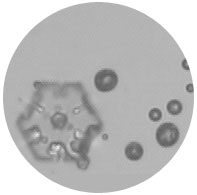
An ice crystal is born
Huddled around a monitor in her laboratory, Margaret Tolbert and two of her graduate students stare intently at a white screen filled with a bunch of black specks, waiting.
A machine pumps water vapor into the sample. Then, as if by magic, little spears of translucent material sprout from a particularly large speck in the lower right-hand corner of the screen. "Look, there one goes!" says Tolbert, Distinguished Professor and CIRES Fellow.
And just like that, an ice crystal is born.
By studying ice formation at this tiniest of scales, Tolbert seeks to understand a much bigger phenomenon: cirrus clouds, which affect climate. "Clouds are one of the largest uncertainties for climate predictions," said Tolbert.
Airplane contrails, fossil fuel emissions, and biomass burning all create particles that can seed the formation of these wispy, ice-laden heat-trapping clouds. Those human activities are changing — while cirrus clouds only occupy 3 percent of busy flyways now, by 2050, scientists expect that number to be closer to 20 percent. So Tolbert and her colleagues are scrutinizing the basic processes that form the clouds, to better understand the influence of human activities.
Clouds are made up of thousands and thousands of ice crystals. Yet, in a batch of particles, ice will only form on one out of every 10,000. Tolbert and her team want to find out what makes the one particle in a batch so ice-worthy.
To do that, Tolbert and her graduate students coat tiny discs with lab-grown versions of air particles found in the tropical tropopause — a cirrus cloud-laden stretch of atmosphere about 12 miles above the tropics. The team then creates pockets of tropical tropopause-like atmosphere in the lab, so they can subject the particles to conditions of temperature, pressure, and water vapor that lead to ice formation.
Then, they wait to see which one will be the most attractive for ice formation.
Size, chemical content, and physique influence why one particle gets the ice crystal and others don't. Larger inorganic particles, for example a speck of soil dust with lots of rough edges, seem to make primo ice surfaces, Tolbert found.
Not only that, but once one particle grows ice, the rest are out of luck. Chances are, all the ingredients have been used up, says Tolbert. "It's like first come, only served."
