3. Petrogenesis Review - The Mid-Tertiary Ignimbrite Flare-Up
Using the composition of an igneous rock to interpret petrogenesis of the rock is a powerful, yet potentially difficult technique. The following is an igneous petrogenesis refresher based on lecture notes (Wilson, 1995) and the course textbook (Wilson, 1989). Techniques discussed include analysis of oxides, trace elements, and isotopes. This discussion assumes a two-layer mantle convection system with convection cells isolated between the upper and lower mantles; the depth of the upper-lower mantle boundary is approximately 670 km.
The upper mantle acts as the main source for oceanic lithosphere and continental crust and lithosphere (at least in the past). Mid-ocean ridge basalt (MORB) is derived from partial melting of the upper mantle. The upper mantle is depleted in incompatible elements (i.e., K, Rb, Sr, Ba, Zr, Th, and other rare earth elements that do not easily fit into the crystal lattice structures of mantle minerals such as olivine, pyroxene, spinel, and garnet). These incompatible elements have gone into making continental crust and lithosphere though time, thus making the continents "enriched" and the upper mantle "depleted" in incompatible elements. The lower mantle may be less affected by partial melting processes and thus is more enriched in incompatible elements. However, subducting slabs composed of enriched material contaminate the depleted upper mantle (making it less depleted) and bring material into the lower mantle.
Oxides
 Two fundamental
processes affect the composition of igneous rocks: partial melting and
fractional crystallization. Additional processes that can influence the
composition of igneous rocks include magma mixing and crustal contamination.
Partial melting can occur due to heating, decompression, and the addition
of fluids and contaminants. Partial melting tends to increase the percentage
of alkalic oxides in rocks, while fractional crystallization tends to
increase the percentage of silica in rocks. Fractional crystallization,
magma mixing, and crustal contamination often occur in high-level magma
chambers. A common way to categorize igneous rocks is to plot weight percentage
of oxides Na2O + K2O against weight percent SiO2,
a plot termed a Harker diagram (Figure 1). The plot is divided into alkalic
and sub-alkalic fields, and also by color index (SiO2).
Two fundamental
processes affect the composition of igneous rocks: partial melting and
fractional crystallization. Additional processes that can influence the
composition of igneous rocks include magma mixing and crustal contamination.
Partial melting can occur due to heating, decompression, and the addition
of fluids and contaminants. Partial melting tends to increase the percentage
of alkalic oxides in rocks, while fractional crystallization tends to
increase the percentage of silica in rocks. Fractional crystallization,
magma mixing, and crustal contamination often occur in high-level magma
chambers. A common way to categorize igneous rocks is to plot weight percentage
of oxides Na2O + K2O against weight percent SiO2,
a plot termed a Harker diagram (Figure 1). The plot is divided into alkalic
and sub-alkalic fields, and also by color index (SiO2).
Figure 1. Harker diagram of alkalic and sub-alkalic extrusive igneous rocks (from Wilson, 1995). Gray field indicates alkalic rocks and red field sub-alkalic rocks.
 Another characterization method is to plot oxides of K and Na verses Si (Figure 2). Sub-alkalic, and low-K sub-alkalic basalts are defined by fields in the Na2O verses SiO2 plot, and alkalic and sub-alkalic basalts can also be defined in K2O verses SiO2 plots.
Another characterization method is to plot oxides of K and Na verses Si (Figure 2). Sub-alkalic, and low-K sub-alkalic basalts are defined by fields in the Na2O verses SiO2 plot, and alkalic and sub-alkalic basalts can also be defined in K2O verses SiO2 plots.
Figure 2. Oxide plots of K verses Si (left) and Na verses Si (right) (from Wilson, 1995)
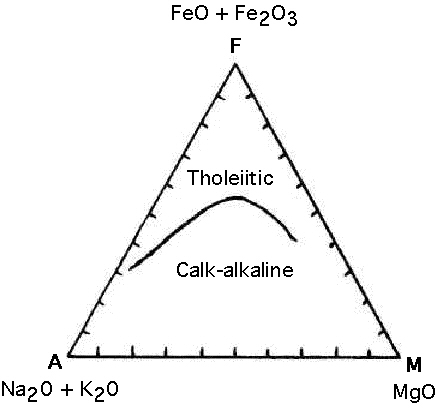 Considering a few more oxides, an AFM plot is useful in defining the composition of tholeiitic and calc-alkaline magmas (Figure 3). Each vertex of the triangle represents an oxide or group of oxides. The "A" vertex represents Na2O + K20, the "F" vertex represents FeO+Fe2O3, and the "M" vertex represents MgO.
Considering a few more oxides, an AFM plot is useful in defining the composition of tholeiitic and calc-alkaline magmas (Figure 3). Each vertex of the triangle represents an oxide or group of oxides. The "A" vertex represents Na2O + K20, the "F" vertex represents FeO+Fe2O3, and the "M" vertex represents MgO.
Figure 3. AFM diagram used to define tholeiitic and calc-alkaline magmas (from Wilson, 1995).
However, analysis of oxides does not guarantee that given a set of weight percentages of particular oxides a unique parent magma can be identified. As can be seen in the table below, tholeiitic and calc-alkaline magmas can form in a variety of tectonic settings.
| TECTONIC SETTING |
Convergent Plate Margin |
Divergent Plate Margin |
Intra-Oceanic |
Intra-Continental |
| COMMON MAGMA(S) |
Tholeiitic, calc-alkaline, alkaline |
Tholeiitic |
Tholeiitic, alkaline |
tholeiitic, alkaline |
Trace Elements
 Trace elements can provide a more unique "fingerprint" of magmas (Figure 4). The behavior of certain trace elements can be related to tectonic environments. Data are usually plotted in a "spiderdiagram" and are typically normalized to mantle composition. For example, positive spikes of Ba, K, and Sr are related to fluid flow in a subduction zone and are commonly observed in calc-alkaline subduction zone samples.
Trace elements can provide a more unique "fingerprint" of magmas (Figure 4). The behavior of certain trace elements can be related to tectonic environments. Data are usually plotted in a "spiderdiagram" and are typically normalized to mantle composition. For example, positive spikes of Ba, K, and Sr are related to fluid flow in a subduction zone and are commonly observed in calc-alkaline subduction zone samples.
Figure 4. Spiderdiagram of a calc-alkaline and MORB rock (from Wilson, 1995).
Isotopes
Not only are isotopes a powerful tool for obtaining the absolute age of crystallization, isotopes can be used to infer tectonic environments when compared with standard samples. The following summary is from Fowler (1991).
Rubidium-Strontium
87Rb decays to 87Sr with a half life of 48800*106years.

When using the rubidium-strontium system, one must be careful of an open system transporting rubidium and strontium into and out of the system. By plotting 87Sr/86Sr against 87Rb/86Sr values for a sample, the y-intercept of the data is termed Isr, or the initial ratio of 87Srinitial to 86Srinitial.
Uranium-Lead
There are two main uranium-lead systems, 238U decays to 206Pb with a half life of 4468*106years, and 235U decays to 207Pb with a half life of 704*106years. By combining the decay relations, an age estimate can be determined from:

Samarium-Neodymium
The samarium-neodymium system is a powerful system for not only age dating minerals, but also for interpreting the source region of a magma. 147Sm decays to 143Nd with a half life of 106000*106years.

By comparing Nd isotope values of a sample against the primitive mantle reference (CHUR - chondritic uniform reservoir), an end value can be obtained.

A rock derived from the primitive lower mantle will have end = 0, suggesting that the rock has not undergone any partial melting. Rocks derived from partial melts of primitive mantle will have end < 0 while rocks derived from the residue of CHUR melts (i.e., not the partial melt of CHUR) will have end > 0. Common end values for the following settings are common: continental crust (-15), continental flood basalt (0), magmatic arc (+8), ocean island basalts (OIB) (+6), mid-ocean ridge basalts (MORB) (+10).
Next Page |
Previous Page |
References |
Main Page
 Two fundamental
processes affect the composition of igneous rocks: partial melting and
fractional crystallization. Additional processes that can influence the
composition of igneous rocks include magma mixing and crustal contamination.
Partial melting can occur due to heating, decompression, and the addition
of fluids and contaminants. Partial melting tends to increase the percentage
of alkalic oxides in rocks, while fractional crystallization tends to
increase the percentage of silica in rocks. Fractional crystallization,
magma mixing, and crustal contamination often occur in high-level magma
chambers. A common way to categorize igneous rocks is to plot weight percentage
of oxides Na2O + K2O against weight percent SiO2,
a plot termed a Harker diagram (Figure 1). The plot is divided into alkalic
and sub-alkalic fields, and also by color index (SiO2).
Two fundamental
processes affect the composition of igneous rocks: partial melting and
fractional crystallization. Additional processes that can influence the
composition of igneous rocks include magma mixing and crustal contamination.
Partial melting can occur due to heating, decompression, and the addition
of fluids and contaminants. Partial melting tends to increase the percentage
of alkalic oxides in rocks, while fractional crystallization tends to
increase the percentage of silica in rocks. Fractional crystallization,
magma mixing, and crustal contamination often occur in high-level magma
chambers. A common way to categorize igneous rocks is to plot weight percentage
of oxides Na2O + K2O against weight percent SiO2,
a plot termed a Harker diagram (Figure 1). The plot is divided into alkalic
and sub-alkalic fields, and also by color index (SiO2).