Oxygen Isotope Methods
Isotope concentrations of precipitation, surface water, or carbonates can be used as a proxy for paleoelevation. Several isotopes may be used; here, only 18O will be discussed. For a given isotope, concentration is reported as a deviation from a standard isotopic measure:
![]()
where d is the deviation (in parts per thousand), asample is the isotope concentration in the sample, and astandard is the isotope concentration in the standard (Dansgaard, 1964). For example, d18O is calculated (in parts per thousand) as:
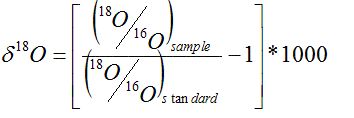
(Morrill and Koch, 2002). The standard for water is Standard Mean Ocean Water
(SMOW), a mean concentration of present-day ocean water, maintained by the International
Atomic Energy Agency in Vienna, Austria. SMOW oxygen atoms are about 99.76%
16O and 0.20% 18O (NIST website). Carbonate samples are
compared to a standard Peedee belemnite (PDB).
Several processes lead to the depletion of d18O
in precipitation. Of greatest concern here are equilibrium processes, which
are laid out in detail in Dansgaard (1964). These processes include condensation,
temperature effects, and rain-out effects. To describe condensation, consider
an air mass that has the isotopic composition of SMOW. As water condenses out
of the air mass, the heavier isotopes condense first, leaving isotope-depleted
moisture in the air mass. Precipitation from the air mass becomes more depleted
(d becomes more negative) with time. Condensation
has the largest effect in the isotopic composition of precipitation (Dansgaard,
1964).
Isotopic composition of the condensate also decreases with temperature. As
temperature of the air mass decreases, it cannot hold as much water as before.
The air mass looses moisture and becomes more depleted in d18O
when its temperature drops (Dansgaard, 1964).
Another factor that leads to differences in isotopic concentration with elevation
is amount effects. Evaporation and isotopic exchange tend to increase d18O
in small amounts of rain, so areas that receive less rain tend to have higher
d18O values than areas that receive more
rain.
To understand condensation, temperature, and amount effects, the path the air
mass follows from the moisture source (usually the ocean) to the region of study
must be known. Present-day storm tracks are seasonal; areas in the Basin and
Range, for example, receive moisture from the Pacific Ocean that travels over
the Sierra Nevada in the winter, and moisture from the Gulf of California that
travels over the Colorado Plateau in the summer (Fig.
2) (Poage and Chamberlain, 2002). This seasonal storm variation causes variation
in isotopic composition. Thus, when considering isotopic compositions of the
past, it is important to understand the source and track of the measured isotopes
to establish expected condensation, temperature, and amount effects.
Applications
Studies of paleoelevation using oxygen isotopes can exploit the concentration
and temperature effects on the lee side of mountain ranges (i.e., Poage and
Chamberlain, 2002). The windward side of a mountain range tends to receive greater
precipitation than the lee side. Consequently, samples of precipitation from
the rain-shadow are expected to be more depleted d18O
than would normally be expected for the distance from the moisture source.
Another way to use oxygen isotopes to infer paleoelevation is to find evidence of snowmelt in the isotopic signal (i.e., Norris et al., 1996). To rise over a mountain, an air mass may loose moisture, so precipitation falling on top of the mountain is depleted in d18O. Spikes of low d18O values in a basin may indicate an influx of snowmelt. If the basin is thought to be too low to have received snow, the snowmelt must be runoff from surrounding highlands. If the paleoelevation of the basin and a lapse rate can be estimated, a minimum elevation of the peaks of the mountain range can be inferred (Norris et al., 1996).
Paleoelevation of the Sierra Nevada
Paleoelevation of the Rocky Mountains
Back to Title page
Back to Introduction