Rainer Volkamer

Ph.D. Physics, University of Heidelberg, Germany
Assistant Professor
Chemistry and Biochemistry
E-mail: rainer.volkamer@colorado.edu
Office: Ekeley M325
Phone: 303-492-1843
Web: Prof. Rainer Volkamer & Group webpage
Research Interests
Rainer’s general interest is the study of atmospheric chemistry in air quality and climate science, using a combination of in-situ and remote sensing measurement techniques, which he and his group are developing and deploying in polluted urban and pristine atmospheric environments from ships, research aircrafts, and autonomous ground-based networks, and in simulation chamber experiments to develop and test the mechanistic understanding represented in atmospheric models used to manage air resources and climate.
Current Research: Atmospheric waters: multiphase chemistry in clouds and aerosols
Water is a major metastable component of the lower atmosphere (see photo) and present at most atmospheric interfaces, including aerosols. We are interested in understanding the multiphase chemistry of glyoxal, an unexplained component in arctic, marine, forest, and urban aerosols, and its indicator properties to understand phase partitioning and chemical processes in aerosol water. Multiphase chemistry in aqueous aerosols is largely missing in atmospheric models for lack of data, missing information

Clouds and aerosol water are chemical reactors for multiphase chemistry.
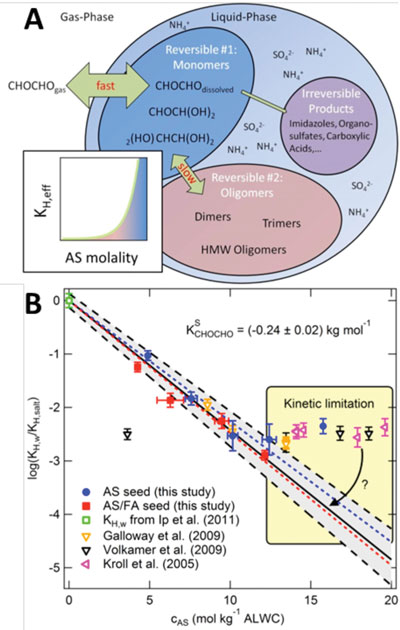
The Henry's law partitioning coefficient is an exponential function of salt concentration. As the ammonium sulfate (AS) concentration exceeds the AS solubility (12 mol kg-1), a phase transition is observed (adopted from Kampf et al. 2013).
Publications
Click here for a complete list of published works »
Honors and Awards
- NSF CAREER Award recipient, 2009
- Feodor-Lynen Fellow (2005-2007)
- Henry & Camille Dreyfus Fellow (2002-2004)
- Marie Curie Fellow (1998-2000)
- Erasmus Fellow (1992-1993)

