|
|
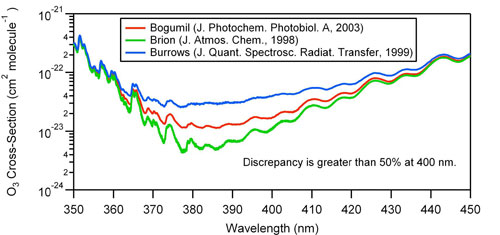
A winning proposal for the Innovative Research Program, 2010: Measurements of weak absorptions by O3 and O3:H2O clusters using cavity enhanced spectroscopyInvestigators:V. Vaida (CIRES/University of Colorado, Boulder, CO) C. Young (CIRES/University of Colorado, Boulder, CO & NOAA/ESRL Chemical Sciences Division, Boulder, CO) R. Washenfelder (CIRES/University of Colorado, Boulder, CO & NOAA/ESRL Chemical Sciences Division, Boulder, CO) G. J. Frost (CIRES/University of Colorado, Boulder, CO & NOAA/ESRL Chemical Sciences Division, Boulder, CO) Objective:We propose to develop a laboratory instrument and method for measuring weak, temperature dependent absorption cross sections in the near-ultraviolet spectral regions for important atmospheric trace gases and their complexes with water vapor. We will focus on ozone and ozone-water, where large discrepancies have been reported for the near-UV cross-section. This work will provide the basis for establishing a program to investigate the weak cross-sections and the effect of hydration of important atmospheric trace gases (aldehydes, ketones and keto acids) whose atmospheric lifetime is photochemically determined. Introduction and background:Weak spectral absorptions play an important role in the radiative transfer of the Earth’s atmosphere and are necessary for accurate satellite retrievals (1). In addition, weak electronic features in the near-UV are important for tropospheric radical production (2, 3). However, these weak absorptions can be difficult to quantify in the laboratory, because traditional spectroscopic instruments lack the necessary sensitivity. The problem is exacerbated by the likelihood of cluster formation between atmospheric trace species and water via hydrogen bonding (4), leading to experimental artifacts at the chromophore concentrations required for detection (5). Surprisingly, even ozone, arguably the most important atmospheric absorber in the visible and UV, has a near UV spectrum that is poorly characterized (see Fig. 1), with reported discrepancies up to one order of magnitude in the weakly absorbing spectral region between 350 – 450 nm.
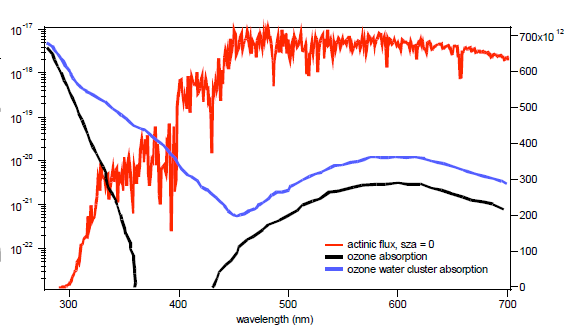
Figure 1: Measured absorption cross sections of ozone between 350-450 nm. Theoretical calculations predict that ozone will form O3:H2O clusters that absorb strongly in the region between 350 and 450 nm (4). This process is important in the atmosphere because the complex may act as an atmospheric source of hydroyxal radicals (3). The effects of such a complex would be evident in the electronic spectrum, particularly in the region between 350 and 450 nm. Frost and Vaida (4) calculate this region is where the O3:H2O cluster will absorb much more strongly than the O3 monomer (Figure 2). The predicted increase lies in the range of 1 – 4 orders of magnitude, but has never been measured experimentally.
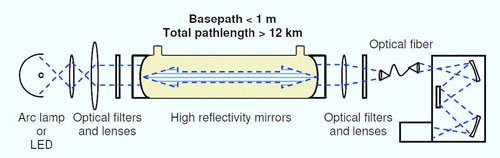
Figure 2: Absorption cross sections of pure ozone and ozone-water complex shown with respect to the actinic flux at the surface of the Earth. Although the value of high accuracy cross sections and J values of weak absorbers has been well understood for some time, only very recently has the technology become available to facilitate such measurements (6, 7). Incoherent broad-band cavity-enhanced absorption spectrometry (IBBCEAS) takes advantage of long optical pathlengths within optical cavities, similar to laser-based cavity ring-down spectrometry (CRDS). IBBCEAS instruments use continuous broadband sources, which are much less expensive than monochromatic lasers used in CRDS and also allow collection of data at multiple wavelengths. The advent of this technique makes it possible to design high sensitivity measurements of absorption cross sections in the near-UV, even for dilute trace gas levels (6, 7). What makes this innovative?Measurements of ozone cross sections have focused on obtaining high-resolution spectra of the higher-intensity bands of ozone absorption (8-10). Little focus has been placed on an accurate cross section for the low-intensity absorption regions, with measurements varying over an order of magnitude (Figure 1). The formation of complexes may be contributing to this variability, as lowsensitivity methods require high concentrations of ozone for detection. The high sensitivity of the IBBCEAS will allow accurate cross sections to be determined in dilute mixtures of ozone, and as a function of different bath gases and relative humidity. The effective path length of current IBBCEAS instrument range up to tens of kilometers, allowing extinction coefficient (i.e., a = concentration × absorption cross section) measurements at 10-10 cm–1 and lower in a laboratory setting, enabling accurate measurements of cross sections and small changes in absorption due to complex formation. Expected outcome and impact:This work will provide an accurate, temperature-dependent absorption cross-section for ozone and the ozone-water complex in the region of 350 to 450 nm. The former is critical for satellites in order to define a background extinction against which NO2 retrievals are performed, while the latter will allow accurate assessments of the importance of ozone-water complexes in the hydroxyl radical budget of the atmosphere. The techniques developed here will also provide a basis for further measurements of near-UV cross sections of other atmospheric radical sources, such as nitric acid, peroxides and carbonyls, along with water complexes of these compounds. Research plan: The experimental research will be conducted as a collaboration between a graduate student in Veronica Vaida’s group and two CIRES employees at NOAA. Dr. Frost (CIRES at NOAA) will assist with the interpretation of the experimental findings. 1. Design, construction and characterization of IBBCEAS instrumentThe proposed laboratory instrument (Figure 3) will be similar to an IBBCEAS field instrument currently in operation. The instrument would have 2-3 channels and utilize LED or arc lamp light sources to obtain full spectral coverage over the range 350 – 470 nm. It will be constructed with jacketed sample cells to allow temperature-controlled measurements. 2. Ozone spectroscopy and water complexesInvestigate changes in the wavelength-dependent electronic cross section between 350 to 450 nm as a function of temperature, pressure, bath gas and relative humidity. 3. Measurement of weak cross sections and hydration of atmospheric trace gasesExtension of the technique to other potential sources of atmospheric radicals, such as nitric acid, peroxides and carbonyls.
Figure 3: Schematic of a single-channel, incoherent broadband cavity enhanced spectrometer (IBBCEAS), showing the light source, optical cavity, and detector. Sources cited:
|