Shelley D. Copley
.jpg)
Ph.D. Harvard University, 1987
Professor; Molecular, Cellular and Developmental Biology
E-mail: shelley.copley@colorado.edu
Office: CIRES 241
Phone: 303-492-6328
Copley Research Group
Research Interests
Biodegradation of recalcitrant pollutants; mechanistic studies of enzymes involved in biodegradation.
Current Research: Adaptation of E. coli to use a novel pathway for vitamin B6 synthesis
Pyridoxal 5’-phosphate (PLP, aka vitamin B6) is a cofactor required for 40 different enzymes in Escherichia coli, including the transaminase enzymes that generate amino acids needed for protein synthesis. Deletion of a gene required for PLP synthesis prevents growth of E. coli on glucose as a sole carbon source because the cells cannot make amino acids. We have adapted a strain of E. coli (lacking an enzyme in the PLP biosynthesis pathway) to grow on glucose as a sole carbon source nearly as well as wild type E. coli. This strain, which we call the “champion,” has reconstituted a pathway for synthesis of PLP via a novel route as a consequence of only six mutations. By reconstructing strains containing various combinations of these mutations, we found that the order in which the mutations occur is critical; some of the mutations are beneficial in some genetic backgrounds, but detrimental in others.
We are currently investigating the mechanisms by which the six mutations enhance growth rate. This effort requires consideration of the effects of mutations on specific proteins, as well as effects that are propagated through the complex metabolic and regulatory networks. One mutation increases the concentration of an enzyme required in the novel and relatively inefficient pathway for PLP synthesis, presumably resulting in increased production of PLP. Two other mutations appear to act by redirecting fluxes through the metabolic network. These mutations may increase the concentrations of the alpha-keto acid precursors of amino acids and compensating, to some degree, for the decreased level of PLP-containing transaminases.
This project is providing new insights into the ways in which mutations can reprogram the metabolic networks of bacteria and allow cells to adapt to new challenges.
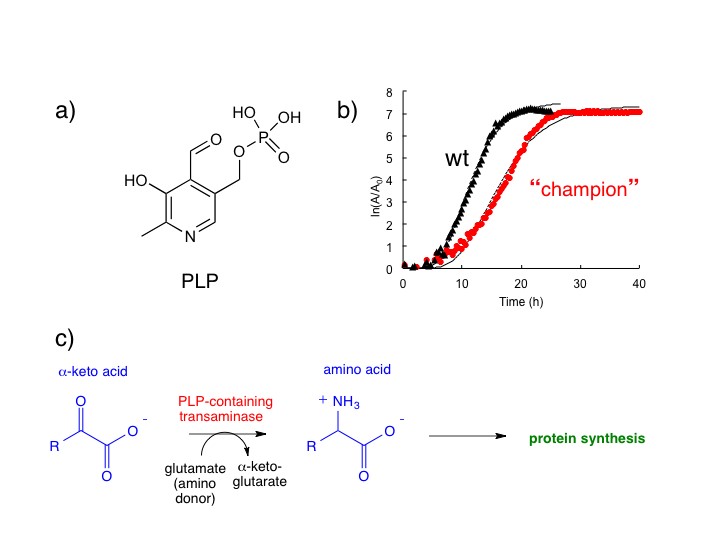
The structure of pyridoxal 5'-phosphate (PLP). b) The "champion" grows nearly as fast as wild type (wt) E. coli. c)
Publications
Click here for a complete list of published works »
Shelley Copley is a member of the CIRES Professor.

