|
|
A winning proposal for the Innovative Research Program, 2007: Optimizing a "Green Chemistry" Bioprocess: Decreasing Toxicity to Microbes During Conversion of Naphthalene to 1-NaphtholInvestigators: Shelley D. Copley* and Ryan T. Gill** Naphthalene is a polycyclic aromatic hydrocarbon found in crude oil and coal tar. It is a priority environmental pollutant because of its toxicity and mutagenicity. Microbes expressing the enzyme toluene 4-monooxygenase can convert naphthalene to 1-naphthol (see Figure 1),1 a valuable chemical that is generally produced using environmentally damaging processes. Over 15,000 tons of 1-naphthol are produced each year in the U.S. alone.2 The bioconversion of naphthalene from contaminated soils to 1-naphthol offers the opportunity to both recoup part of the cost of bioremediation and to generate a commodity chemical via an environmentally benign process.
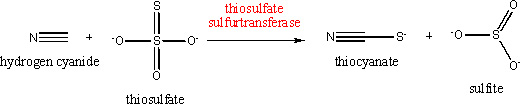
Figure 1. Conversion of naphthalene to 1-naphthol by toluene 4-monooxygenase. Unfortunately, the toxicity of 1-naphthol limits the efficiency of the bioconversion process. Ryan Gill's group has used a novel microarray technology called SCALES3 to identify genes that, when overexpressed, allow E. coli cells to resist the toxicity of 1-naphthol. One of these genes is pspE, which encodes a protein called thiosulfate sulfurtransferase. We will abbreviate this protein as PspE. The goal of this proposal is to elucidate the mechanism of this protective effect. PspE is a mysterious protein. It is expressed when cells are subjected to a variety of stresses, including exposure to solvents such as ethanol and hexane, high temperatures, and infection by phages (viruses that infect bacteria). It has been shown to catalyze the reaction shown in Figure 2.4 Its ability to catalyze the reaction shown below is not impressive. The KM (which corresponds to the concentration required to achieve half the maximal enzyme activity) for hydrogen cyanide is 27 mM, and for thiosulfate is 4.6 mM. These high values suggest that catalysis of this reaction may not be the physiological role of the enzyme. Furthermore, in the experiments carried out in Ryan Gill's lab, hydrogen cyanide and thiosulfate were not present in the reaction medium.
Figure 2. A reaction catalyzed by thiosulfate sulfurtransferase. We will explore two hypotheses for the mechanism of the protective effect of PspE. First, we will examine whether PspE converts 1-naphthol to a less toxic metabolite. This will be accomplished by purifying the protein and determining whether it modifies 1-naphthol, and, if it does, identifying the product. Second, we will determine whether overexpression of PspE protects cells from perturbation of membrane properties by 1-naphthol. Partitioning of 1-naphthol into the hydrophobic membrane would be expected to alter membrane fluidity, and cells might compensate by altering the composition of the membrane lipids. We will analyze the membrane lipids of cells exposed to 1-naphthol in the presence and absence of overexpressed PspE. We will also examine the effect of 1-naphthol on the proton gradient normally maintained across the cell membrane that is used to generate ATP and drive transport processes. The toxicity of 1-naphthol may be due to its ability to dissipate this proton gradient. If this is the case, we will determine whether overexpression of PspE allows cells to maintain the proton gradient in the presence of 1-naphthol. An understanding of the mechanism by which PspE decreases susceptibility to the toxic effects of 1-naphthol would allow us to engineer strains in which this protective effect is optimized and would enhance the utility of the bioconversion process. It would also have broader significance because of this protein's role in response to other stressful processes, as well.
|