueous aerosol particles. Here this model is applied to predict SOA formation due to glyoxal processing in Mexico City. We present an initial case study where the model was constrained with measurements from Mexico City during MCMA 2003 in a first attempt to bridge between laboratory and field observations, and an early assessment of our understanding of the processes and parameters that determine the amount of SOA formed from glyoxal.
Monday, February 14, 2011
Roles of Subvisible Particles in Protein Aggregation Pathways
John Carpenter
University of Colorado, Health Sciences Center School of Pharmacy
Abstract
Typically, protein aggregates are arbitrarily divided in subclasses: 1) soluble aggregates are oligomers that are found in solution and include species ranging in size from dimers to those large enough to elute in the void volume of a size exclusion chromatography (SEC) column; 2) insoluble aggregates are those that can be separated from the native protein and soluble aggregates by centrifugation or filtration; and 3) particles are aggregates that are usually too large to be analyzed by SEC and are at too low of a mass percent to be quantified by mass loss due to filtration or centrifugation. Particles typically are counted for quantification, and the size range examined is from about 1 micron to 125 microns. The connections between all of these species in the aggregation pathways of proteins are poorly understood. However, recent studies in which all three types were quantified during pharmaceutically relevant stresses (e.g., freeze-thawing, agitation and agitation in the presence of silicone oil) have shown that the first detectable aggregate type is subvisible particles. These can be quantified at mass percent far below the limit of detection for loss of monomer. Thus, particle counting provides the most sensitive method to date to observe and quantify protein aggregation. Also, it appears that when there is sufficient mass of aggregate such that insoluble aggregates can be quantified due to loss of monomer, these aggregates are composed of agglomerates of subvisible particles. Therefore, subvisible particles are fundamentally important components on the protein aggregation pathway. Finally, when particles are formed during a unit operation such a filling of vials, the particles can foster enhanced protein aggregation and particle formation during subsequent stress (e.g., agitation after vial filling).
Monday, February 7, 2011
Atmospheric Spectroscopy
Peter Bernath Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK
Abstract
An overview of the spectroscopy of the Earth’s atmosphere with the Atmospheric Chemistry Experiment (ACE) satellite will be presented. The primary instrument is a high-resolution infrared Fourier Transform Spectrometer (FTS) operating from 2 to 13 microns that measures the vertical distribution of trace gases, and the meteorological variables of temperature and pressure. Secondary instruments include a UV/Visible spectrometer called MAESTRO and two filtered solar imagers. Although now in its eighth year, the ACE satellite is still operating nominally. Ground-based atmospheric solar absorption spectra recorded with a copy of the ACE-FTS will also be discussed. The analysis of our spectra requires new laboratory measurements as will be illustrated with satellite retrievals of a wide range of organic molecules associated with air pollution.
Monday, January 31, 2011
Segregating Primary Biopolymer Associations with Airborne Particulate Matter: A Sanitary Engineering Perspective on Bioaerosol Traffic in the Atmospheric Environment
Prof. Mark Hernandez, Environmental Engineering, UCB
Abstract
While generally considered oligotrophic, the atmosphere carries microcellular hallmarks of life – both in primary and weathered forms. In this context, bioaerosols are defined as a generic class of airborne particulate matter comprised either in whole or in part of macromolecular compounds of biological origin. The contribution of the most common primary biopolymers — DNA, lipids, carbohydrates and proteins — to the pool of atmospheric organic carbon remains relatively unknown, as is their potential to weather and participate in secondary aerosol formation. We report here, phylogenetic characterization of atmospheric DNA pools juxtaposed to carbohydrate, proteins and lipids, as normalized by size segregated organic carbon mass.
Fall 2010
Monday, November 29, 2010
Ground based FTIR and MAXDOAS column observations of atmospheric trace gases
Ivan Ortega
Abstract
Carbon monoxide (CO) and Nitrogen dioxide (NO2) are important pollutants in urban agglomerations. Even though surface concentrations of those pollutants have been monitored routinely, the quantification of the total load (vertical column integral of concentration over the boundary layer height) is challenging because surface concentrations may not be representative over height of the boundary layer; the vertical distribution may further be variable. This presentation consists of two parts describing the measurement and retrieval techniques as well as results for CO and NO2 in two very different urban environments, Mexico City and Los Angeles, CA.
The diurnal trend of columnar CO in the boundary layer of Mexico City has been measured during one year with ground-based infrared absorption spectroscopy. Daytime CO total columns are retrieved from measured solar spectra and for the first time, nocturnal CO total columns using moonlight have been retrieved within a megacity. The measurements were taken at the Universidad Nacional Autonoma de Mexico (UNAM) campus located in Mexico City (19.33N, 99.18W, 2260 m.a.s.l.) from October 2007 to October2008 with a Fourier-transform infrared spectrometer (FTIR) at 0.5 cm-1 resolution.
An overview of measurements with a MAXDOAS instrument deployed at the Caltech supersite in Pasadena, CA this year will be presented in the second part of this talk. Validation of real time slant column densities of NO2 will be presented from the period of May to June 2010 in Pasadena, CA as part of CALNEX campaign. Using a geometric approximation NO2 vertical column densities are retrieved. The degree of mixing in the boundary layer is assessed by comparison of the NO2 vertical column densities with NO2 mixing ratios as measured from a cavity enhanced DOAS instrument located on top of Millikan Library, and independent measurements of mixing height by ceilometers.
Monday, November 15, 2010
Isotopic Labeling of Early Earth Haze Analogs
Raea Lessard
Abstract
The Faint Young Sun paradox predicts that the surface temperature of the early Earth was below the freezing point of water. A cold Earth is the expected outcome of an atmosphere composed of nitrogen and trace amounts of carbon dioxide coupled with a sun whose intensity was only 70% of its current luminosity. However, a cold Earth contrasts with the geologic record, which suggests that the early Earth was warm and had liquid water on its surface. The paradox can be resolved by adding methane, a strong greenhouse gas, to the weakly warming atmosphere assumed in the paradox. We have shown that atmospheres composed of nitrogen and trace amounts of methane and carbon dioxide will produce a haze when irradiated by light at the Lyman-a wavelength (121.6 nm). Such a haze would have implications for the early climate, as well as for the origin and evolution of life.
Now that the possibility of haze has been demonstrated, some questions remain: What is its composition? How is it made? To answer these questions, we label each of the carbon sources with 13C and use an aerosol mass spectrometer (AMS) to observe the haze fragments generated by electron impact ionization. We aim to use the information generated by the AMS, coupled with isotopic labeling, to elucidate the chemical pathway(s) by which the haze is made. In this talk, we share preliminary results from these experiments, demonstrate the feasibility of the proposed methods in elucidating the pathway(s), and touch on future work.
Monday, November 1, 2010
Levoglucosan, S-Phenylmercapturic Acid, and S-Benzylmercapturic Acid as Urinary Biomarkers of Wood Smoke Exposure
Callie Cole
Abstract
Wood smoke (WS) inhalation is a source of adverse health effects in humans due to its toxic and carcinogenic constituents. Monitoring exposure levels to WS through the use of urinary biomarkers can support epidemiological studies involving the direct health effects of exposure and help evaluate the effectiveness of efforts to limit human exposure in the future. Studies on levoglucosan showed that the diet has too large of an influence on its urinary concentration to conclusively quantify WS exposure. This project focuses on two other potential urinary biomarkers, s-phenylmercapturic acid (SPMA) and s-benzylmercapturic acid (SBMA), that have been previously successful for monitoring environmental tobacco smoke and industrial smoke exposure in humans, and studies their effectiveness in quantifying WS exposure. The hypothesis of this study is that both SPMA and SBMA will be effective biomarkers of WS exposure. To test this hypothesis, methods for the extraction and analysis of these compounds from urine were developed, evaluated and applied to the urine of exposed and control human subjects. The analytical method was found to have a limit of detection of 10ppb and a limit of quantitation of 50 ppb. Analyses of urine from individuals before and after exposure to WS showed quantifiable levels of the markers in 60% of samples, did not reveal a significant increase or decrease in concentrations of biomarkers in post-exposure relative to pre-exposure samples. The results imply that these biomarkers may not be suitable to determine exposure to WS. Additional studies to improve the extraction and detection sensitivity and reproducibility, as well as to monitor urinary levels over longer periods of time pre- and post exposure are needed to conclusively determine the utility of these markers.
A Molecularly Imprinted Polymer as a Sensor for Nicotine
Brett Palm
Abstract
Molecularly imprinted polymers (MIP) have been shown to preferentially bind molecules with specific shape or functional groups. This technology is discussed as it relates to the development of a nicotine sensor. Qualitative results are shown using an Atomic Force Microscope and FTIR spectra, and progress is made towards quantitative analysis using a GC.
Monday, October 25, 2010
Copper Binding by Dissolved Organic Matter using Competitive Ligand Exchange- Solid Phase Extraction and Application to the Biotic Ligand Model
Alison Craven
Abstract
The toxicity of copper to aquatic organisms is dependent on the aqueous copper speciation. The free copper concentration (Cu2+) primarily controls the toxicity of copper to fish due to its adsorption in the gill (a “biotic ligand”). Copper binding by dissolved organic matter plays a significant role in copper speciation and toxicity by reducing the amount of free copper that can bind to the biotic ligand. The biotic ligand model (BLM) is a model that predicts the concentration of free copper as a function of pH, dissolved organic matter concentration, and water hardness. While the model includes dissolved organic matter, there is still much uncertainty as to the correct copper-dissolved organic matter binding constants to include in the model. Much work has been done to measure the conditional stability constants for the copper-dissolved organic matter complex, but the methods used to measure these stability constants have “detection window” limitations that are not always recognized by those incorporating the stability constants into toxicity models. In this study, the competitive ligand exchange-solid phase extraction (CLE-SPE) method was used to measure the stability constants at pH 6.5 and 0.01 ionic strength over a wide range of Cu:dissolved organic matter concentration ratios. A comparison of the CLE-SPE stability constants with those measured by other methods revealed that the most important factor to determine the binding strength is the ratio of Cu:dissolved organic matter concentration, even for dramatically different environmental and experimental conditions. The Cu:dissolved organic matter concentration ratio is important because as the ratio decreases, the binding constant increases due to the heterogeneity of binding sites present in dissolved organic matter. Results obtained from this study can be used in the BLM to more accurately predict the role of dissolved organic matter in copper speciation.
Monday, October 4, 2010
Eddy-covariance measurements with time-of-flight mass spectrometry: A new approach to chemically-resolved organic particle and gas fluxes
Delphine K. Farmer
Abstract
Although laboratory studies show that biogenic VOCs yield significant secondary organic aerosol, emission of biogenic SOA fluxes has not yet been conclusively observed over forests. Further, while aerosols are known to deposit to forest surfaces, the chemical identity and ecosystem implications of this deposition flux remains undetermined. To better constrain sources and sinks of biogenic SOA, we have developed a new technique to directly measure fluxes of chemically resolved aerosol over forests using the High Resolution Time-of-Flight Aerosol Mass Spectrometer in a new, fast eddy covariance mode. This approach allows us to quantitatively identify both organic and inorganic components, including NH4+ , SO4_2- and NO3- , and has been deployed over both temperate and tropical forests. Results show both upward and downward fluxes of aerosol, including deposition of NH4+ . Differences in direction and magnitude of different chemical components provide insight on aerosol chemistry and potential ecosystem effects. In particular, we observe evidence for rapid formation or growth of biogenic SOA within both temperate and tropical forest canopies.
Aerosol Composition in Los Angeles During the 2010 CalNex Campaign Studied by High Resolution Aerosol Mass Spectrometry
Patrick L. Hayes
Abstract
Submicron atmospheric aerosols impact climate and human health, but their sources and composition are poorly understood. To address this knowledge gap, high-resolution time-of-flight aerosol mass spectrometry (AMS) and other advanced instrumentation were deployed during the CalNex field campaign in May and June 2010 to characterize the composition of aerosols in the Los Angeles area. Utilizing AMS, the concentrations for both organic and non-refractory inorganic (sulfate, nitrate, ammonium, chloride) submicron aerosols were quantified at the Pasadena ground site 15 km NE of downtown Los Angeles. Nitrate aerosols appear to dominate in the cooler mornings, but their concentration is reduced in the afternoon when organic aerosols (OA) increase and dominate. The diurnal variations in concentration are strongly influenced by vertical dilution from the rising planetary boundary layer in the afternoon. The concentrations of oxygenated OA (OOA), and hydrocarbon-like OA (HOA) are estimated, and it is found that OOA is consistently the largest type of OA present (~75% of the total OA concentration). This result indicates that the air mass over the site has undergone substantial chemical aging. The correlations between OOA and Ox (O3 + NO2) concentrations, as well as between HOA, CO and black carbon concentrations are consistent with previous studies. Ultimately, the goal of this research will be to test the accuracy of recently-proposed SOA models by combining the characterization of SOA with measurements of precursor and oxidant concentrations carried out concurrently during CalNex.
Monday, September 27, 2010
Update on RASEI, Solar Fuels, and Critical Areas of Electrochemical Energy Research
Carl A. Koval
Abstract
Since 2006, my primary responsibility has been the creation and development of CU-Boulder’s Energy initiative, which became the Renewable and Sustainable Energy Institute (RASEI). The first part of my presentation will be to reflect how RASEI got to where it is and, hopefully, where it is going. The second part will focus on how large-scale solar production of hydrogen is likely to be the first step towards creating a solar fuels industry. Finally, I will briefly describe three critical areas of energy research that the (photo)electrochemistry community needs to address.
Organic Aerosol Sources and Processing in the Atmosphere
Jose L. Jimenez
Abstract
Organic aerosols (OA) account for about 1/2 of the submicron particle mass in the atmosphere, but their sources, sinks, and evolution are poorly understood. OA is comprised of primary OA (emitted in the particle phase) and secondary OA (formed from chemical reactions of gas-phase species). In this talk I will summarize research in OA by our group over the last year, including a summary of the recent CalNex-LA field project in Pasadena (CA), research on the SOA formation and photochemical evolution of biomass burning emissions, on SOA model/measurement comparisons in different environments, and on collaborative work with global modelers to constraint the global OA budget. I will also discuss upcoming projects including characterization and use of semicontinuous GC-MS and chemical ionization mass spec. to analyze OA, and field projects in the Rocky Mountains, India, and Southeast Asia.
Monday, September 20, 2010
Nanoscale Therapeutics in the Treatment of Multidrug Resistant Pathogens
Daniel Feldheim
Abstract
In the past century, great strides have been made in the design and synthesis of pharmaceutical compounds for the treatment of human disease. Diseases such as breast cancer and chronic mylogenous leukemia, once thought to be completely untreatable, are now survived by a large percentage of patients. However, there is a large number of pressing medical issues related to the use of small-molecule drugs that must be addressed. For example, the emergence of drug-resistant bacterial and viral strains represent significant global medical challenges to overcome in the 21st century. To provide the tools to potentially treat infectious diseases, we have been developing ligand-coated gold nanoparticles as a new paradigm for drug discovery. Our work to date has offered some hope that the gold nanoparticle therapeutic platform may be a promising one in the search for new treatments for viral and bacterial disease. A few examples from our labs are the use of 2.0 nm diameter ligand-coated gold nanoparticles in the prevention of HIV fusion to human cells, and in the growth inhibition of E. coli and methicillin-resistant S. aureus. These results have inspired us to think in new ways about gold nanoparticle-based therapeutic design and the molecular-level design parameters that may influence activity. This talk will discuss some of these considerations.
The First Inhalable Dry Powder Measles Vaccine Entering Clinical Trials
Robert E. Sievers
Abstract
Ten percent of all pre-school deaths in India are measles-related. We have developed a Measles Vaccine, Dry Powder (MVDP) and PuffHaler® a novel dry powder inhaler for respiratory delivery to overcome the safety and vaccine wastage problems associated with the current injectable vaccine. MVDP was manufactured from bulk liquid, live attenuated, measles vaccine using CAN-BD® to yield a stable, dry powder, with fine particles suitable for aerosolization and delivery to the pulmonary tract by inhalation. This was achieved by substituting myo-inositol for sorbitol as a stabilizing sugar that is less hygroscopic. Contact with water is detrimental to the stability of measles vaccine and the microparticles are reconstituted only when they deposit in the moist surfaces of the respiratory tract. There is no need for purified water to be carried to remote locations. The particles dried by CAN-BD are homogeneous; the vaccine viral activity distribution in the 1 to 5 micron aerodynamic diameter microparticles is the same as the mass distribution, within experimental error. The shelf life is excellent at 2 to 8 °C with only about 0.5 log loss of activity after more than 2 years. At 25 °C, there is approximately 0.8 log loss after 6 months of storage. The immunogenicity and protective efficacy of a primary dose of MVDP delivered was tested in non-anesthetized, free breathing, Rhesus macaques. MVDP induced MV-specific humoral and T-cell responses at least as robust as subcutaneous measles vaccine and completely protected macaques from infection with wild type measles virus without adverse effects. Phase I clinical trials are now scheduled.
Monday, September 13, 2010
Sulfur Particles on Early Earth
Margaret A Tolbert
Understanding the atmosphere of the early Earth during the Archean, the period of time approximately 4 – 2.45 billion years ago, is an important part of understanding the conditions under which life originated and developed. While the atmospheric composition in highly uncertain during this time, it is likely that volcanic activity released sulfur dioxide into the air. Because of the lack of oxygen on the early Earth, the atmospheric chemistry of sulfur is expected to be quite different than on present day Earth. It has been suggested that atmospheric reactions in a reducing atmosphere could favor S8 particles over sulfuric acid particles. Here we use aerosol mass spectrometry to probe the chemical composition of particles formed from reactions of sulfur dioxide under a range of atmospheric conditions. Implications for the early Earth are discussed.
A heterogeneous open ocean source for glyoxal and iodine oxide
Rainer Volkamer
The climate relevance of biologically active ocean upwelling regions has primarily been studied in terms of the air-sea partitioning of long-lived greenhouse gases (e.g., CO2, CH4, N2O etc), and the release of the reactive gas DMS, which can form aerosols as a result of atmospheric transformations. Considerably less attention has been paid to open ocean sources of other reactive gases that, like DMS, can form aerosols. Such molecules are glyoxal (CHOCHO) and iodine oxide (IO). Glyoxal is an indicator for oxidative hydrocarbon chemistry, and a building block for secondary organic aerosol (SOA). SOA modifies the hygroscopic properties of organic aerosols, and potentially also adds to the growth of small particles to sizes that can more easily activate to form cloud droplets. Iodine oxide (IO) can nucleate new particles, and/or add to the growth of pre-existing particles. Due to the very high solubility of the glyoxal molecule, concentrations in excess of 100ppt over the open ocean like we found over the Pacific Ocean require an airborne source mechanism (Sinreich et al., 2010). We have investigated the source mechanism further during a ship campaign in 2009, as well as a first research flight aboard the NSF/NCAR GV research aircraft (HIAPER). Both campaigns give clues about the sources of both gases over the remote tropical Pacific Ocean, and reveal a surprising impact on the composition of the free troposphere.
Spring 2010
Tuesday, April 27, 2010
Laboratory Studies of Titan Tholins
Raea Lessard
Saturn’s moon Titan is enshrouded in a thick orange haze. A similar haze may have formed on the early Earth and provided the organic compounds necessary for the emergence of life. This talk will present results from several laboratory studies focusing on the manipulation of Titan aerosols, also known as tholins, for chemical analysis and simulation of prebiotic processing. Analysis techniques include nuclear magnetic resonance (NMR) and sizing by the scanning mobility particle sizer (SMPS). This will be followed by preliminary results from in-situ analysis by the Aerodyne quadrupole aerosol mass spectrometer (Q-AMS) of tholins formed at a range of pressures.
Tuesday, April 13, 2010
DOAS Measurements of Halogen Oxides
Hilke Oetjen
Halogens species are of outmost importance to the atmosphere due to their potential to destroy ozone and the DOAS (differential optical absorption spectroscopy) method is a powerful tool to measure halogen oxides like IO, BrO, but also OClO. After a brief introduction to the longpath-DOAS and multi-axes-DOAS techniques, this talk will present results from several field studies focusing mainly on the observations of iodine and bromine oxides and their impact on the marine boundary layer. This will be followed by a brief excursion to the stratosphere and the chlorine chemistry of the polar ozone hole. The third part of the talk will give an outlook on the future deployment of an airborne multi-axis (AMAX) DOAS instrument in the CalNex campaign in California this summer.
Tuesday, April 6, 2010
Single Particle Studies of Aerosol Hygroscopicity, Aging and Mass Accommodation
Professor Jonathan P. Reid, School of Chemistry, University of Bristol, UK
Abstract
Studies of the processes that govern the physical and chemical transformation of aerosol particles are crucial for improving our understanding of the properties of atmospheric aerosol. In particular, the equilibrium state is governed by hygroscopicity, the vapour pressures of semi-volatile organic components, and mixing state. Aerosol optical tweezers can provide a method for isolating single particles, or multiple particles of distinct composition for comparison. When combined with cavity enhanced Raman spectroscopy, particle size, composition and morphology can be characterised in detail. We will first examine how such an approach can allow an examination of the equilibrium state of aerosol. Measurements of the chemical aging of mixed component aerosol (oleic acid/sodium chloride/water) by ozone will then be reported. Finally, a novel approach for probing the kinetics of the mass transfer accompanying condensation or evaporation of water will be described and first measurements reported.
Tuesday, March 30, 2010
Development and Application of a Metastable Atom Bombardment (MAB) Source for Penning Ionization Time-of-Flight Aerosol Mass Spectrometry
Carly Robinson
Abstract
The Aerodyne time-of-flight aerosol mass spectrometer (ToF-AMS) utilizes thermal vaporization followed by electron ionization (EI) to convert aerosol components to gas-phase ions. The method enables quantification of chemical classes, but the extensive fragmentation caused by EI limits the specificity of both chemical analysis and source identification by factor analysis. To better identify the molecular components of aerosols, we have constructed a metastable atom bombardment (MAB) ionization source that can be interfaced to standard ToF-AMS hardware. A beam of metastable rare gas atoms is produced by a low-voltage DC discharge and focused toward the vaporization plume, yielding Penning Ionization of the analyte molecules. By changing gases, the excited energies of the metastables can be adjusted between 20.61 eV (He) and 9.92 eV (Kr). Source parameters, including pressures, current, geometry, and materials, were optimized for He, Ar, and Kr. Instrument sensitivity and induced fragmentation was characterized for each using lab-generated oleic acid particles. The demonstrated sensitivities are 0.1% of EI (3% of the SNR of EI in the V-mode, comparable to the Q-AMS SNR), which is sufficient for ambient monitoring. A metastable flux of 2.6e14 sr-1sec-1 has been achieved. The MAB-AMS has been deployed to the FLAME-3 campaign at the USDA Fire Sciences Laboratory in Missoula, MT, and used to sample smoke from open burning of different biomass samples.
Tuesday, March 16, 2010
Hugh Coe, Prof. of Atmospheric Composition, University of Manchester, UK
Title: "Secondary Aerosol Composition and Properties: A contrast between European Pollution and Tropical Biogenic Environments"
Abstract: This talk will describe a number of recent studies of secondary aerosol properties and processes in two contrasting environments - European pollution, sampled during the EUCAARI Intensive study in May 2008, and a clean tropical biogenic environment, sampled during the Oxidants and Particle Production Potential (OP3) study in Borneo, also in 2008. The EUCAARI project aims to advance our understanding of climate and air quality by integrating laboratory studies, both short and long term field investigations, satellite data, and modeling at regional and global scales. I will present airborne observations taken during an intensive study to provide a local and regional scale overview of the general composition trends of atmospheric aerosol over northern Europe.
The time dependent measurements obtained by aerosol mass spectrometry enable the chemical transformation time scales of aerosol material to be explored. In particular, factor analyses of the observed organic mass spectral fingerprint demonstrates the continuum nature of chemical processing. Ammonium nitrate is an important aerosol component in NW Europe. It exists in a temperature dependent equilibrium which favours particulate formation in the moist, cool upper portion of the boundary layer. We have shown that this results in aerosol optical depths inferred from ground based measurements are biased low by up to 50% by comparison with AERONET, aircraft measurements, and lidar data during periods of high AOD in anticyclonic conditions. The ubiquitous nature of black carbon (BC) was also demonstrated and how this is processed by secondary particulate will be shown using single particle soot photometry. These topics will all be explored and comparison with global model studies will also be discussed. In contrast, tropical environemnts are dominated by transport of aerosol into the region, by primary production of biological material in the coarse mode aerosol or by secondary organic aerosol produced within the region by photochemical oxidation of precursors. These will presented and the properties of secondary organic material compared with other tropical sites and with global models.
Tuesday, March 9, 2010
Nathalie Carrasco, Laboratoire Atmosphères, Milieux, Observations Spatiales, IPSL, Verrières le Buisson, France
Title: Experimental simulation of Titan’s atmosphere by a radio-frequency plasma
Titan is the biggest satellite of Saturne (about half the Earth). Its dense atmosphere of 1.5 bar at the surface is mainly composed of nitrogen and methane. So Titan is considered as an interesting analog of a frozen primitive Earth. Despite the very low temperature, between 100 and 200 K, atmospheric layers undergo efficient reaction chains, initiated by the solar flux and the Saturn magnetospheric electrons. Complex organic matter made of nitrogenated hydrocarbons is produced into the gaseous phase leading to a brownish photochemical fog surrounding the whole satellite.
Several techniques have been developed to simulate lab analogs, called tholins, of Titan’s haze. Most efficient methods presently known involve plasma techniques. Such a plasma device, named PAMPRE, has been developed in LATMOS, using a RF plasma discharge (13.6 MHz) in a stationary flux of nitrogen and methane gaz mixture, at various temperature and pressure conditions. Recent results will be presented concerning in situ mass spectrometry analysis of the gaseous phase and ex-situ chemical and morphological analysis of the tholins.
Tuesday, March 2, 2010
Dry powder formulation development for nasal vaccination
Regina Westmeier, Department of Pharmaceutics and Biopharmaceutics, Christian Albrecht University, Kiel, Germany
Abstract
I will briefly talk about some aspects of the mucosal immune system, which are important for the nasal vaccination strategy. I will then focus on the nose as delivery location, introduce a nasal cast model for the determination of formulation deposition in the different parts of the nose and show some delivery devices I have been characterizing. Further on I will shortly review the vaccine delivery systems described in literature and will then talk about our formulation strategies. Besides I will introduce a rapid in vitro test to assess the respiratory toxicity of both excipients and complete formulations.
Tuesday, February 16, 2010
Observations of iodine oxide and reactive gaseous mercury at a coastal site in Pensacola, FL
Sean Coburn
Abstract
An increasing body of evidence suggests that atmospheric halogens, marked largely by the presence of BrO and IO, are ubiquitous components of the lower troposphere in coastal and oceanic areas. The role of halogen chemistry in mercury oxidation in coastal regions remains unknown. Simulations of atmospheric mercury oxidation suggest that chemistry initiated by atomic Br may have been underestimated. Synergistic effects of the iodine compounds can be expected and little is known about the possible direct role I atoms may play to oxidize mercury. Finally, other reactive trace gases, such as CH2O and C2H2O2, can suppress the oxidation of Hg by converting bromine-radicals into chemically inert reservoir species. Here we present data from several months of MAX-DOAS measurements of halogen oxides, O4, CH2O and C2H2O2 in parallel with measurements of speciated mercury (Hg0, Hg2+) that are currently being conducted along the U.S. Gulf Coast.
Tuesday, February 9, 2010
Light Emitting Diode Cavity Enhanced Differential Optical Absorption Spectroscopy (LED-CE-DOAS): a novel technique for monitoring atmospheric trace gases
Ryan Thalman and Rainer Volkamer
Abstract
The combination of Cavity Enhanced Absorption Spectroscopy (CEAS) with broad-band light sources (e.g. Light-Emitting Diodes, LEDs) lends itself to the application of cavity enhanced DOAS (CE-DOAS) to perform sensitive and selective point measurements of multiple trace gases with a single instrument. In contrast to other broad-band CEAS techniques, CE-DOAS relies only on the measurement of relative intensity changes, i.e., does not require knowledge of the light intensity in the absence of trace gases and aerosols (I0) and is therefore insensitive to lamp drifts and/or the presence of aerosols. We have built a prototype LED-CE-DOAS instrument in the blue spectral range (420-490nm) to measure nitrogen dioxide (NO2), glyoxal (CHOCHO), methylglyoxal (CH3COCHO), iodine monoxide (IO), water (H2O) and oxygen dimers (O4). Aerosol extinction is retrieved at two wavelengths by means of observing water and O4 and measuring pressure, temperature and relative humidity independently. The instrument components are presented, and the approach to measure aerosol extinction is demonstrated by means of a set of experiments where laboratory generated monodisperse aerosols are added to the cavity. The aerosol extinction cross section agrees well with Mie calculations, demonstrating that our setup enables measurements of the above gases in open cavity mode.
Tuesday, February 2, 2010
Environmental Analysis of Water and Food Samples by Advanced Mass Spectrometry Techniques
Imma Ferrer and E. Michael Thurman
Environmental, Civil, and Architectural Engineering
CU, Boulder
Abstract
The analysis of pharmaceuticals and pesticides in water and food is one of the hot topics in environmental analysis. Recently the Associated Press published an article that much of the drinking water of the US is affected by pharmaceuticals coming from our wastewater. This article was backed up by numerous scientific studies since the late 1990s showing pharmaceuticals occurring in surface waters of the US. We have established a Laboratory for Environmental Mass Spectrometry soon to be a Center for Environmental Mass Spectrometry that uses an array of state of the art MS, including LC/time-of-flight MS, triple quadrupole MS with Jetspray, LC/MS ion trap, and other instrumentation to examine questions such as pharmaceuticals in drinking water and pesticides in food. Our seminar will discuss our new Center of Environmental MS, instrumentation, and applications to pharmaceuticals in drinking water and pesticides in food.
Friday, January 22, 2010 (note special day)
Pedro Campuzano-Jost
University of British Columbia, Canada
CIRES Auditorium @ 4:00 p.m University of Colorado, Boulder
Abstract
Trapping organic aerosol ions for molecular identification: Some lessons learned
The incapability of off-line techniques to resolve a significant part of the organic fraction of atmospheric aerosols has resulted in a stronger focus in recent years on developing real time aerosol mass spectrometers that are able to identify not just compound classes, but specific molecules. The approach chosen in most cases has been reducing fragmentation by use of soft-ionization techniques. While use of soft ionization can greatly simplify the mass spectra of complex mixtures, it also tends to erase the chemical information provided by the fragmentation pattern. Therefore, the ability to isolate and obtain tandem MS spectra of individual molecular ions is key to be able to id individual compounds.
Ion traps are compact, versatile devices that are similarly well suited for use in single particle mass spectrometers (SP-MS) than the more ubiquitous reflectron time-of-flight-MS; however, unlike reTOFs, they can also be used for tandem MS experiments. We have recently build a single particle ion trap mass spectrometer (SPIT-MS) that combines in-trap IR laser desorption of particles with a state-of-the-art laser based tabletop tunable VUV source. This allows separate optimization of both the desorption and ionization step for minimal fragmentation and -at high enough IR fluencies- a quantitative response. At the same time, measurements of the first ionization energy of the molecular ion together with their tandem MS spectrum can be used for molecular identification.While the SPIT-MS works as intended with certain types of test aerosol, it mostly fails with mixtures of aliphatic compounds, its intended target. The reasons for this failure will be examined in detail and some of its implications for alternative designs discussed.
In the second part of the talk, two other simpler SPIT-MS designs recently developed at UBC will be discussed:
- A laboratory prototype that uses pulsed IR laser desorption and conventional pulsed 70 eV electron impact for ionization. While its overall performance is comparable to the Aerodyne TOF in single particle mode, in addition it allows for analysis of refractory material as well as tandem MS analysis of larger ions.
- A new field capable ITMS that combines a novel aerosol beam alignment design with a mass spectrometer with dual polarity detection. Ionization occurs by laser ablation at 266 nm. This instrument is currently being deployed to the top of Whistler Mountain, BC to study long range transport of Asian dust.
Fall 2009
Monday, November 30, 2009
4:00 p.m. CIRES Auditorium University of Colorado, Boulder
Synthesis of Catalyst Arrays from Nanoparticle Precursors
Bryan Tienes
Abstract
The desire to develop novel, more efficient heterogeneous catalysts is a vital area of research in renewable energy applications. Due to the huge compositional and structural parameter space under which solid state materials can exist, it is nearly impossible to develop new heterogeneous catalysts a-priori. Therefore, many research groups have turned to combinatorial methods to identify new materials. While methods of synthesizing combinatorial arrays of bulk materials are well established, combinatorial arrays of nano-materials have been more elusive. Here we present a novel synthesis methodology for arrays of nanoparticles on solid supports using nanoparticles as synthetic precursors. The combinatorial arrays are generated by utilizing multidirectional nanoparticle gradients created by exploiting the kinetics of electrostatic adhesion of nanoparticles to a solid support. Subsequent reaction of the solid supports at elevated temperatures causes adjacent particles to alloy yielding nanoparticles with a gradient of compositions. These reactions have been demonstrated to take place on solid supports in both gaseous and liquid environments. Nanoparticle arrays adsorbed onto indium tin oxide (ITO) coated glass have been shown to alloy in a similar fashion. The arrays on ITO glass were subsequently fabricated into working electrodes for an electrochemical cell. The electrodes were then demonstrated as a catalyst screening tool for methanol fuel cell catalysts.
Monday, November 2, 2009
4:00 p.m. CIRES Auditorium University of Colorado, Boulder
A Novel Method for Analysis of Respirable Particle Size Distributions using an Andersen Cascade Impactor
Lia Rebits
Andersen cascade impactors (ACIs) are widely used throughout the pharmaceutical industry to obtain aerodynamic particle size distributions for inhalable products. An ACI separates aerosolized particles by passing them through a series of orifice‐containing stages and plates; the plate on which a certain particle will deposit depends on its velocity and aerodynamic diameter. A limitation encountered with the use of this instrument is that the analytical method chosen to quantify the deposition of particles on each plate must be precise, accurate, and have a low detection limit. A method that measures the total organic carbon (TOC) content on each plate has been found to fulfill many of the requirements needed to obtain accurate particle size distributions.
Amperometric S-Nitrosothiol Sensors: Applications and Methods
Candice Smith
It has been discovered that the right amount of S-nitrosothiol (RSNO) species in blood prevents the activation of clotting agents. A new RSNO detection technique, based on an electrochemical sensor, rapidly measures the level of RSNOs in whole blood. Use of this S-nitrosothiol sensor during surgical procedures, like Extra Corporeal Life Support (ECLS), could provide physicians with a novel method of monitoring a patient’s health. This seminar is an opportunity to describe the methods of the S-nitrosothiol biosensor’s assembly and in vivo testing.
Monday, October 26, 2009
4:00 p.m. CIRES Auditorium University of Colorado, Boulder
Practical Analytical Chemistry - Colorimetric Wet Chemistry Methods for Wastewater, Drinking Water, and Natural Water
Kristin Boles
Abstract
Analytical chemistry is an essential tool in drinking water and wastewater regulation. Wet Chemistry specifically refers to analytical chemistry performed in aqueous solution. It is therefore easily performed in the same matrix as wastewater and drinking water. This presentation will cover three wastewater testing methods: EPA 420.1, which tests for all phenols/phenolic compounds; EPA 365.3 dissolved, which tests for the orthophosphate ion in a filtered solution; and SM 3500-Cr B, which tests for hexavalent chromium in natural or treated water. Each of these tests is colorimetric, and the chemistry of their respective color-producing reaction will be discussed, as well as typical matrix interferences.
Monday, October 19, 2009
4:00 p.m. CIRES Auditorium University of Colorado, Boulder
Sources of organic aerosols: atmospheric transformations of emissions from combustion systems
Allen Robinson
Carnegie Mellon University
Abstract
Atmospheric particles play an important role in climate forcing; they are also strongly associated with adverse human health effects. Organic aerosols account for a large fraction of ambient fine particle mass, but their sources and transformation processes are still poorly understood. Motor vehicles, wood stoves, and other combustion systems are major sources of organic aerosols. This talk discusses recent field, laboratory, and modeling results on organic particle emissions from combustion systems. The results reveal a dynamic picture in which low-volatility organics evaporate, oxidize, and recondense as they are transported away from the source. This new picture alters our understanding of the contribution of combustion sources to urban and regional pollution and brings chemical transport model predictions into better agreement with field observations. The talk concludes with a discussion of the implications of these recent findings on human exposures and the design of regulations to control organic aerosols.
Monday, October 12, 2009
4:00 p.m. CIRES Auditorium University of Colorado, Boulder
New Insights Into the Mechanism of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase
Gregory Schill
Abstract
Class II 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate limiting enzyme of the mevalonate pathway, represents an attractive potential target for the development of antibiotics the against multiple drug-resistant strains of Gram-positive cocci. However, recent steady-state spectrofluorimetric studies suggest that the mechanism of catalysis does not go through a mevaldehyde intermediate as accepted in the literature. The objective of this study was to investigate HMG-CoA reductase catalysis prior to steady-state conditions to determine if this incongruence was the result of a fast equilibrium. Stopped-flow kinetic assays, with dead times of 8 ms, were conducted to probe for the elusive mevaldehyde intermediate. Results found that a fast equilibrium can not account for the absence of mevaldehyde, suggesting a revision of the current literature-accepted mechanism of catalysis to one where coenzyme A plays a more integral role.
A NEGATIVE ION PHOTOELECTRON SPECTROSCOPIC STUDY OF Mo2-.
Sunil Baidar
Abstract
The homonuclear diatomic metal ion Mo2- was studied using negative ion photoelectron spectroscopy. This bare, homonuclear group 6 transition metal dimer allows the study of multiple metal-metal bonding free of ligand effects. The photoelectron spectra, obtained at 488 nm with an instrumental resolution of about 6 meV (50 cm-1), provide measurements of the electron affinities, vibrational frequencies for both the anion and the neutral states, and bond length changes upon electron detachment. The Mo2- spectra displays a transition to the multiply-bonded 1Σg+(dπ)4(dδ)4(dσ)2(sσ)2 ground state of the neutral molecule. In the anion, the extra electron occupies the vacant antibonding sσ* orbital, giving a 2Σu+ ground state.
Monday, October 5, 2009
4:00 p.m. CIRES Auditorium University of Colorado, Boulder
Aerosols of the Past: Lessons from Saturn’s Moon Titan
Margaret Tolbert
Dept. Chem & Biochem., and CIRES, CU-Boulder
Abstract
An organic haze layer in the upper atmosphere of Titan plays a crucial role in the atmospheric composition and climate of that moon. Photochemistry in the atmosphere of the early Earth shortly after the time of planetary formation may have led to a similar haze. However, due to differences in atmospheric chemistry, the aerosols formed on the early Earth may have had distinct chemical and optical properties from those suspected to make up the hazes on Titan. We have conducted laboratory experiments simulating photochemical haze production on Titan and early Earth. The resulting aerosols are analyzed using an Aerosol Mass Spectrometer (AMS) to obtain compositional information and extinction cavity ring down (CRD) spectroscopy to determine the optical properties of the particles. The hazes on the two worlds will be compared and the implications of haze on the climate and habitability of ancient Earth will be discussed.
What have we learned about organic aerosol sources and processing? What’s next?
Jose-Luis Jimenez
Dept. Chem & Biochem., and CIRES, CU-Boulder
Abstract
Aerosols play major roles in climate forcing and the hydrological cycle, and also on human health effects, visibility degradation, and deposition of acids, toxics, and nutrients to ecosystems. Organic aerosols (OA) account for a large fraction (~50%) of ambient submicron aerosol mass, but their sources and transformation processes are still poorly understood. I will summarize recent findings about the sources, processing and budget of OA arising from multiple field and lab studies, as well as comparisons to 1D and 3D models. OA from all sources evolves in the atmosphere by becoming more oxygenated and hygroscopic and less volatile, and partially losing the chemical signature of the original source and gaining a common signature of atmospheric oxidation. Secondary OA (SOA) formation in urban areas is greatly underestimated by traditional models, in contrast with biogenic SOA formed under pristine conditions which is reasonably predicted. New models close the gap and even exceed the observed SOA in urban areas, but it is not clear whether this is for the right reasons. Further progress necessitates more complete high-quality measurements at one location as well as new measurement & analysis techniques, and we are preparing for two such campaigns in Los Angeles, CA in 2010 and Manitou Springs, CO in 2011.
Monday, September 28, 2009
4:00 p.m. CIRES Auditorium University of Colorado, Boulder
Water and photon mediated chemistry relevant to atmospheric aerosol formation
Veronica Vaida
Dept. Chem & Biochem., and CIRES, CU-Boulder
Abstract
Research in the Vaida group explores the interface between physical chemistry and atmospheric science using tools of spectroscopy, photochemistry and photoreaction dynamics. This presentation will exemplify discuss water catalyzed photochemical reactions of oxidized organic species (acids, alcohols, aldehydes and ketones) relevant to atmospheric chemistry. Our approach utilizes electronic and vibrational spectroscopy to explore chemical reactions. Theoretical modeling in collaboration with the skodje group elucidates the roles of cluster conformation and dynamical effects in the photochemical processes. The specific example of methyl glyoxal, and pyruvic and glyoxilic acids and of their hydrates will be presented. The discussion will point to the role of water and photon mediated chemistry of organic molecules to the generation and processing of secondary organic aerosols and, more generally, to climate.
Instrument development for use in field and laboratory experiments in the ATMOSpeclab
Rainer Volkamer
Dept. Chem & Biochem., and CIRES, CU-Boulder
Abstract
The Atmospheric Trace Molecule Spectroscopy Laboratory (ATMOSpeclab) was established in 2007 at CU Boulder to develop and apply optical spectroscopic instruments to measure atmospheric composition of reactive trace gases in polluted urban, and pristine natural atmospheric environments. Specific atmospheric chemistry projects in the ATMOSpeclab will be discussed, and include: - Impact of halogen chemistry on Mercury deposition in the coastal environment - Airborne MAX-DOAS from research aircraft - Ship based MAX-DOAS in the Southern Hemisphere Pacific Ocean - Laboratory studies of SOA formation from glyoxal in simulation chambers - Development of a detailed model to represent SOA formation via the aerosol aqueous phase
Monday, September 21, 2009
4:00 p.m. CIRES Auditorium University of Colorado, Boulder
CU Inhalable Dry Powder Vaccines have Provided Protection Against Infection
R. Sievers, S. Cape, J. Burger, D. McAdams, J. Manion, L. Rebits, S. Winston, B. Quinn, J. Searles, D. Krank, P. Bhagwat, P. Pathak, A. Genosar, R. Dhere, V. Vaidya, R. Muley, D. Griffin, W-H Lin, and P. Rota
Abstract
Protection against infection with measles virus has been demonstrated in animal models immunized 14 months earlier by inhalation of CU-invented dry powder vaccine microparticles. Needle-free aerosol delivery of dry powder vaccines may provide an effective and low-cost means of immunization. Powder manufacturing with gentle, rapid drying by Carbon Dioxide Assisted Nebulization with a Bubble Dryer (CAN-BD) appears to be an attractive alternative to freeze drying of sensitive biologicals like live attenuated Edmonston-Zagreb measles virus vaccine. Stability of measles virus in glassy, dry, respirable microparticles made by CAN-BD with less than 1% water sealed in unidose aluminum foil has been demonstrated. The dry powder aggregate, upon dispersion from an active dry powder inhaler, deposits in the moist respiratory tract where the microparticles rapidly dissolve within minutes. Antibiotics are also being studied and delivered as microparticles against infectious diseases.
Funded by FNIH Grant 1077.
Improving the Performance of Li Ion Batteries Using Atomic Layer Deposition
Steven M. George
Depts. of Chemistry and Chemical Engineering University of Colorado, Boulder, CO 80309 Steven.George@Colorado.Edu
Abstract
Lithium ion batteries (LIBs) are emerging as the dominant power source for portable electronics. Improvement in their capacity lifetime during charge-discharge cycles must be achieved before LIBs can be used for plug-in-hybrid and electric vehicles. LiCoO2 and graphite are common cathode and anode materials, respectively, for LIBs. The capacity loss of LIBs is linked to electrode deterioration caused by interfacial reactions with the electrolyte, dissolution of the metal oxide cathodes and structural instabilities caused by volume expansion during lithium intercalation. Atomic layer deposition (ALD) is based on the strategy of sequential, self-limiting surface reactions to deposit inorganic materials. ALD can deposit thin, conformal films on cathode and anode materials in LIBs to provide chemical protection, restrict dissolution and stabilize structural changes. Our recent work has demonstrated that ultrathin Al2O3 ALD films can improve the capacity stability of LiCoO2 cathodes and graphite anodes. Additional work is developing new protective coatings that will serve as an artificial solid-electrolyte interphase (SEI) for further stability improvement.
Monday, September 14, 2009
4:00 p.m. CIRES Auditorium University of Colorado, Boulder
Renewable and Sustainable Energy Institute (RASEI): What does this mean for the Dept. of Chemistry and Biochemistry (and other departments and institutes)?
Carl A. Koval, Co-Director
Abstract
In June 2009, the University of Colorado’s Board of Regents approved the formation of a new campus institute: RASEI (pronounced ‘racy”). This formation of this institute grew out of the former CU-Boulder Energy Initiative (EI), which was created in early 2006. In addition, administrators at CU-Boulder and the National Renewable Energy Laboratory (NREL) in Golden signed a MOU to operate RASEI as a joint institute. In this presentation, I will focus on:
• the transition of the EI to RASEI, from initiative to institute;
• opportunities for faculty to be involved in RASEI governance and programs;
• opportunities for students to be involved RASEI’s research, teaching, commercialization and outreach activities.
For more information about RASEI, go to http://rasei.colorado.edu.
Research at the Interface of Nanoscale Materials and Biology
Daniel L. Feldheim
Dept. Chem & Biochem., CU-Boulder
Abstract
Between the size scale of individual biomacromolecules and cellular organelles lies a size regime―the few nm to 100 nm range―that is not readily probed using existing technologies. Thus, while a vast inventory of RNA and protein sequences and structures are being catalogued, a quantitative cellular context for these structures is lagging. Yet to understand this context would be to know how collections of individual macromolecules assemble into the dynamic machines that form a living cell. Once these interactions are revealed, a new picture of the cell and its disease states is sure to emerge.
With achievable 2 nm resolution and perfect preservation of cellular structure, electron tomography (ET) now represents the highest resolution technique for examining biomolecules in their native cellular context. Indeed, cell biologists now dream of generating 3D images containing the entire proteome of a living cell. This dream remains out of reach, not because ET cannot visualize individual proteins, but because it is simply not possible currently to know which protein is which in a tomographic cellular reconstruction.
This presentation will describe methods being developed in our lab for creating electron dense nanoparticle tags for identifying cellular biomolecules by ET. These methods rely on the discovery of materials ribozymes and enzymes―RNA and protein/peptide sequences that can catalyze the formation of inorganic nanoparticles and control nanoparticle growth. The isolation and structure-function relationships of a number of materials ribozymes and enzymes will be shown.
In addition to visualizing biomolecule interactions in cells, we are working on ways to mimic and disrupt such interactions for disease treatment. We view this largely as a materials problem as well, and have been learning how to design metal nanoclusters to effectively interact with cellular biomolecules. A few lessons learned in our studies of cell and nuclear targeting and the prevention of viral infection will be described.
Thursday, September 10, 2009
4:00 p.m. CIRES Auditorium (note change of location) University of Colorado, Boulder
What controls the diurnal variability of carbon dioxide and reactive species? (a.k.a. "Boundary Layers for Chemists")
Jordi Vilà-Guerau de Arellano
Meteorology and Air Quality Section
Wageningen University (The Netherlands)
e-mail: jordi.vila@wur.nl
Abstract
We examine the main physical and chemical processes that determine the diurnal variability of atmospheric compounds in the boundary layer. In addition to surface processes, turbulent mixing and reactivity, we put special emphasis on investigating the role of the exchange of heat, water and chemical species between the free troposphere and the atmospheric boundary layer, namely the entrainment process. This process enhances the dilution of compounds and introduces free tropospheric air masses with different characteristics into the atmospheric boundary layer.
In the seminar, I will discuss several cases where entrainment plays a major role in the evolution of atmospheric compounds. By analyzing observational evidence or performing numerical experiments by the large eddy simulation technique and a conceptual (mixed-layer theory) model, we are able to find the contribution of entrainment to the diurnal variability of carbon dioxide or isoprene. Particular emphasis is placed on the need to maintain a balance in dynamic processes and the specific characteristic of each atmospheric compound.